Abstract
Background
Artemisia splendens from the Asteraceae family is a new source of biologically active compounds. The current study investigated to evaluate antimicrobial and cytotoxicity activity of methanolic extracts and their fractions obtained from aerial parts by agar disk diffusion and MTT methods, respectively. The active fractions were subjected to preparative HPLC for isolating the pure compounds, which were structurally elucidated, by 1H and 13C NMR.
Results
The results showed that the methanolic extract and its 60% SPE fraction have the anti-proliferative activity on A549 cell line in comparison with the control group. Meanwhile, the methanolic extract and its 40% SPE fraction can inhibit the growth of Gram-positive strains as anti-microbial activity. The 60% SPE fraction also illustrated anti-proliferative activity on the HT-29 cell line compared to the control group. Chromatographic separations via preparative HPLC yielded 5 flavonoids and three flavonoid glycosides.
Conclusion
Based on the results it can be concluded that A. splendens as a potential source of cytotoxic and antimicrobial compounds can be used in pharmaceutics.
Similar content being viewed by others
Introduction
The genus Artemisia is one of the largest and most important genera of the Asteraceae family used in traditional medicine worldwide [1]. Biologically active secondary metabolites such as phenolic compounds (flavonoids, phenylpropanoids, and coumarins), and terpenoids (sesquiterpene lactones) [2], which were isolated from plants of this genus, exhibited various pharmacological activities such as antimalarial, antitumor, antioxidant [3], anti-microbial [4], and anti-inflammatory [5]. It is also a large genus (with about 400 species), distributed in different parts of the world particularly in Europe, North America, Asia, and South Africa [1]. Thirty-four species of this genus are distributed in Iran. Among them, Artemisia splendens Wild. is commonly known as “Asia minor wormwood” and “Dermane derakhshan” in Persian. This species is one of the most popular herbs in traditional medicine and is mostly used for the treatment of diseases like malaria, hepatitis, cancers, and inflammations. Afshar et al. reported that the main volatile compounds of A. splendens were 1,8-cineol (4.7%), caryophyllene oxide (3.8%), valencene (3.5%) and α–terpinyl acetate (3.4%) [6]. In addition, Kazemi et al,. indicated that the major constituents of A. splendens were 1,8-cineole (14.5%), germacrene D (14.3%), α-pinene (11.3%) and bicyclogermacrene (11.3%)[7]. However, their chemical composition has not hitherto been described. Thus, the aim of this study was to analyze the chemical constituents of methanolic fractions obtained from the aerial parts of A. splendens to exert antimicrobial activity and cytotoxic effects on cancerous cells.
Material and methods
Plant material
Artemisia splendens aerial parts were collected from Arasbaran, at E: 31° 49′ 46″, N: 10° 49′ 38″ (altitude of 2470) East Azerbaijan Province, Iran in 2015. The plant material was identified by botanist Dr. Fatemeh Ebrahimi and the voucher specimen of the plant was deposited at the Herbarium of Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran, under the registry code Tbz-FPh 717.
Extraction
Air-dried aerial parts (100 g) were ground and extracted in a Soxhlet apparatus using MeOH. The extract was concentrated under the vacuum by a rotary evaporator at 45 °C and stored under refrigeration before analysis and biological evaluation.
Fractionation of methanolic extract
Two grams of the MeOH extract were fractionated on Sep-Pak™ Vac 35 cc (10 g) C18 cartridge (Waters) eluting with a step gradient of MeOH-H2O mixtures (10:90, 20:80, 40:60, 60:40, 80:20, 100:0). A rotary evaporator was used to remove solvents at 45 °C under a vacuum. The procedure was repeated to increase the amount of SPE fractions.
Isolation and identification of compounds
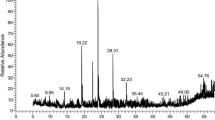
The pure compounds of (60:40) methanolic fractions were collected according to the method described by Afshar et al. [8], using preparative reversed-phase HPLC (Knauer, preparative pump 1800), with photodiode array detector (PDA) (Fig. 1). The flow rate was 8 ml/min and the injection volume was 1 ml. Furthermore, 40% SPE fraction was subjected to preparative reversed-phase HPLC using the following gradient elution: 0–30 min, linear gradient of 40–60% MeOH in water; 30–35 min, maintained in 60% MeOH in water; 35–38 min, linear gradient from 60 to 100% MeOH in water; 38–41 min, maintained in 100% MeOH; 41–43 min, linear gradient from 100 to 40% MeOH in water; 43–45 min, maintained in 40% MeOH in water; flow-rate: 8.0 mL/min and injection volume was 1 ml. Detection was done by a photo-diode-array detector (PDA) at 220 and 280 nm. This method allowed us to obtain astragalin [6], isorhamnetin-3-O-beta-d-glucoside [7] and narcisin [9]. Structures of compounds were elucidated by the literature data of respective compounds [3, 6,7,8,9]. Moreover, shift reagents in UV method were used [10] as follows: sodium methoxide (NaOMe) (Merck), aluminum chloride (AlCl3), aluminum chloride/ hydrochloric acid (AlCl3/HCl) (Merck) sodium acetate (NaOAc) and sodium acetate/boric acid (NaOAc/H3BO3) (Merck).
Anti-proliferative activity
Cell culture
HT-29 (colon carcinoma cell line) and A549 (adenocarcinoma human alveolar basal epithelial cells) cell lines were prepared and cultured according to described method by Afshar et al. [11]. At 75% confluence, the cells were washed by phosphate-buffered saline (PBS) and cultured in 96-well plates (Nunc, Denmark) for MTT experiment.
MTT assay
The anti-cancer effect of the MeOH extract of A. splendens and its fractions were evaluated by MTT reduction colorimetric assay. The cells were cultured in a 96-well plate at a density of 1 × 104 cells/well and incubated for 24 h. Cells were treated with different concentrations of extract and fractions which were prepared in dimethyl sulfoxide (DMSO). After 48 h of incubation, the medium was discarded and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml in PBS) was added to each well and incubated at 37 °C and 5% CO2 for 4 h [12]. The medium was removed and then DMSO was added to dissolve purple insoluble formazan. The wells were shaken for 5 min and then the optical density of each well was determined at 570 nm wavelength by a microplate reader (ELISA plate reader, Bio Teck, Bad Friedrichshall, Germany). Each assay was accomplished three times for each cell line. Antiproliferative activity of extracts and their fractions were measured by relative viability according to the method described by Afshar et al. [11].
Antimicrobial activity
Microbial strains
The following lyophilized forms of microbial cultures purchased from Pasture Institute, Tehran, Iran were used in this study: Gram-negative Pseudomonas aeruginosa (ATCC 9027) and Escherichia coli (ATCC 8739), Gram-positive Staphylococcus epidermidis (ATCC 12228), Staphylococcus aureus (ATCC 6538), and yeast Candida albicans (ATCC 10231).
Disc diffusion test
Activated microorganisms were transferred into Muller-Hinton broth medium (Merck, Germany) and incubated overnight at 37 °C. For providing an optical density of 0.5 McFarland (108 CFU/mL as a standard optical density) of bacterial concentration, the centrifuged pellets (3000 rpm for 15 min) were washed twice and suspended in saline solution. The ultimate concentration of inocula was set up to 106 CFU/mL with sterile saline solution. To achieve unified microbial growth, 10 mL of prepared inoculum suspensions were seeded in the autoclaved Muller-Hinton agar medium. Sterile discs (Whatman paper no. 6 mm diameter) located on the surface of media, were impregnated with 50 µL of different concentrations of extracts (1:1, 1:5, 1:10) which were dissolved in 50% aqueous DMSO. Then they were imbued with a 0.5 McFarland’s standard of mentioned bacteria covering 3 disks for test samples, one disk was used for negative control (including antimicrobial extracts and culture medium) and one disk was used for the positive control (including 0.5 McFarlend of the pathogen and culture medium). Afterward, for incubation and analysis, 100 µL of test solutions were poured into respective wells (200 mg/mL). The plates were transferred into the refrigerator to allow the diffusion of extracts approximately for 30 min, and then petri dishes were incubated at 37 °C for 24 h. Finally, the diameter of the inhibition zones obtained nearby each well (excluding well diameter), showing no bacterial growth, was measured with venire caliper. Doublet plates were prepared for each sample. Extracts and fractions displaying considerable anti-microbial effects were further evaluated for their minimum inhibitory concentration (MIC), which is the lowest concentration of each product for completely inhibition of the bacterial growth [13]. Successively, two-fold dilutions of MeOH extract and its fractions were utilized in broth. The pure sterile nutrient broth was used as control. MIC values were calculated after 24 h incubating the plates at 37 °C [14].
Statistical analysis
All statistical analyses were done using Graph Pad Prism 8.01 and SigmaPlot 11 software. One Way ANOVA with a post hoc Tukey test was used for evaluating significant differences between groups. The experiments were performed in triplicates (n = 3) and all data were presented as mean ± S.D. P values < 0.05 which are regarded as significant.
Results
In the present study, the pure compounds [1,2,3,4,5,6,7,7, 9] obtained from SPE fractions have been assayed. Moreover, anti-proliferative and anti-microbial activities of the MeOH extract and its different SPE fractions from aerial parts of A. splendens were determined and the results are indicated in Tables 1, 2. (NMR spectroscopic data of compounds 1–8 (CD3OD, chemical shift δ in ppm, coupling constant J in Hz in parentheses):
Compound 1: 1HNMR and 13CNMR of the isorhamnetin-3-O-rutinoside was as follows: 1HNMR (200 MHz, CD3OD): Aglycone moiety: δ 7.95(1H, d, J = 2 Hz, H2′), δ 7.65 (1H, dd, J = 8.48,2 Hz, H6′), δ 6.91(1H, d, J = 8.48 Hz, H5′), δ 6.42 (1H, d, J = 2 Hz, H8), δ 6.22 (1H, d, J = 2 Hz, H6), δ 3.59 (3H, S, 3′-OMe). Glucose moiety: δ 5.24 (1H, d, J = 7.59 Hz, H1″), 3.2–3.6 (signal patterns unclear due to over lapping, H2″-3″-4″-5″), δ 3.90 (2H, H6″), δ 4.54 (l H, S, Hl‴), 3.2–3.6 (signal patterns unclear due to over lapping, H2‴-3‴-4‴-5‴) δ 1.16 (3H, d, J = 5.61 Hz, H 6‴). 13CNMR (50 MHz, CD3OD). Aglycone moiety: δ 177.5 (C4), δ 164.6 (C7), δ 162.1 (C5), δ 157.3 (C9), δ 156.8 (C2), δ 149.8 (C3′), δ 146.8 (C4′), δ 134.3 (C3), δ 122.7 (C6′), δ 121.3 (C1′), δ 113.4 (C5′), δ 115.3 (C2′), δ 104.8 (C10), δ 98.3 (C6), δ 93.5 (C8), Glucose moiety: δ 102.7 (C1″), δ 76.7 (C5″), δ 76.1 (C3″), δ 72.5 (C2″), δ 71.0 (C4″), δ 67.1 (C6″), δ 55.3 (OMe), δ 101.2 (C1‴), δ 74.5 (C4‴), δ 70.8 (C2‴), δ 70.5 (C3‴), δ 68.3 (C5‴), δ 16.5 (C6‴).
Compound 2: 1HNMR of quercetin was as follows: 1HNMR (200 MHz, CD3OD): δ 7.74(1H, d, J = 1.91 Hz, H2′), δ 7.66 (1H, dd, J = 8.40,1.91 Hz, H6′), δ 6.90 (1H, d, J = 8.40 Hz, H5′), δ 6.40 (1H, d, J = 1.5 Hz, H8), δ 6.18 (1H, d, J = 1.5 Hz, H6).
Compound 3: 1HNMR and 13CNMR of luteolin was as follows: 1HNMR (200 MHz, CD3OD): δ 7.38 (l H, d, H6′), δ 7.37 (1H, S, H 2′), δ 6.90 (1H, d, J = 8.92 Hz, H5′), δ 6.54(1H, S, H3), δ 6.44 (1H, S, H8), δ 6.21(1H, S, H6).13CNMR (50 MHz, CD3OD): δ 182.4 (C4), δ 164.7 (C7), δ 164.6 (C2), δ 162.1 (C5), δ 158.0 (C9), δ 104.1 (C10), δ 102.4 (C3), δ 98.7 (C6), δ 93.6 (C8), δ 149.6 (C4′), δ 145.6 (C3′), δ 122.2 (C6′), δ 118.9 (C1′), δ 115.3 (C5′), δ 112.7 (C2′). UV spectrum bands II and I respectively (MeOH, λmax, nm): 257, 368; + NaOMe 249, 405; + AlCl3 272, 440; + AlCl3/HCl 268, 420; + NaOAc 270,390; + NaOAc/H3BO3 262, 380. The UV spectrum of compound 5 with MeOH as a solvent is characteristic of flavones derivatives. Studying UV spectra data after the addition of NaOMe and production of 37 nm band I bathochromic shift is indicative of 4′-OH. After 5 min, no destruction of band I is showed that no moiety on C3 was observed. The addition of AlCl3 produced 72 nm band I bathochromic shift, so ortho di-hydroxyl structure of a compound can be elucidated and free 5-OH position of flavonoid is indicated as well. The addition of NaOAc and production of band II bathochromic shift of 13 nm indicated the free 7-OH position of flavonoid. After the addition of H3BO3, and production of 12 nm bathochromic shift on band I confirmed the presence of ortho di-hydroxyl structure.
Compound 4: 1HNMR and 13CNMR of kaempferol was as follows: HNMR (200 MHz, CD3OD): δ 8.10(2H, d, J = 8.70 Hz, H2′, 6′), δ 6.90 (2H, d, J = 8.70 Hz, H3′, 5′), δ 6.41(1H, d, J = 1.44 Hz, H8), δ 6.19 (1H, d, J = 1.83 Hz, H6). 13CNMR (50 MHz, CD3OD): δ 129.3 (C2′, 6′), δ 114.9 (C3′, 5′), δ 98.2 (C6), δ 93.1 (C8),
Compound 5: 1HNMR and 13CNMR of genkwanin was as follows: 1HNMR (200 MHz, CD3OD): 7.85(2H, d, J = 8.80 Hz, H2′, 6′), δ 6.93 (2H, d, J = 8.80 Hz, H3′, 5′), δ 6.66 (1H, S, H3), δ 6.48 (1H, d, J = 1.74 Hz, H8), δ 6.21 (1H, d, J = 1.74 Hz, H6), δ 3.97 (3H, S, OMe). 13CNMR (50 MHz, CD3OD): δ 164.0 (C7), δ 157.1 (C5), 128.0 (C2′, 6′), δ 115.6 (C3′, 5′), δ 103.8 (C3), δ 98.8 (C6), δ 94.4 (C8), δ 55.6 (C3 OMe). UV spectrum bands II and I respectively (MeOH, λmax, nm): 271, 350; + NaOMe 266, 405; + AlCl3 276, 390; + AlCl3/HCl 278, 387; + NaOAc 273,363; + NaOAc/H3BO3 270, 348. UV spectrum of compound 5 confirmed the structure of Genkwanin as well.
Compound 6: 1HNMR and 13CNMR of Astragaline was as follows: HNMR (200 MHz, CD3OD): δ 8.07 (2H, d, J = 8.81 Hz, H2′, 6′), δ 6.93 (2H, d, J = 8.81 Hz, H3′, 5′), δ 6.41(1H, d, J = 2.00 Hz, H8), δ 6.21 (1H, d, J = 2.00 Hz, H6). 13CNMR (50 MHz, CD3OD): δ 130.93 (C2′, 6′), δ 114.81 (C3′, 5′), δ 98.2 (C6), δ 93.1 (C8), δ 62.10- 76.40 (C6″–C2″).
Compound 7: 1HNMR and 13CNMR of Isorhamnetin-3-O-beta-D-glucoside was as follows: HNMR (200 MHz, CD3OD): δ 7.94 (1H, d, H2′), δ 7.59 (1H, d, J = 7.54 Hz, H6′), δ 6.91 (1H, d, J = 7.54 Hz, H5′), δ 6.41(1H, d, J = 2.00 Hz, H8), δ 6.21 (1H, d, J = 2.00 Hz, H6), δ 5.43 (1H, d, J = 6.35 Hz, H1″). δ 3–4 (5H, d, 5 H2″-H6″). 13CNMR (50 MHz, CD3OD): δ 178.80 (C4), δ 164.60 (C7), δ 158.80 (C2), δ 163.50 (C5), δ 160.00 (C9), δ 103.90 (C10), δ 130.90 (C3), δ 98.40 (C6), δ 93.30 (C8), δ 146.80 (C4′), δ 151.90 (C3′), δ 122.30 (C6′), δ 121.10 (C1′), δ 114.60 (C5′), δ 112.9 (C2′), δ 102.10 (C1″), δ 61.90- 72.90 (C6″–C2″).
Compound 8: This compound was named as Narcisin. The NMR data was the same as compound 1.
Cytotoxic assay
In our study, the MeOH extract of A. splendens and its fractions were investigated for their cell growth inhibitory effect in human tumor cell lines such as HT-29 and A549 by MTT assay. The IC50 values of corresponding extract and fractions are reported in Table 1. The results indicated that the methanolic extract had weak to moderate anti-proliferative activity on the A549 cell line in comparison with DMSO control (P < 0.001) with an IC50 value of 617.72 ± 292.90 μg/mL. Whereas (10:90), (20:80), (40:60) MeOH: water SPE fractions did not affect the growth of A549 cells, 60% fraction inhibited cell growth of A549 cell line compared with control (P < 0.001) with an IC50 value of 98.63 μg/mL. In terms of colon cancer cell line, although, MeOH extract, could not decrease the amounts of cancer cells, its 60% SPE fraction significantly indicated anti-proliferative activity (P < 0.001).
Anti-microbial activity
Among all the strains, only inhibition zones were observed against gram-positive S. epidermidis and S. aureus (Table 2). The MIC values for S. epidermidis was 200 mg/mL and for S. aureus 100 mg/mL which considered as very weak activity compared to the control by MeOH extract and 40% SPE fraction.
Discussion
Cancer is one of the diseases with the highest mortality rate in developed countries and the second leading cause of death in developing countries [15]. Among all cancer types, lung and colon cancers are the most commonly diagnosed cancers in both genders [16]. Furthermore, due to the adverse effects of anti-microbial chemical drugs, the use of plants with anti-microbial effects is recommended to reduce side effects. Different studies have revealed that herbal medicines have anti-proliferative and anti-microbial activities through different functional mechanisms [17]. In the current study, the cytotoxic and anti-microbial effects of MeOH extract and its SPE fractions of A. splendens on A549 and HT-29 cells and different microorganisms have been investigated.
The results showed that 60% SPE fraction of MeOH extract had weak to moderate cytotoxic effect on both tumor cells in comparison with other fractions and MeOH extract as well as control (P < 0.001). It is worth mentioning that even though HT-29 was not influenced by MeOH extract, its 60% SPE fraction inhibited the growth of cells (P < 0.001). It can be concluded that the main anti-cancer ingredients of this extract have been accumulated in this fraction. Chromatographic separation of this fraction afforded 5 known flavonoids, namely isorhamnetin-3-O-rutinoside, quercetin, luteolin, kaemferol and genkwanin which are reported in this species for the first time. The anti-proliferative activity of various species of Artemisia has been evaluated by several researchers. These extracts inhibited the growth of different cell lines such as T47D, MCF-7 by inducing apoptosis [18]. Furthermore, previous studies have shown that quercetin and luteolin have cytotoxic effects on A549 with an IC50 value of 10.0 and 3.1 µM, respectively [19]. Luteolin induces apoptosis through the down-regulation of several apoptotic proteins in HT-29 [20]. It has been reported that kaempferol treatment promotes apoptosis of HT-29 human colon cancer cells through both extrinsic and intrinsic pathways [21]. Additionally, the IC50 value of genkwanin on A549 was determined as 9 µM [22]. A combination of solid-phase-extraction (SPE) and reversed-phase prep-HPLC analysis of the aerial parts of the SPE fraction (60%) afforded five flavonoids, which were isorhamnetin-3-O-rutinoside, quercetin, luteolin, kaempferol, and genkwanin. Structural identification of isolated phytochemicals was carried out by UV and NMR analysis and comparison of the data with the reported values. According to our knowledge, none of these phenolic compounds were isolated from A. splendens. Although in prior studies some compounds such as melilotoside have been reported from this plant [23].
The 1H-NMR spectrum of compound 1, revealed a doublet resonance at δ 7.95 (1H, d, 2.0) was specified for H-2′. The doublets at 7.65 (J = 1.70, 8.40 Hz) and 6.91 (J = 8.40 Hz) indicated ortho-coupled aromatic H-atoms assignable to H (6′) and H (5′), respectively. Besides, doublet resonances at δ 6.22 (1H, 2.0) and δ 6.42 (1H, 2.0) for the H (6) and H (8), indicated the meta coupled connection. Furthermore, doublet resonance at δ 5.24 showed the presence of an anomeric proton of glucose. The presence of singlet resonance at δ 4.54 and doublet resonance at δ 1.11(J = 6.04 Hz) is indicative of anomeric proton and CH3 (C6) of rhamnose, respectively. Mentioned data are consistent with the isorhamnetin-3-O-rutinoside (Narcissin) structure which has been proved by the previously published papers. The presence of this compound has been observed in other species of Artemisia such as A. sublessingiana, A. absinthium, A. vulgaris, and A. incana [24, 25].
In terms of compound 2 (quercetin), the UV spectrum of this compound indicated the presence of OH-4′ and 3 moieties due to bathochromic shift of band I and destruction of it after 5 min. The addition of AlCl3 reagent and observing a bathochromic shift in band I has been proved the presence of sensitive and resistant moieties of this structure. Subsequently, by adding HCL, the hypsochromic shift compared to the AlCl3 graph as well as a bathochromic shift to the MeOH graph illustrated the existence of both sensitive moieties (ortho dihydroxy of 3′, 4′) and resistant ones (free 3-OH and 5-OH). Furthermore, 1H and 13C-NMR spectrum have been proved this structure as well. All of the mentioned data are consistent with many previous investigations as well [26]. Spectrum data of compound 4, completely are in line with compound 2, except little difference in B ring with a symmetric structure in which the spectrum data are in line with published papers [27]. In the case of compounds (3) and (5), UV spectra were identical with flavone structure, (which was supported by 1H-NMR and 13C-NMR spectrums, showing characteristic signals appeared at δ 6.54 (1H, s, H-3), 6.66 (1H, s, H-3), luteolin, and genkwanin, respectively [26, 28].
MeOH extract and 40% SPE fraction of this plant showed inhibition zones against S. epidermidis and S. aureus. Although the MIC values for these pathogens are 100 and 200 mg/ml which considered as weak to inactive. It seems that it can be achieved potent anti-microbial agents via more isolating compounds from MeOH extract and 40% SPE fraction. Furthermore, in extract and fractions due to there are broad ranges of compounds, different biological activity might be observed (because the compounds have synergist or antagonist activity with each other). Previous studies showed MeOH extract of A. nilagirica indicated inhibitory activity against Gram-positive, as well as Gram-negative with DIZ value of 12 mm [29]. Furthermore, the MeOH extract of A. nilagirica was active against M. smegmatis [30]. Buffered methanol (80% methanol and 20% PBS) and acetone extracted substances from A. absinthium were active against E. coli, S. infantis, S. aureus, and L. monocytogenes [31]. A. capillaris Thunb. and A. caruifolia Buch. demonstrated inhibitory activity against B. cereus and L. monocytogenes [32]. A. annua and A. vulgaris L. illustrated the most prominent anti-bacterial activity against S. mutans [33].
To the best of our knowledge, no data were available on the antimicrobial activity of A. splendens MeOH extract. 40% SPE fraction for bearing moderate inhibitory effect on microorganisms was selected for further investigation using prep-HPLC afforded three main compounds as following structure. Compound 6 was isolated as yellow needles. HRESIMS analyses (positive and negative ion modes) of compound 1 revealed the pseudo-molecular ions at m/z 471 [M+Na]+ and 447 [M−H]−, which was in agreement with the molecular formula C21H20O11Na and C21H19O11, respectively. The 1H-NMR spectrum of compound 1 revealed the doublets resonance at δ 8.07 (2H, d, J = 8.81 Hz) and δ 6.93 (2H, d, J = 8.81 Hz) were specified for H-2′, H-6′ and H-3′, H-5′, respectively. In addition, doublet resonances at δ 6.41 (1H, d, J = 2.00 Hz) and δ 6.21 (1H, d, J = 2.00 Hz) for the H-8 and H-6, indicated the meta coupled connections, correspondingly. The above-mentioned data suggesting the presence of a flavonoid structure with a para pattern in the B ring. In addition, the 1H-NMR spectra of compound 6 confirmed the presence of an overlapping signal for five protons (δ 3–4 ppm) and an anomeric proton (δ 5.45, 1H, d, J = 7.48 Hz) corresponding to a glucosyl moiety with β configuration. The UV spectrum of compound 1 with MeOH as a solvent is characteristic of flavonols derivatives. Studying UV spectra data after the addition of NaOMe and production of 60 nm band I bathochromic shift is indicative of 4′-OH. Remaining of band I after 5 min indicated that C-3 has substitution except for OH. The addition of AlCl3/HCl did not cause any hypsochromic shift in the AlCl3 spectrum. Therefore, it can be concluded there is no ortho-dihydroxy group (3′ and 4′-OH) in the B ring. Furthermore, it leads to the presence of OH in C-5. Addition of NaOAc and production of band II bathochromic shift of 7 nm indicative of free 7-OH. In studying the 13C-NMR, the presence of four signals at δ 60–75, confirms the presence of glucose in the composition. Moreover, signals at δ 130.93 and 114.81 corresponded to C-2′, C-6′ and C-3′, C-5′, respectively. Thus, compound 6 was identified as Astragalin. All spectroscopic data were in good agreement with the literature data of respective compounds [34, 35]. While Astragalin [6] was previously reported from A. transiliensis [36] and A. annua, [3] this is the first report on the presence of Astragalin in the A. splendens.
Compound 7 was isolated as a yellow amorphous powder. ESIMS and FABMS analyses (negative and positive ion modes) of compound 7 revealed the pseudo-molecular ions at m/z 477 [M−H]− and 479 [M+H]+, which was in agreement with the molecular formula C22H21O12 and C22H23O12, respectively. The 1H-NMR spectrum of compound 7, revealed a singlet resonance at δ 7.94 (1H, s) was specified for H-2′. The doublets at δ 7.59 (1H, d, J = 7.54 Hz) and δ 6.91 (1H, d, J = 7.54 Hz) indicated ortho-coupled aromatic H-atoms assignable to H-6′ and H-5′ in the B ring of flavonoid, respectively. In addition, doublet resonances at δ 6.41 (1H, s) and δ 6.21 (1H, s) for the H-8 and H-6 indicating the presence of the meta coupled connection. There was also a methoxy signal (δH 3.95, 3H, s) that is indicative of 3′-OCH3. Although an anomeric proton (δ 5.43, 1H, d) and some of the glycosidic protons overlapped (δ 3–4 ppm) in the 1H-NMR spectrum, in studying the 13C-NMR, the presence of five signals at δ 60–75 and a resonance at δ 102.1 (anomeric carbon of glucose), confirms the presence of glucose in the composition. The presence of a signal at δ 55.3 ppm in the 13C-NMR spectrum indicating a methoxy group which is confirmed by the presence of a singlet resonance at δ 3.95 (3H) in the 1H-NMR spectrum. In 13C -NMR, signals at δ 178.80, 164.60, 163.50, 160.00, 158.80, 130.90, 103.90, 98.40 and 93.30 corresponded to C-4, C-7, C-5, C-9, C-2, C-3, C-10, C-6 and C-8, respectively. Furthermore, signals at δ 151.90, 146.80, 122.30, 121.10, 114.60 and 112.9 corresponded to C-3′, C-4′, C-6′, C-1′, C-5′ and C-2′, respectively. Thus, compound 7 was identified unequivocally as isorhamnetin-3-O-beta-d-glucoside. Spectroscopic data were in good agreement with the literature data of the respective compound [37]. While isorhamnetin-3-O-beta-d-glucoside was previously reported from A. halodendron, [38] this is the first report for reporting this compound from A. splendens. Compound 8 was also isolated as a light yellowish powder from a 60% fraction as well. FABMS analyses (negative and positive ion modes) of compound 8 revealed the pseudo-molecular ions at m/z 623 [M−H]− and 647 [M+Na]+, which was in agreement with the molecular formula C28H31O16 and C28H32O16Na, respectively. The 1H-NMR and 13C-NMR spectra of compound 8 were similar to those of compound 7, with the exception that one more rhamnose moiety has been regarded to this compound with four protons signals (δ 3.2–3.6), doublet resonance at δ 1.11 (3H, d, J = 6.04 Hz) and singlet resonance at δ 4.54 (1H, s), which is attributed to anomeric proton of H-1‴ of rhamnose moiety. Furthermore, the 1H-NMR spectra of compound 8 verified the existence of one isorhamnetin nucleus. Our claim has been supported by methoxy group shift (δ 3.95, 3H, s) in 1H-NMR as well as methoxy carbon signal (δ 55.30) at 13C-NMR; In 1H -NMR two aromatic spin systems, 7.95 (1H, d, J = 1.70 Hz), 7.64 (1H, dd, J = 8.40 and 1.70 Hz) and δ 6.95 (1H, d, J = 8.40 Hz) corresponded to H-2′, H-6′ and H-5′, respectively. Remaining peaks have been considered for 6.41 (1H, s) H-8 and 6.22 (1H, s) H-6.
The 1H-NMR spectrum of compound 3 showed the presence of one glucosyl moieties with characteristic doublet resonance at δ 5.24 (1H, d, J = 7.18 Hz) for the anomeric proton of β-glucose. In addition, the 1H-NMR spectra of compound 1 confirmed the presence of overlapping signals for four protons (δ 3.2–3.6 ppm), an anomeric proton (δ 4.54, 1H, s) and δ 1.11 (3H, d, 6.04) (methyl group of rhamnose), corresponding to a rhamnose moiety in the composition. In studying the 13C-NMR spectrum, the presence of five signals at δ 60–75 and a resonance at δ 102.7 (anomeric carbon of glucose), confirms the presence of glucose in the composition as well as the presence of four signals at δ 60–75, presence of a signal at δ 101.2 (anomeric carbon of rhamnose) and δ 16.5 (methyl group of rhamnose), was confirmed the presence of rhamnose in the composition. Furthermore, signals at δ 177.50, 164.60, 162.10, 157.30, 156.80, 134.30, 104.80, 98.10 and 93.50 corresponded to C-4, C-7, C-5, C-9, C-2, C-3, C-10, C-6 and C-8, respectively; as well as, signals at δ 149.80, 146.80, 122.70, 121.30, 115.30 and 113.40 corresponded to C-3′, C-4′, C-6′, C-1′, C-2′ and C-5′, respectively. On the basis of these data, the structure of 8 was elucidated as narcissin. Finding a literature review of previous works was compatible with our results [39]. While isorhamnetin-3-O-beta-D-glucoside was previously reported from A. sublessingiana, A. absinthium, A. vulgaris [40] and A. incana, [41] to the best of our knowledge, the respective compound was detected in this specie for the first time.
Conclusion
In the current study, we reported the anti-proliferative and anti-microbial activities as well as chemical compositions of 60% and 40% SPE fractions which indicated maximum cytotoxic (against A549 and HT-29 cancerous cells) and inhibition zones against Gram-positive strains, respectively for the first time. Further analysis of both potent fractions afforded 5 flavonoids: narcisin (1), quercetin (2), luteolin (3), kaempferol (4) and genkwanin (5) from 60% SPE fraction as well as three known flavonoid glycosides, astragalin (6), isorhamnetin-3-O-beta-D-glucoside (7) as well as narcisin (8) from 40% SPE fraction for the first time from the A. splendens.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article.
References
Valant-Vetschera K, Fischer R, Wollenweber E (2003) Exudate flavonoids in species of Artemisia (Asteraceae—Anthemideae): new results and chemosystematic interpretation. Biochem Syst Ecol 31(5):487–498
Lee SH, Lee M-Y, Kang H-M, Han DC, Son K-H, Yang DC et al (2003) Anti-tumor activity of the farnesyl-protein transferase inhibitors arteminolides, isolated from Artemisa. Bioorg Med Chem 11(21):4545–4549
Ferreira JF, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules. 15(5):3135–70
Habibi Z, Yousefi M, Mohammadi M, Eftekhar F, Biniyaz T, Rustaiyan A (2010) Chemical composition and antibacterial activity of the volatile oils from Artemisia turcomanica. Chem Nat Compd 46(5):819–821
Moscatelli V, Hnatyszyn O, Acevedo C, Megías J, Alcaraz MJ, Ferraro G (2006) Flavonoids from Artemisia copa with anti-inflammatory activity. Planta Med 72(01):72–74
Zhou Y, Wang W, Tang L, Yan X, Shi L, Wang Y et al (2009) Lignan and flavonoid glycosides from Urtica laetevirens Maxim. J Nat Med 63(1):100–1
Mabry TJ, Markham KR, Thomas MB (1970) Reagents and procedures for the ultraviolet spectral analysis of flavonoids. Springer, The systematic identification of flavonoids, pp 35–40
Afshar FH, Delazar A, Nazemiyeh H, Khodaie L, Moghaddam SB (2015) Phenolic Derivatives of Artemisia Spicigera C. Koch Growing in Iran. Iran J Pharm Res 14(4):1241
Hartwig UA, Maxwell CA, Joseph CM, Phillips DA (1990) Chrysoeriol and luteolin released from alfalfa seeds induce nod genes in Rhizobium meliloti. Plant Physiol 92(1):116–122
Andersen OM, Markham KR (2005) Flavonoids: chemistry, biochemistry and applications. CRC Press, New York
Heshmati Afshar F, Asgharian P, Khodaie L, Delazar A, Lotfipour F, Baradaran B. Anti-proliferative and antimicrobial activity of methanolic extract and SPE fractions of Artemisia spicigera (2017 ). Jundishapur J Nat Pharm Prod 12(2)
Tarhriz V, Eyvazi S, Musavi M, Abasi M, Sharifi K, Ghanbarian H et al (2019) Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem 120(11):18854–18861
Tarhriz V, Eyvazi S, Shakeri E, Hejazi MS, Dilmaghani A (2020) Antibacterial and antifungal activity of novel freshwater bacterium Tabrizicola aquatica as a prominent natural antibiotic available in Qurugol Lake. Pharm Sci 26(1):88–92
Asgharian P, Afshar FH, Asnaashari S, Lotfipour F, Baradaran B (2017) Evaluation of various biological activities of the aerial parts of Scrophularia frigida growing in Iran. IJPR 16(1):277
Mathers C (2008) The global burden of disease: 2004 update. World Health Organization, Berlin
Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics, 2000. CA J Clin. 50(1):7–33
Safarzadeh E, Delazar A, Kazemi T, Orangi M, Shanehbandi D, Esnaashari S. The cytotoxic and apoptotic effects of Scrophularia atropatana extracts on human breast cancer cells. 2017.
Hajdú Z, Hohmann J, Forgo P, Máthé I, Molnár J, Zupkó I (2014) Antiproliferative activity of Artemisia asiatica extract and its constituents on human tumor cell lines. Planta Med 80(18):1692–1697
Kawaii S, Tomono Y, Katase E, Ogawa K, Yano M (1999) Antiproliferative activity of flavonoids on several cancer cell lines. Biosci Biotechnol Biochem 63(5):896–899
Lim DY, Jeong Y, Tyner AL, Park JH (2007) Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 292(1):G66–G75
Lee HS, Cho HJ, Yu R, Lee KW, Chun HS, Park JHY (2014) Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int J Mol Sci 15(2):2722–2737
Pichette A, Eftekhari A, Georges P, Lavoie S, Mshvildadze V, Legault J (2010) Cytotoxic phenolic compounds in leaf buds of Populus tremuloides. Can J Chem 88(2):104–110
Afshar FH, Delazar A, Nazemiyeh H, Nahar L, Moghaddam SB, Mbaebie BO et al (2017) Melilotoside Derivatives from Artemisia splendens (Asteraceae). Records of Natural Products 11(1):43
Duong TH, Beniddir MA, Nguyen VK, Aree T, Gallard JF, Mac DH et al (2018) Sulfonic acid-containing flavonoids from the roots of Phyllanthus acidus. J Nat Prod. 81(9):2026–31
Ryakhovskaya T, Manadilova A, Sapko O (1985) Flavonoids of Artemisia sublessingiana. Chem Nat Compd 21(3):381–382
Galati G, Moridani MY, Chan TS, O’Brien PJ (2001) Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: glutathione oxidation and conjugation. Free Radical Biol Med 30(4):370–382
Mabry T, Markham KR, Thomas MB (2012) The systematic identification of flavonoids. Springer, Berlin
Avula B, Wang Y-H, Smillie TJ, Mabusela W, Vincent L, Weitz F et al (2009) Quantitative determination of flavonoids by column high-performance liquid chromatography with mass spectrometry and ultraviolet absorption detection in Artemisia afra and comparative studies with various species of Artemisia plants. J AOAC Int 92(2):633–644
Ahameethunisa AR, Hopper W (2010) Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complement Altern Med 10(1):6
Naik SK, Mohanty S, Padhi A, Pati R, Sonawane A (2014) Evaluation of antibacterial and cytotoxic activity of Artemisia nilagirica and Murraya koenigii leaf extracts against mycobacteria and macrophages. BMC Complement Altern Med 14(1):87
Alzoreky N, Nakahara K (2003) Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol 80(3):223–230
Shan B, Cai Y-Z, Brooks JD, Corke H (2007) The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol 117(1):112–119
Chen C-P, Lin C-C, Tsuneo N (1989) Screening of Taiwanese crude drugs for antibacterial activity against Streptococcus mutans. J Ethnopharmacol 27(3):285–295
Deng S, Deng Z, Fan Y, Peng Y, Li J, Xiong D et al (2009) Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (Lotus) by high-speed counter-current chromatography. J Chromatogr B 877(24):2487–2492
Wei Y, Xie Q, Fisher D, Sutherland IA (2011) Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography. J Chromatogr A. 1218(36):6206–11
Chumbalov T, Fadeeva O (1969) Flavonoids of Artemisia transiliensis. Chem Nat Compounds. 5(5):364
Lee YS, Lee S, Lee HS, Kim B-K, Ohuchi K, Shin KH (2005) Inhibitory effects of isorhamnetin-3-O-β-d-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol Pharm Bull 28(5):916–918
Wang Y, Yin J, Qiao Y, Zhang H, Lu X. Studies on antioxidant activity and chemical constituents of Artemisia halodendron. 亚洲传统医药. 2007;2(1):30–3.
Dehaghani ZA, Asghari G, Dinani MS (2017) Isolation and Identification of Nicotiflorin and Narcissin from the Aerial Parts of Peucedanum aucheri Boiss. J Agric Sci Technol A. 54:45
Ruakhovskaya T, Manadilova A, Sapco O. The flavonoids of Artemisia sublessingiana. Akademiya nauk uzbekskol SSR UL kuibysheva 15, Tashkent, Uzbekistan; 1985. p. 407.
Agrawal PK, Rastogi R (1981) C-13 NMR-spectroscopy of flavonoids. Heterocycles 16(12):2181–2236
Acknowledgements
We would like to thank Dr. Baradaran for their supportive help in performing the anti-proliferative part.
Funding
This work was supported and funded scheme by Faculty of Pharmacy, Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
FAH and MZ performed the experiments; ZR and AD analyzed of data; FAH, MZ, ZR and AD prepared the manuscript; PA and VT wrote and edited the manuscript; PA designed the experiments; PA led and supervised the project. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
There is no involvement of human or animal in this study.
Consent for publication
All other authors declare no conflict of interest.
Competing interests
We declare that we have no significant competing financial, professional, or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Heshmati Afshar, F., Zadehkamand, M., Rezaei, Z. et al. Chemical compositions, antimicrobial effects, and cytotoxicity of Asia minor wormwood (Artemisia splendens Willd.) growing in Iran. BMC Chemistry 15, 33 (2021). https://doi.org/10.1186/s13065-021-00759-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-021-00759-w