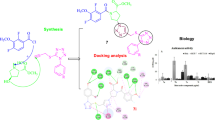
Abstract
Background
1,2,4-oxadiazole derivatives exhibited significant anti-cancer activity when they were evaluated, against human cancer cell lines. They also showed anti-inflammatory, analgesic, diabetic, immunosuppressive, α,β3-receptor antagonist, antimicrobial, anti-helminthic, histamine-H3 and antiparasitic properties. A pyrimidine analog, 5 fluoro-uracil is a chemotherapeutic drug used for treating multiple solid malignant tumors. But its application is limited, as it has side effects like low bioavailability and high toxicity. Molecular docking is an exemplary tool, helps in identifying target and designing a drug containing high bio-availability and minimum toxicity.
Results
A set of 1,2,4-oxadiazole linked 5-fluoruracil derivatives (7a–j) were synthesized and their structures were confirmed by 1HNMR, 13CNMR and Mass spectral analysis. Further, these compounds were investigated for their anticancer activity towards a panel of four human cancer cell lines such as (MCF-7, MDA MB-231), lung cancer (A549) and prostate cancer (DU-145) by using MTT method. Among them, compounds 7a, 7b, 7c, 7d and 7i demonstrated more promising anticancer activity than standard.
Conclusion
Synthesized derivatives (7a–j) of 1,2,4-oxadiazole linked 5-fluorouracil and investigated for their anticancer activity towards a panel of four human cancer cell lines.
Similar content being viewed by others
Background
Over the past few decades, heterocyclic rings containing nitrogen atoms have played a significant role in medicinal chemistry. They are considered as key templates for the development of new therapeutic agents [1]. Among all the nitrogenated compounds, pyrimidines are a more privileged class of six-membered heterocyclic organic units. They occupy a unique position in medicinal chemistry due to their wide range of biological applications [2,3,4,5,6,7,8,9,10,11,12]. Pyrimidines exist as an essential component in several nucleic acids and therapeutic drugs, such as 5-Fluorouracil (1, 5-FU, Fig. 1) [13,14,15,16]. The USFDA-approved drug, 5-FU, is one of the most distinguishable chemotherapeutic drugs available. It was first synthesized by Heidelberger and co-workers [17]. It shows antitumor activity by inhibition of thymidylate synthetase enzyme leading to prevention of DNA synthesis [18], and has been used frequently for the treatment of various solid malignant tumors [19,20,21]. However, it has limited clinical applications because of several side effects, including poor tumor selectivity, toxicity, lower drug-resistance, gastrointestinal toxicity, and adverse effects on central nervous system [22, 23]. Previously, many researchers have developed several 5-FU contained compounds to overcome such side effects [24]. On the other hand, oxadiazoles are a unique class of nitrogen and oxygen atoms containing five-membered ring heterocyclic core units [25]. They are frequently found in marine organisms [26]. These are more attractable heterocyclic structural framework to medicinal chemist [27], due to their broad spectrum of biological properties including (2S)cannabinoid receptor 2 (CB2) [28], immunosuppressive [29], muscarinic [30], α,β3-receptor antagonist [31], antimicrobial [32], insecticides [33], histamine-H3 [34], anti-inflammatory [35], analgesic [36], diabetic, anticancer, antiparasitic and anti-helminthic properties. The US FDA approved drug such as ataluren (2), contains 1,2,4-oxadiazole framework as a part of the structure and is used for the treatment of muscular dystrophy [37,38,39].
The above biological findings and the continuous of demand for the new anticancer agents prompted us to design and synthesize a set of 1,2,4-oxadiazole linked 5-fluorouracil derivatives (7a–j). Their structures were confirmed by 1HNMR, 13CNMR and mass spectral data. Their anticancer activity towards four human cancer cells such as breast cancer (MCF-7, MDA MB-231), lung cancer (A549) and prostate cancer (DU-145) were evaluated.
Results and discussion
The synthesis of 1,2,4-oxadiazole linked 5-fluorouracil derivatives (7a–j) described in this study are outlined in Scheme 1. Commercially available 5-fluorouracil (1) was treated with 4-(bromomethyl)benzonitrile (3) in the presence of a base, DBU and anhydrous DMF at room temperature for 12 h to give an intermediate compound 4 with 67% yield. The resulting nitrile intermediate 4 was reacted with hydroxylamine hydrochloride in triethyl amine base in methanol at reflux for 6 h to afford pure amidoxime compound 5 with 82% yield. Further, intermediate 5 was subjected to cyclization with substituted aromatic carboylic acids (6a–j) in presence of a coupling reagent, EDC·HCl and a base sodium acetate in ethanol at reflux for 3 h to afford pure final compounds 7a–j in good yields.
Experimental section
Materials and methods
All chemicals and reagents were obtained from Aldrich (Sigma-Aldrich, St. Louis, MO, USA), Lancaster (Alfa Aesar, Johnson Matthey Company, Ward Hill, MA, USA) and were used without further purification. Human cancer cell lines such as (MCF-7, MDA MB-231 were collected from Sigma-Aldrich. Reactions were monitored by TLC, performed on silica gel glass plates containing 60 F-254, and visualization on TLC was achieved by UV light or iodine indicator. 1H and 13C NMR spectra were recorded on Gemini Varian-VXR-unity (400 MHz, 300 MHz) instrument. Chemical shifts (d) are reported in ppm downfield from internal TMS standard. ESI spectra were recorded on Micro mass, Quattro LC using ESI+ software with capillary voltage 3.98 kV and ESI mode positive ion trap detector. Melting points were determined with an electro thermal melting point apparatus and are uncorrected.
4-((5-Fluoro-3,4-dihydro-2,4-dioxopyrimidin-1(2H)-yl)methyl)benzonitrile (4)
Compound 3 (14.9 g, 0.076 mol) was dissolved in 10 ml of dry DMF and added dropwise to a solution of 5-fluorouracil (1) (10 g, 0.076 mol) and DBU (12.6 ml, 0.084 mol) in dry DMF (40 ml) under N2 atmosphere at 4 °C. The reaction mixture was allowed to stir at room temperature overnight, and then concentrated. The crude product was purified using flash chromatography (hexane/ethyl acetate = 1:1) to afford corresponding product 4 as 12.6 g with 67% yield. IR (KBr): 3265 (N–H stretching vibration), 3021 (Ar–C–H str.), 2893 (C–H str.), 1632 (NH(C=O)–C=C– stretching vibration of amide), 1562, 1469 (C=N and C=C stretching vibration of pyridine Nucleus), 988 (C–F stretching vibration), 782 (2-Substituted pyridine),1H-NMR (300 MHz, DMSO-d6): \(\updelta \) 4.86 (s, 2H), 7.34 (d, 2H, J = 8.0 Hz), 7.72 (d, 2H, J = 8.0 Hz), 8.23 (d, 1H, J = 6.1 Hz), 11.89 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 156.8, 149.3, 141.6, 141.0, 134.3, 130.1, 126.1, 118.3, 109.1, 49.7; MS (ESI): m/z 246 [M+H]+.
(1E)-4-((5-Fluoro-3,4-dihydro-2,4-dioxopyrimidin-1(2H)-yl)methyl)-N'-hydroxybenzamidine (5)
To a solution of compound 4-((5-fluoro-3,4-dihydro-2,4-dioxopyrimidin-1(2H)-yl) methyl)benzonitrile (4) (12 g, 0.049 mol) in methanol was added Et3N (20 ml, 0.146 mol) and NH2OH·HCl (10.2 g, 0.146 mol), and the reaction mixture was refluxed for 6 h. The reaction mixture was concentrated under vacuum; the residue was diluted with water and extracted with ethyl acetate. The combined organic layers were dried over anhydrous Na2SO4, concentrated in vacuo and purified by flash column chromatography on silica gel by using ethyl acetate/hexane (9:1) to afford the pure product 5 as 11.2 g with 82% yield. IR (KBr): 3314 (N–H stretching vibration), 3052 (Ar–C–H str.), 2962 (C–H str.), 1684 (NH(C=O)–C=C– stretching vibration of amide), 1684, 1485 (C=N and C=C stretching vibration of pyridine Nucleus), 974 (C–F stretching vibration), 766 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 4.82 (s, 2H), 5.88 (s, 2H), 7.26 (d, 2H, J = 8.0 Hz), 7.70 (d, 2H, J = 8.0 Hz), 8.22 (d, 1H, J = 6.0 Hz), 11.89 (s, 1H), 14.35 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 179.6, 156.7, 150.3, 141.3, 140.7, 134.1, 131.0, 129.3, 126.2, 49.2; MS (ESI): m/z 279 [M+H]+.
1-(4-(5-Phenyl-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7a)
A solution of substituted benzoic acid (6a) (0.16 ml, 0.0017 mol) in anhydrous CH2Cl2 (50 ml) was cooled to 0 °C and ethyl-(Nʹ,Nʹ-dimethylamino)propylcarbodiimide hydrochloride (EDC·HCl) (418 mg, 0.00269 mol) was added to it under nitrogen atmosphere. The reaction mixture was stirred at 0 °C for an additional half an hour. To this mixture, (1E)-4-((5-fluoro-3,4-dihydro-2,4-dioxopyrimidin-1(2H)-yl)methyl)-Nʹ-hydroxybenzamidine (5) (500 mg, 0.0017 mmol) was added and stirring was continued for another half an hour at 0 °C. The reaction mixture was slowly allowed to attain room temperature with continuous stirring. Then it was heated to 110 °C for 2 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled down to the room temperature, to obtain the solid product, which was then filtered and washed with CH2Cl2. The resultant white solid was heated for 3 h by dissolving it in a mixture of ethanol (20 ml), sodium acetate (83 mg, 0.0017 mmol) and water (3 ml). The reaction mixture was then cooled down to room temperature. The resultant solid was filtered and purified by recrystallization using ethanol to obtain the desired solid compound 7a with 32% (210.4 mg) yield.
Mp: 316–318 °C, IR (KBr): 3176 (N–H stretching vibration), 2965 (Ar–C–H str.), 2944 (C–H str.), 1642 (NH(C=O)–C=C– stretching vibration of amide), 1598, 1425 (C=N and C=C stretching vibration of pyridine Nucleus), 1065 (C–F stretching vibration), 776 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 4.98 (s, 2H), 7.34–7.45 (m, 3H), 7.61 (d, 2H, J = 8.1 Hz), 7.94 (d, 2H, J = 7.6 Hz), 8.24 (d, 1H, J = 6.1 Hz), 8.28 (d, 2H, J = 8.1 Hz), 11.88 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 52.8, 127.5, 128.4, 128.7, 129.5, 130.4, 131.5, 131.8, 134.2, 134.7, 138.4, 140.3, 145.5, 151.7, 155.6, 157.8, 158.3, 163.7; MS (ESI): m/z 365 [M+H]+.
1-(4-(5-(3,4,5-trimethoxyphenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7b)
The compound, 7b was prepared following the method described for the preparation of the compound 7a, employing 5 (500 mg, 0.0017 mol) with 3,4,5-trimethoxybenzoic acid (6b) (361 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mmol) to afford pure compound 7b, 308.4 mg in 38% yield. Mp: 330–332 °C,IR (KBr): 3181 (N–H stretching vibration), 3062 (Ar–C–H str.), 2923 (C–H str.), 1657 (NH(C=O)–C=C– stretching vibration of amide), 1602, 1403 (C=N and C=C stretching vibration of pyridine Nucleus), 1093 (C–F stretching vibration), 773 (2-Substituted pyridine), 1HNMR (300 MHz, DMSO-d6): \(\delta\) 3.88 (s, 3H), 3.91 (s, 6H), 4.90 (s, 2H), 7.63 (d, 2H, J = 8.0 Hz), 7.69 (s, 2H), 8.23 (d, 1H, J = 6.1 Hz), 8.28 (d, 2H, J = 8.0 Hz), 11.87 (s, 1H); 13CNMR (75 MHz, DMSO-d6): \(\delta\) 52.7, 57.6, 61.5, 106.4, 12.6, 128.5, 128.7, 129.4, 130.4, 131.5, 134.7, 138.2, 140.2, 143.5, 145.4, 151.7, 155.6, 155.8, 157.6, 158.4, 165.7; MS (ESI): m/z 455 [M+H]+.
1-(4-(5-(3,5-dimethoxyphenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7c)
The compound, 7c was prepared similar to the preparation of the compound 7a, in which compound 5 (500 mg, 0.0017 mol) was treated with 3,5-dimethoxybenzoic acid (6c) (309 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg 0.0017 mol) to afford pure compound 7c, 284.2 mg in 37% yield. Mp: 345–347 °C, IR (KBr): 3181 (N–H stretching vibration), 3065 (Ar–C–H str.), 2912 (C–H str.), 1621 (NH(C=O)–C=C– stretching vibration of amide), 1612, 1423 (C=N and C=C stretching vibration of pyridine Nucleus), 1053 (C–F stretching vibration), 762 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 3.79 (s, 6H), 4.98 (s, 2H), 6.98 (s, 1H), 7.61 (d, 2H, J = 8.1 Hz), 7.67 (s, 2H), 8.24 (d, 1H, J = 6.1 Hz), 8.29 (d, 2H, J = 8.1 Hz), 11.89 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 52.6, 57.6, 104.6, 105.7, 127.6, 128.4, 128.7, 130.4, 134.5, 134.8, 138.5, 140.2, 145.8, 151.7, 155.9, 157.4, 158.7, 162.6, 165.7; MS (ESI): m/z 425 [M+H]+.
1-(4-(5-(4-Methoxyphenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7d)
The compound, 7d was prepared following the method described for the preparation of the compound 7a, employing 5 (500 mg, 0.0017 mol) with 4-methoxybenzoic acid (6d) (258 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mol) to afford pure compound 7d, 291.3 mg in 41% yield. Mp: 342–344 °C, IR (KBr): 3181 (N–H stretching vibration), 3021 (Ar–C–H str.), 2895 (C–H str.), 1641 (NH(C=O)–C=C– stretching vibration of amide), 1618, 1386 (C=N and C=C stretching vibration of pyridine Nucleus), 1093 (C–F stretching vibration), 784 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 3.96 (s, 3H), 5.06 (s, 2H), 7.17 (d, 2H, J = 7.7 Hz), 7.63 (d, 2H, J = 8.1 Hz), 8.09 (d, 2H, J = 7.7 Hz), 8.24 (d, 1H, J = 6.1 Hz), 8.28 (d, 2H, J = 8.1 Hz), 11.89 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 52.8, 57.8, 118.4, 118.9, 127.4, 128.6, 128.9, 130.5, 134.5, 138.2, 140.6, 145.6, 151.8, 155.6, 157.3, 158.4, 162.5, 165.8; MS (ESI): m/z 395 [M+H]+.
1-(4-(5-(4-Chlorophenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7e)
This compound, 7e was prepared following similar method used for the preparation of the compound 7a, employing 5 (500 mg, 0.0017 mol) with 4-chlorobenzoic acid (6e) (266 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mol) to afford pure compound 7e, 313.5 mg in 44% yield. Mp: 338–340 °C,IR (KBr): 3163 (N–H stretching vibration), 3075 (Ar–C–H str.), 2897 (C–H str.), 1691 (NH(C=O)–C=C– stretching vibration of amide), 1610, 1422 (C=N and C=C stretching vibration of pyridine Nucleus), 1094 (C–F stretching vibration), 794 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 5.19 (s, 2H), 7.61 (d, 2H, J = 8.1 Hz), 7.72 (d, 2H, J = 7.8 Hz), 8.12 (d, 2H, J = 7.8 Hz), 8.25 (d, 1H, J = 6.2 Hz), 8.30 (d, 2H, J = 8.1 Hz), 11.89 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 53.2, 127.6, 128.7, 128.9, 129.5, 130.4, 130.8, 133.4, 134.5, 134.7, 138.5, 140.3, 145.6, 151.7, 155.6, 157.4, 158.7, 165.8; MS (ESI): m/z 399 [M+H]+.
1-(4-(5-(4-Bromophenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7f)
The compound 7f was prepared using the method described for the preparation of the compound 7a, employing 5 (500 mg, 0.0017 mol) with 4-bromobenzoic acid (6f) (341 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mol) to afford pure compound 7f, 372.4 mg in 47% yield. Mp: 362–364 °C, IR (KBr): 3203 (N–H stretching vibration), 3055 (Ar–C–H str.), 2965 (C–H str.), 1643 (NH(C=O)–C=C– stretching vibration of amide), 1632, 1403 (C=N and C=C stretching vibration of pyridine Nucleus), 1093 (C–F stretching vibration), 769 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 5.17 (s, 2H), 7.64 (d, 2H, J = 8.1 Hz), 7.76–7.88 (m, 4H), 8.23 (d, 1H, J = 6.2 Hz), 8.31 (d, 2H, J = 8.1 Hz), 11.89 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 52.9, 126.7, 127.6, 128.7, 128.9, 129.2, 130.6, 133.4, 134.5, 134.8, 138.6, 140.6, 145.8, 151.6, 155.6, 157.8, 158.9, 165.7; MS (ESI): m/z 445 [M+H]+.
1-(4-(5-(4-Nitrophenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7g)
The compound, 7g was prepared as described above, employing 5 (500 mg, 0.0017 mol) with 4-nitrobenzoic acid (6g) (284 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mol) to afford pure compound 7g, 367.3 mg in 50% yield. Mp: 370–372 °C, IR (KBr): 3176 (N–H stretching vibration), 2906 (Ar–C–H str.), 2966 (C–H str.), 1668 (NH(C=O)–C=C– stretching vibration of amide), 1631, 1396 (C=N and C=C stretching vibration of pyridine Nucleus), 1112 (C–F stretching vibration), 791 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 5.28 (s, 2H), 7.65 (d, 2H, J = 8.2 Hz), 7.96 (d, 2H, J = 8.1 Hz), 8.14 (d, 2H, J = 8.1 Hz), 8.24 (d, 1H, J = 6.2 Hz), 8.33 (d, 2H, J = 8.2 Hz), 11.91 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 53.5, 126.7, 127.3, 127.8, 128.4, 128.7, 130.5, 134.7, 138.4, 139.6, 140.6, 145.8, 149.4, 151.7, 155.6, 157.3, 158.4, 165.8; MS (ESI): m/z 410 [M+H]+.
1-(4-(5-(3,5-Dinitrophenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7h)
The compound 7h was prepared as per the method used for the preparation of the compound 7a, in which compound 5 (500 mg, 0.0017 mol) treated with 3,5-dinitrobenzoic acid (6h) (360 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mol) to afford pure compound 7h, 342.8 mg in 42% yield. Mp: 376–378 °C, IR (KBr): 3206 (N–H stretching vibration), 3112 (Ar–C–H str.), 2887 (C–H str.), 1648 (NH(C=O)–C=C– stretching vibration of amide), 1603, 1403 (C=N and C=C stretching vibration of pyridine Nucleus), 1114 (C–F stretching vibration), 769 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 5.37 (s, 2H), 7.66 (d, 2H, J = 8.3 Hz), 8.25 (d, 1H, J = 6.3 Hz), 8.37 (d, 2H, J = 8.3 Hz), 8.52 (s, 2H), 8.89 (s, 1H), 11.91 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 53.7, 121.6, 127.6, 128.5, 128.9, 129.3, 130.7, 134.5, 138.6, 138.9, 140.4, 145.8, 149.7, 151.7, 155.8, 157.7, 158.7, 165.9; MS (ESI): m/z 455 [M+H]+.
1-(4-(5-p-Tolyl-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7i)
The compound 7i was prepared as described above. In this, compound 5 (500 mg, 0.0017 mol) was reacted with 4-methylbenzoic acid (6i) (231 mg, 0.0017 mmol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mol) to afford pure compound 7i, 239.6 mg in 35% yield. Mp: 356–358 °C, IR (KBr): 3224 (N–H stretching vibration), 3102 (Ar–C–H str.), 2986 (C–H str.), 1643 (NH(C=O)–C=C– stretching vibration of amide), 1632, 1405 (C=N and C=C stretching vibration of pyridine Nucleus), 1087 (C–F stretching vibration), 756 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 2.69 (s, 3H), 5.10 (s, 2H), 7.37 (d, 2H, J = 7.8 Hz), 7.62 (d, 2H, J = 8.1 Hz), 7.86 (d, 2H, J = 7.8 Hz), 8.23 (d, 1H, J = 6.1 Hz), 8.31 (d, 2H, J = 8.1 Hz), 11.89 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 35.8, 52.7, 127.5, 128.7, 128.9, 129.5, 130.5, 131.7, 133.4, 134.8, 138.6, 140.6, 141.8, 145.7, 151.7, 157.8, 158.9, 165.6; MS (ESI): m/z 379 [M+H]+.
1-(4-(5-(4-Bromo-3,5-dinitrophenyl)-1,2,4-oxadiazol-3-yl)benzyl)-5-fluoropyrimidine-2,4(1H,3H)-dione (7j)
This compound 7j was prepared following the method described for the preparation of the compound 7a, in which compound 5 (500 mg, 0.0017 mol) was made to react with 4-bromo-3,5-dinitrobenzoic acid (6j) (494 mg, 0.0017 mol), EDC·HCl (418 mg, 0.00269 mol) and sodium acetate (83 mg, 0.0017 mol) to afford pure compound 7j, 356.9 mg in 37% yield. Mp: 386–388 °C, IR (KBr): 3125 (N–H stretching vibration), 3098 (Ar–C–H str.), 2995 (C–H str.), 1664 (NH(C=O)–C=C– stretching vibration of amide), 1609, 1409 (C=N and C=C stretching vibration of pyridine Nucleus), 1076 (C–F stretching vibration), 748 (2-Substituted pyridine), 1H NMR (300 MHz, DMSO-d6): \(\delta\) 5.40 (s, 2H), 7.65 (d, 2H, J = 8.3 Hz), 8.25 (d, 1H, J = 6.3 Hz), 8.37 (d, 2H, J = 8.3 Hz), 8.68 (s, 2H), 11.92 (s, 1H); 13C NMR (75 MHz, DMSO-d6): \(\delta\) 53.7, 122.7, 27.6, 128.5, 128.9, 130.6, 132.6, 134.8, 136.7, 138.4, 140.6, 145.8, 151.7, 157.6, 158.9, 166.8; MS (ESI): m/z 534 [M+H]+.
Biological evaluation
In vitro cytotoxicity
The target compounds (7a–j) were examined for their anticancer activity against a panel of four human cancer cell lines including breast cancer (MCF-7, MDA MB-231), lung cancer (A549) and prostate cancer (DU-145) by using MTT method. Etoposide was used as reference standard and the obtained results were summarized in Table 1.
Among the compounds (7a–j) synthesized 7a, 7b, 7c, 7d and 7i possessed good activity with IC50 values ranging from 0.011 ± 0.009 to 19.4 ± 8.11 µM and standard reference showed IC50 values range from 1.91 ± 0.84 to 3.08 ± 0.135 µM, respectively. The structure–activity relationship (SAR) studies indicated that the compound 7a without substituent on the phenyl ring attached to 1,2,4-oxadiazole moiety has showed good anticancer activity against four cell lines (MCF-7 = 0.76 ± 0.044 µM; A549 = 0.18 ± 0.019 µM; DU145 = 1.13 ± 0.55 µM and MDA MB-231 = 0.93 ± 0.013 µM). Up on introduction of electron-donating 3,4,5-trimethoxy group on the phenyl ring resulted compound 7b, showed more significant anticancer activity (MCF-7 = 0.011 ± 0.009 µM; A549 = 0.053 ± 0.0071 µM; DU145 = 0.017 ± 0.0062 µM and MDA MB-231 = 0.021 ± 0.0028 µM) than standard against cancer cells presented in Table 1. When one methoxy group is removed, resulting compound 7c displayed slightly decreased activity on four cell lines (MCF-7 = 0.88 ± 0.073 µM; A549 = 1.44 ± 0.32 µM; DU145 = 1.28 ± 0.27 µM and MDA MB-231 = 1.95 ± 0.19 µM) when compared with 7b. Where, compound 7d with 4-methoxy substituent has showed reduced anticancer activity (MCF-7 = 1.78 ± 0.22 µM; A549 = 1.67 ± 0.49 µM; DU145 = 2.10 ± 1.09 µM and MDA MB-231 = 2.34 ± 1.10 µM) than 7c. Instead of 4-methoxy group with weak electron-donating 4-methyl group (7i) exhibited acceptable activity (MCF-7 = 2.17 ± 1.66 µM; A549 = 1.88 ± 0.25 µM; DU145 = 2.65 ± 1.26 µM and MDA MB-231 = 2.14 ± 0.94 µM). Further, the compounds 7e, 7f, 7g, 7h and 7j, which possess electron-withdrawing substituents on the phenyl ring, showed moderate activities compared to those compounds 7a, 7b, 7c, 7d and 7i, without electron withdrawing and with electron-donating substituents.
Molecular docking
The docking studies of the potent 1,2,4-Oxadiazole linked 5-Fluorouracil derivatives (7a–7j) were performed using Molegro Virtual Docker (MVD). The crystal structure of Human VGEFR-2 enzyme (PDB ID: 1YWN) along with the crystal ligand imatinib was downloaded from protein databank [40]. Human VGEFR-2 enzyme is the key enzyme in angiogenesis, hematopoiesis and vasculogenic. All the chemical structures were prepared by using Marvin sketch and minimized and saved in a single file as SDF format. MVD was used to perform computational studies, cavity prediction, assigning bond orders, defining the active binding sites of the Human VGEFR-2 enzyme, structure refinement and preparation. The protein preparation was carried out with MVD and the chain was treated to add missing hydrogen, assign proper bond orders and deleted water molecules. The structure output format was set to pose viewer file so as to view the output of resulting docking studies and hydrogen bond interactions of different poses with the protein. The 2D and 3D interactions were generated by using Discovery Studio Visualizer.
In silico ADMET prediction
In silico ADMET screened for 5-fluoro-1-(4-(5-substituted phenyl-1,2,4-oxadiazol-3-yl)benzyl)pyrimidine-2,4(1H,3H)-dione derivatives (7a–7j) was assessed by using DataWarrior Software [41]. It calculates the properties of the datasets of ligands to determine the violation of Lipinski’s rule of 5 and toxicity parameters. All the calculated chemical properties are represented in Table 2.
Molecular docking studies
The docking studies of the potent compounds 7a–7j were performed using Molegro Virtual Docker (MVD). The crystal structure of human Vascular Endothelial Growth Factor Receptors (VEGFR-2) enzyme (PDB ID: 1YWN) along with the co-crystal ligand was downloaded from protein databank. Design of VEGFR-2 could be potential target for inhibition of blood vessel formation and leads to the development of anti-angiogenesis agents. All the chemical structures were prepared by using Marvin sketch and minimized. Mol file format structures were converted into Mol2 file format by using Discovery studio. MVD was used to perform computational studies, cavity prediction, assigning bond orders, structure refinement, defining the active binding sites of the VEGFR-2 and structure preparation. The protein preparation was carried out with MVD and the chain was treated to add missing hydrogen, assign proper bond orders and deleted water molecules. The structure output format was set to pose viewer file so as to view the output of resulting docking studies and hydrogen bond interactions of different poses with the protein. The 2D interactions were generated from Protein Plus and 3D interactions were generated from MVD.
In the present study, the synthesized compounds were docked into X-ray crystal structures of Human VEGFR-2 enzyme (PDB ID: 1YWN) to understand the possible target mechanism of action. The cavities were detected with MVD and the following are the active residues involved with co-crystal ligand, as it forms hydrogen bond interactions with Glu883, Glu915, Cys917, Asp1044. Arg1049 residues and hydrophobic interactions with Leu838, Val846, Val897, Val914, Cys1043, and Asp1044, residues. The docking has validated and found same interactions with Moldock score − 199.37 and H-bond energy − 6.24 kcal/mol (Fig. 2).
All the synthesized compounds form hydrogen bond interactions with Asp1044 and have shown same interaction with crystal ligand. The binding affinity of the docked compounds were expressed in negative binding energy kcal/mol (Moldock score). The ligands with more negative value of Moldock score will have more affinity with protein binding. 3,5-dinitro substituted compounds (7j and 7h) established a hydrogen bond (NH—ON) with amino group of Asp1044 and oxygen atom of oxadiazole (NH—O) with amino group of Lys866 with a H-bond energy − 7.40 and − 7.34, respectively and with Moldock score values are − 156.20 and − 157.88, respectively. The 7j and 7h also form same hydrophobic interactions with Arg840, Val846, Asp1044, Gly1046, and Arg1049 amino acid residues (Figs. 3, 4). Compound 7c oxygen of oxadiazole group forms a hydrogen bond (NH—O) with amino hydrogen of Gly1046 with a H-bond energy of − 2.59 kcal/mol (Fig. 5) and hydrophobic interactions with Val846, Arg840, Gly1046, Arg1049 amino acid residues. All the docked ligands have exhibited same interactions with active site of VEGFR. From the data it is revealed that compounds of the series 7j (Moldock score − 156.20 kcal/mol) and 7h (Moldock score − 157.88 kcal/mol) showed good inhibitory constant and excellent free energy of binding, which might be the reason for anticancer activity.
Pharmacophore prediction
All the synthesized compounds generated pharmacophore features by using PharmaGist web server [42]. It will predict the spatial arrangement of features like hydrogen bond acceptor (HBA), hydrogen bond donor (HBD), hydrophobic center (HY) and positive ionisable (PI) which are essential for a ligand to interact with specific target protein. All compounds were submitted to PharmaGist and the pharmacophoric score is found to be 75.17 with 10 Spatial Features, 4 Aromatic, 1 Donor and 5 Acceptor features for all set of ligands.
In silico ADMET properties
The in silico ADMET properties of 5-fluoro-1-(4-(5-substituted phenyl-1,2,4-oxadiazol-3-yl)benzyl)pyrimidine-2,4(1H,3H)-dione derivatives (7a–7j) was assessed by using DataWarrior Software (Fig. 6). All the compounds were determined molecular descriptors for Rule of 5 (Lipinski rule) which states the oral bioavailability and drug like properties. The determinants possess Molecular weight ≤ 500, no of H-Acceptors ≤ 10, no of H-Donor ≤ 5. Among the calculated chemical descriptors, all the ligands have passed the Lipinski rule which states ligands did not violate more than one Rule of 5, except compound 7j substituted with 4-bromo-3,5-dinitro has violated Molecular weight and H-Acceptor.
MTT assay
Individual wells of a 96-well tissue culture micro titer plate were inoculated with 100 µl of complete medium containing 1 × 104 cells. The plates were incubated at 37 °C in a humidified 5% CO2 incubator for 18 h prior to the experiment. The results are shown in Table 3.
Conclusion
In conclusion, we have synthesized a library of 1,2,4-oxadiazole linked 5-fluorouracil derivatives (7a–j) and all these compounds were characterized by 1HNMR, 13CNMR and Mass spectral analysis. Further, these compounds were investigated for their anticancer activity towards a panel of four human cancer cell lines such as (MCF-7, MDA MB-231), lung cancer (A549) and prostate cancer (DU-145) by using MTT method. Among them, compounds 7a, 7b, 7c, 7d and 7i were demonstrated more promising anticancer activity than standard. Further docking studies of all the compounds were presented, where the designed compounds showed good anticancer activity and excellent free energy of binding interactions with VEGFR-2 kinase domain.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its Additional file 1).
References
Ghomi JS, Ghasemzadeh MA (2010) An efficient route to the synthesis of pyrimidine-2-ones under ultrasound irradiation. Dig J Nanomater Biostruct 5:303–306
Wagner E, Al-Kadasi K, Zimecki M, Sawka-Dobrowolska W (2008) Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur J Med Chem 43:2498–2504
Ukrainets IV, Tugaibei IA, Bereznykova NL, Karvechenko VN, Turov AV (2008) 4-Hydroxy-2-quinolones 144. Alkyl-, arylalkyl- and arylamides of 2-hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-3-carboxylic acid and their diuretic properties. Chem Heterocycl Compd 5:565–575
Ballell L, Field RA, Chung GAC, Young RJ (2007) New thio pyrazolo [3, 4-d] pyrimidine derivatives as anti-mycobacterial agents. Bioorg Med Chem Lett 17:1736–1740
Fujiwara N, Nakajima T, Ueda Y, Fujita H, Kawakami H (2008) Novel piperidinylpyrimidine derivatives as inhibitors of HIV-1 LTR activation. Bioorg Med Chem 16:9804–9816
Amr AE, Nermien MS, Abdulla MM (2007) Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh Chem 138:699–707
Kurono M, Hayashi M, Miura K, Isogawa Y, Sawai K (1988) A series of substituted pyrimidine has been discovered as a new class of potent antioxidant. Chem Abstr 109:37832
Cordeu L, Cubedo E, Bandres E, Rebollo A, Saenz X, Chozas H, Domínguez MV, Echeverria M, Mendivil B, Sanmartin C, Palop JA, Font M, García-Foncillas J (2007) Biological profile of new apoptotic agents based on 2,4-pyrido[2,3-d] pyrimidine derivatives. Bioorg Med Chem 15:1659–1669
Prakash O, Kumar R, Kumar R, Tyagi P, Kuhad RC (2007) Organo iodine(III) mediated synthesis of 3,9-diaryl- and 3,9-difuryl-bis-1,2,4-triazolo[4,3-a][4,3-c]pyrimidines as antibacterial agents. Eur J Med Chem 42:868
Katiyar SB, Bansal I, Saxena JK, Chauhan PMS (2005) Syntheses of 2,4,6-trisubstituted pyrimidine derivatives as a new class of anti-filarial topoisomerase II inhibitors. Bioorg Med Chem Lett 15:47
Patel RB, Desai PS, Desai KR, Chikhalia KH (2006) Synthesis of pyrimidine based thiazolidinones and azetidinones. Antimicrobial and antitubercular agents. Indian J Chem 45(B):773–778
Cox RA. Quart Rev. 1968;22:934.
Callery P, Gannett P (2002) Cancer and cancer chemotherapy. In: Williams DA, Lemke TL (eds) Foye’s principles of medicinal chemistry. Philadelphia, Lippincott Williams and Wilkins, pp 934–935
Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Sheiner J (1957) Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 179(4561):663–666
Dando EE, Lim GFS, Lim SJM (2020) Intralesional 5-fluorouracil for the nonsurgical management of low-risk, invasive squamous cell carcinoma. Dermatol Surg 46(1):126–130
Moorkoth D, Nampoothiri KM, Nagarajan S, Girija AA, Balasubramaniyan S, Sakthi KD (2021) Star-shaped polylactide dipyridamole conjugated to 5-fluorouracil and 4-piperidinopiperidine nanocarriers for bioimaging and dual drug delivery in cancer cells. ACS Appl Polym Mater 3(20):737–756
Noordhuis P, Holwerda U, Van der Wilt CL, Van Groeningen CJ, Smid K, Meijer S, Pinedo HM, Peters GJ (2004) 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann Oncol 15:1025–1032
Danesi CC, Dihl RR, Bellagamba CB, Andrade RHH, Cunha SK, Guimarães NN, Lehmann M (2012) Genotoxicity testing of combined treatment with cisplatin, bleomycin, and 5-fluorouracil in somatic cells of Drosophila melanogaster. Mut Res 747:228–233
Metterle L, Nelson C, Patel N (2015) Intralesional 5-fluorouracil (FU) as a treatment for nonmelanoma skin cancer (NMSC): a review. J Am Acad Dermatol 74:552–557
Rossi S (ed) (2013) Australian medicines handbook. The Australian Medicines Handbook Unit Trust, Adelaide
Wood PA, Du-Quiton J, You S, Hrushesky WJ (2006) Circadian clock coordinates cancer cell cycle progression, thymidylate synthase, and 5-fluorouracil therapeutic index. Mol Cancer Ther 5:2023–2033
Zhang N, Yin Y, Xu SJ, Chen WS (2008) 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules 13(8):1551–1569
Malet-Martino M, Martino R (2002) Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist 7(4):288–323
Malet-Martino M, Jolimaitre P, Martino R (2002) The prodrugs of 5-fluorouracil. Curr Med Chem 2(2):267–310
Carbone M, Li Y, Irace C, Mollo E, Castelluccio F, Pascale AD, Cimino G, Santamaria R, Guo YW, Gavagnin M (2011) Structure and cytotoxicity of phidianidines A and B: first finding of 1,2,4-oxadiazole system in a marine natural product. Org Lett 13(10):2516–2519
Khan I, Ibrar A, Abbas N (2014) Oxadiazoles as privileged motifs for promising anticancer leads: recent advances and future prospects. Arch Pharm (Weinheim) 347(1):1–20
Zarghi A, Hajimahdi Z (2013) Substituted oxadiazoles: a patent review (2010–2012). Expert OpinTher Pat 23:1209–1232
Pace A, Buscemi S, Piccionello AP, Pibiri I (2015) Recent advances in the chemistry of 1,2,4 oxadiazoles. Adv Heterocycl Chem 116:85–136
Vaidya A, Jain S, Jain P, Jain P, Tiwari N, Jain R, Jain AK, Agrawal RK (2016) Synthesis and biological activities of oxadiazole derivatives: a review. Mini Rev Med Chem 16(10):825–845
Lueg C, Schepmann D, Günther R, Brust P, Wünsch B (2013) Development of fluorinated CB2 receptor agonists for PET studies. Bioorg Med Chem. 21:7481–7498
Bokach NA, Khripoun AV, Kukushkin VY, Haukka M, Pombeiro AJL (2003) A route to 1,2,4-oxadiazoles and their complexes via platinum-mediated 1,3-dipolar cycloaddition of nitrile oxides to organonitriles. Inorg Chem 42(3):896–903
Manfredini S, Lampronti I, Vertuani S, Solaroli N, Recanatini M, Bryan D, McKinney M (2000) Design, synthesis and binding at cloned muscarinic receptors of N-[5-(1’-substituted-acetoxymethyl)-3-oxadiazolyl] and N-[4-(1’-substituted-acetoxymethyl)-2-dioxolanyl] dialkyl amines. Bioorg Med Chem 8:1559
Boys ML, Schretzman LA, Chandrakumar NS, Tollefson MB, Mohler SB, Downs VL, Penning TD, Russell MA, Wendt JA, Chen BB, Stenmark HG, Wu H, Spangler DP, Clare M, Desai BN, Khanna IK, Nguyen MN, Duffin T, Engleman VW, Finn MB, Freeman SK, Hanneke ML, Keene JL, Klover JA, Nickols GA, Nickols MA, Steininger CN, Westlin M, William W, Yi XY, Wang Y, Dalton CR, Norring SA (2006) Convergent, parallel synthesis of a series of beta-substituted 1,2,4-oxadiazole butanoic acids as potent and selective alpha(v)beta3 receptor antagonists. Bioorg Med Chem Lett 16:839–844
dos Santos Filho JM, Leite ACL, de Oliveira BG, Moreira DRM, Lima MS, Soares MBP, Leite LFCC (2009) Design, synthesis and cruzain docking of 3-(4-substituted-aryl)-1,2,4- oxadiazole-N-acylhydrazones as anti-Trypanosoma cruzi agents. Bioorg Med Chem 17(18):6682–6691
de Mel SJ, Sobral AD, Lopes HDL, Srivastava RM (1998) Synthesis of Some 3-aryl-1,2,4-oxadiazoles carrying a protected l-alanine side chain. J Braz Chem Soc 9(5):465–468
Clitherow JW, Beswick P, Irving WJ, Scopes DIC, Barnes JC, Clapham J, Brown JD, Evans DJ, Hayes AG (1996) Novel 1, 2, 4-oxadiazoles as potent and selective histamine H3 receptor antagonists. Bioorg Med Chem Lett 6:833
Ispikoudi M, Amvrazis M, Kontogiorgis C, Koumbis AE, Litinas KE, Hadjipavlou-Litina D, Fylaktakidou KC (2010) Convenient synthesis and biological profile of 5-amino-substituted 1,2,4-oxadiazole derivatives. Eur J Med Chem 45:5635–5645
Chawla G (2018) 1,2,4-oxadiazole as a privileged scaffold for anti-inflammatory and analgesic activities: a review. Mini Rev Med Chem 18(18):1536–1547
Vaidya A, Jain S, Prashantha KB (2020) Synthesis of 1,2,4-oxadiazole derivatives: anticancer and 3D QSAR studies. Monatsh Chem 151:385–395
Miyazaki Y, Maeda Y, Sato H, Nakano M, Mellor GW (2008) Rational design of 4-amino-5,6-diaryl-furo[2,3-d]pyrimidines as potent glycogen synthase kinase-3 inhibitors. Bioorg Med Chem Lett 18(6):1967–1971
Sander T, Freyss J, von Korff M, Rufener C (2015) DataWarrior, An open-source program for chemistry aware data visualization andanalysis. J Chem Inf Model 55:460–473
Inbar Y, Schneidman-Duhovny D, Dror O, Nussinov R, Wolfson HJ (2007) Deterministic pharmacophore detection via multiple flexible alignment ofdrug-like molecules. Annual international conference on research in computational molecular biology. Springer, Berlin, pp 412–429
Acknowledgements
Authors are thankful to the management, Executive Director, Director and Principal of SNIST and GCET for their encouragement.
Funding
No funding has been received from any source.
Author information
Authors and Affiliations
Contributions
RK and SK carried out the experimental work. LE performed thestatistical analysis and drafted the manuscript. RR performed docking studies. All authors have gone through the final manuscript and approved it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Spectra of synthesized compounds.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bommera, R.K., Kethireddy, S., Govindapur, R.R. et al. Synthesis, biological evaluation and docking studies of 1,2,4-oxadiazole linked 5-fluorouracil derivatives as anticancer agents. BMC Chemistry 15, 30 (2021). https://doi.org/10.1186/s13065-021-00757-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-021-00757-y