Abstract
We have synthesized new series of bisindole analogs (1–27), characterized by 1HNMR and HR-EI-MS and evaluated for their anti-leishmanial potential. All compounds showed outstanding inhibitory potential with IC50 values ranging from 0.7 ± 0.01 to 13.30 ± 0.50 µM respectively when compared with standard pentamidine with IC50 value of 7.20 ± 0.20 µM. All analogs showed greater potential than standard except 10, 19 and 23 when compared with standard. Structure activity relationship has been also established for all compounds. Molecular docking studies were carried out to understand the binding interaction of active molecules.

Similar content being viewed by others
Introduction
Leishmaniasis has affected almost 98 countries of the world. Every year approximately in 2 million people leishmaniasis has been reported while 350 million people are at risk [1]. The efficacy of drugs available for leishmaniasis is limited [2]. Leishmaniasis, a parasitic disease unveiled by four syndromes which are cutaneous leishmaniasis, visceral leishmaniasis, muco cutaneous leishmaniasis and kalaazar dermal leishmaniasis. In 90% population of India, Bangladesh, Nepal, East Africa and Brazil visceral leishmaniasis have been reported. The first-line drugs used for the treatment of leishmaniasis are pentavalent antimonial compounds which is not too much effective in almost 60% cases due to drug resistance. Some other treatment has been introduced for visceral leishmaniasis which has serious limitation [3]. Some second line drugs are also used for the treatment like pentamidine amphotericin B, but they have toxicity problems and unavailability [4, 5]. Some vaccine has been introduced for leishmaniasis infections which are effective with low price however effective vaccine is not yet introduce [6, 7]. The growth and survival of leishmanial parasite depend on polyamine bases which are mainly produce during metabolic process. Interaction directly with polyamine or biosynthetic pathways of these bases could result in leishmanial infection [8]. The most challenging task is the introduction of an affordable, effective and alternative antilieshmanial drug.
Bisindole compounds are known to have wide range of pharmacological activities like anticancer and antimicrobial [9,10,11,12,13,14], etc. Hamacanthin A bisindole alkaloid isolated from the sponge Hamacantha sp. and Spongosorites sp. exhibited effective antibacterial activity against Staphylococcus aureus and MRS with MIC of 6.45 mM and antifungal activity against Bacillus subtilis with MIC of 3.22 mM [15,16,17,18]. Additionally, bisindoles compounds have been used in many biological processes such as fluorescent molecular probes [19]. Amongst several antileishmanial scaffolds reported, indole alkaloids [20,21,22,23,24,25] showed promising activity against Leishmania parasite.
Keeping the idea for designing of new antilieshmanial drug, it is important to synthesize molecules having different biological properties based on their structure domain. We have synthesized variety of biologically active compounds for specific biological target [26,27,28,29,30,31]. Herein we report the synthesis of bis-indole derivatives as antilieshmanial agents.
Results and discussions
Chemistry
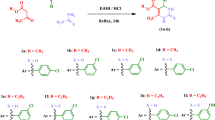
The synthesis of bis-indole analogs (1–27) was carried out in three steps. In the first step, 2 equivalent of indole (I) was mixed with methyl-4-formylbenzoate (II) in acetic acid and reflux for 4–6 h to afford intermediate product III. The Intermediate III was then treated with hydrazine hydrate (3 mL) in ethanol, then reflux for 3–4 h to obtained intermediate IV. The intermediate IV was then mixed with different isothiocyanates to get the pure products (1–27) in good yield. All reactions completion was monitored by periodic TLC. Structures of all synthesized analogs were confirmed with 1HNMR, 13CNMR and HR-EIMS (Scheme 1).
Biological activity
In the continuation of our effort for enzyme inhibition [32,33,34,35,36], we have synthesized series of bisindole derivatives as a new class of anti-leishmanial agents. All compounds (1–27) were screened for their leishmanial activity (Table 1). All these compounds showed outstanding inhibition when compared with standard. Out of 27 analogs, fifteen compounds i.e. 3, 4, 7, 8, 9, 11, 12, 15, 16, 17, 18, 20, 21, 22 and 25 showed excellent inhibitory potential with IC50 values ranging from 0.7 ± 0.01 to 4.30 ± 0.20 µM respectively when compared with standard pentamidine having IC50 value of 7.20 ± 0.20 µM. Compounds 1, 2, 5, 6, 10, 13, 14, 19, 23, 24, 26 and 27 also showed excellent inhibition ranging from 5.20 ± 0.2 to 13.30 ± 0.50 µM when compared with standard.
Structure activity relationship (SAR) has been established for all compounds. The compound 8, a 2,3-dihydroxy analog was found to be the most potent among the series with (IC50 value 0.7 ± 0.01 µM). If we compare analog 8 with other dihydroxy analogs 4, a 2,3-dihydroxy (IC50 value 0.80 ± 0.01 µM) 7, a 2,5-dihydroxy (IC50 values 3.50 ± 0.20) 14, a 3,5-dihydroxy analog (IC50 values 5.80 ± 0.30), and 22 a 2,4-dihydroxy analogs (IC50 values 3.30 ± 0.10) it’s clear that vicinal dihydroxy system i.e. 8 and 4 showed excellent inhibitory potential rather as compared the other dihydroxy analogs. This indicates the vicinal dihydroxy system is conjugated effectively with enzyme Pteridine reductase to cause higher inhibition. Comparing analogs 12, 15 and 27, monohydroxy analogs the 2-hydroxy analog 12 (IC50 value 2.65 ± 0.10 µM) is more potent than 3-hydroxy and 4-hydroxy analogs 15 (IC50 value 3.60 ± 0.20 µM) and 27 (IC50 value 5.8 ± 0.20 µM) showing its effective binding with enzyme. Compound 6 with 2-methyl on phenyl ring showed good active (IC50 value 5.2 0 ± 0.2 µM). The methyl may be involved in interaction through inductive effect. The compound 1 having 4-methoxy showed better activity than compound 2 having 3-methoxy with IC50 value 5.30 ± 0.30 and 6.4 ± 0.20 µM respectively. The 2-nitro analog 21 (IC50 value 3.30 ± 0.20 µM) is more potent when compared with 3-nitro analog 3 (IC50 value 4.30 ± 0.20 µM) and 4-nitro analog 13 (IC50 value 6.8 ± 0.20 µM). This shows that position of substituents plays a vital role in inhibition. Ortho fluoro analog 17 (IC50 value 0.95 ± 0.05 µM) is much superior than meta and para fluoro analogs 9 and 26 with IC50 values 1.50 ± 0.05, and 6.80 ± 0.2 µM respectively. So, it was concluded from this study that the nature, position and number of substituents play a critical role in the inhibitory potential of our designed analogs Table 1.
Molecular docking studies of bis-indole derivatives on pteridine reductase
Docking studies with PTR shows that all the active compounds tend to adopt a similar binding mode as depicted in Fig. 1a. Comparison of the binding mode of the most active compound 8 with standard pentamidine used in the study, shows that the compound 8 interacts with the key residues of the PTR active site establishing hydrophilic and hydrophobic contacts, while in the case of the pentamidine interacts with fewer hydrophobic residues as shown in Fig. 1b. This clearly shows that this class of synthetic derivatives could be potential candidates for therapeutic against leishmaniasis.
The activity profile of these derivatives ranges from IC50 (0.7 μM to 13.30 μM). Therefore, it’s clear that these compounds are good starting point in pteridine reductase inhibitor discovery. In the following section, we limit our self to report only the binding mode of four most active compounds. Binding mode of compound 8 (Fig. 2a) shows that the meta hydroxy group attached to the benzene ring forms hydrogen bonds with side chain of Gly13, Gly19 and Asn109, respectively. While the phenyl ring positioned at compound’s center forms π-π stacking with Phe133. In addition, the di-indole rings form hydrophobic interaction with residues such as Met183, Leu188, Met233 and Leu226, respectively.
Figure 2b shows the binding mode of compound 4, where the meta and para positioned hydroxy moieties forms hydrogen bond with side chains of Gly13, Gly19 and Asn109. Next, the phenyl ring positioned at the center of the compound forms π–π stacking with Phe11 and the di-indole rings forms hydrophobic interaction with residues such as Met183, Leu188, Met233 and Leu226 similarly as in case of compound 8. Interestingly, in the case of compound 17, the entire complex was stabilized by hydrophobic interaction. The 2-fluorobenzene group forms hydrophobic contact with Leu18 and the phenyl ring forms π–π stacking with Phe133 and hydrophobic contact with Met233, respectively. Finally, the di-indole rings form non-polar contact with Met183, Leu188 and Leu226. Likewise, the compound 9 forms hydrophobic contacts with 3-fluorobenzene group with Leu18 and the phenyl ring forms π–π stacking with Phe133 and the di-indole rings interaction with Met183, Leu188, Leu226 and Tyr283 stabilize the complex.
Conclusion
It was concluded from this study that a series of bisindole analogs (1–27) were synthesized, characterized by 1HNMR and HR-EI-MS and evaluated for their anti-leishmanial potential. All compounds showed outstanding inhibitory potential with IC50 values ranging from 0.7 to 13.30 µM respectively when compared with standard pentamidine with IC50 value of 7.20 ± 0.20 µM. Structure activity relationship has been also established for all compounds, which shows that the nature, position and number of substituents on phenyl ring play a critical role. Molecular docking studies were carried out to understand the binding interaction of our synthesized molecules with the active site of this enzyme (Additional file 1).
Materials and methods
NMR experiments were performed on Avance Bruker AM 300 MHz machine. Electron impact mass spectra (EI MS) were recorded on a Finnigan MAT-311A (Germany) mass spectrometer. Thin layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Germany). Chromatograms were visualized by UV at 254 and 365 nm.
Molecular docking studies
In this recent work, we have used pteridine reductase (PTR) as vital drug target against leishmaniasis, a vital enzyme accountable for pteridine salvage in leishmania protozoans. For the molecular docking studies, we have used similar protocol that has been adopted in our previous work for both ligand preparation and docking studies of derivatives of bis-indole against PTR. Molecular docking studies were carried out using glide: a complete solution for ligand-receptor docking in small molecule drug discovery suite. Initially, receptor grid generation was done by generating grid on the Pteridine reductase structure were the grid box was centered on methotrexate (MTX) complexed ligand with 12 Å radius respectively. Both standards precision (SP) mode and extra precision (XP) mode was chosen during the Glide docking process and Glide score was considered for analysis. Further top rank scored binding mode analyzed in Pymol [37].
General procedure for the synthesis of compounds (1–27)
The synthetic scheme towards the synthesis of bis-indole compounds involved mixing of indole with methyl-4-formylbenzoate in acetic acid to afford the ester intermediate which was then reacted with hydrazine hydrate and finally with isothiocyanate to get the final products 1–27.
The synthesis of bis-indole analogs (1–27) was carried out in three steps. In the first step, 2 equivalent of indole (I) was mixed with methyl-4-formylbenzoate (II) in acetic acid and reflux for 4–6 h. to afford intermediate product III. The Intermediate III was then treated with hydrazine hydrate (3 mL) in ethanol, then reflux for 3–4 h to obtained intermediate IV. The intermediate IV was then mixed with different isothiocyanates to get the pure products (1–27) in good yield. All reaction completion was monitored by periodic TLC. Structures of all synthesized analogs were confirmed with 1HNMR, 13CNMR and HR-EIMS.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(4-methoxyphenyl)-1,3,4-thiadiazol-2-amine (1)
Yield 90%, 1H-NMR (500 MHz, DMSO-d6): δ 12.30 (s, 2H, NH), 11.60 (s, 1H, NH), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.52 (d, J = 7.4 Hz, 2H, Ar), 7.48 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.00 (d, J = 7.1 Hz, 2H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.61 (d, J = 7.0 Hz, 4H, Ar), 6.21 (H, CH), 3.83 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ 174.3, 153.1, 152.2, 138.4, 136.1, 136.2, 133.0, 130.2, 129.2, 129.2, 127.2, 127.2, 127.2, 127.2, 123.3, 123.1, 121.9, 121.4, 121.3, 121.1, 119.5, 119.1, 118.6, 118.4, 115.3, 115.2, 112.6, 112.3, 111.4, 111.2, 55.4, 54.2; HR-EIMS: m/z calcd for C32H25N5OS [M]+ 527.1780, Found 527.1768.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(3-methoxyphenyl)-1,3,4-thiadiazol-2-amine (2)
Yield 82%, 1H-NMR (500 MHz, DMSO-d6): δ 10.52 (s, 2H, NH), 10.12 (s, 1H, NH), 7.76 (d, J = 7.5 Hz, 2H, Ar), 7.52–7.49 (m, 3H, Ar), 7.47 (d, J = 7.3 Hz, 2H, Ar), 7.42–7.40 (m, 1H, Ar), 7.22 (dd, J = 8.0, 3.0 Hz, 1H, Ar), 7.20 (d, J = 7.5 Hz, 2H, Ar), 6.85 (dd, J = 8.0, 2.0 Hz, 2H, Ar), 6.80 (d, J = 7.5 Hz, 1H, Ar), 6.70 (dd, J = 7.5, 3.0 Hz, 2H, Ar), 6.58 (d, J = 7.0 Hz, 2H, Ar), 6.20 (s, 1H, CH), 3.90 (s, 3H, OCH3); 13C-NMR (125 MHz, DMSO-d6): δ 174.3, 152.5, 142.1, 139.0, 138.0, 136.3, 136.2, 130.2, 129.8, 129.4, 129.1, 127.7, 127.5, 127.3, 127.1, 123.4, 123.2, 121.7, 121.5, 121.3, 119.5, 119.3, 119.1, 118.6, 118.3, 114.4, 112.4, 112.2, 111.4, 111.2, 54.3, 61.2; HR-EIMS: m/z calcd for C32H25N5OS [M]+ 527.178, Found 527.168.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(3-nitrophenyl)-1,3,4-thiadiazol-2-amine (3)
Yield 79%, 1H-NMR (500 MHz, DMSO-d6): δ 12.60 (s, 2H, NH), 12.10 (s, 1H, NH), 8.10 (s, 1H), 8.00 (d, J = 7.8 Hz, 1H, Ar), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.70–7.65 (m, 3H, Ar), 7.48 (d, J = 7.3 Hz, 2H, Ar), 7.45 (dd, J = 8.2 3.4 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.82 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.68 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.62 (d, J = 7.0 Hz, 2H, Ar), 6.21 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.3, 152.4, 148.3, 143.1, 138.4, 136.3, 136.2, 130.3, 130.2, 129.2, 129.1, 127.1, 127.0, 127.0, 126, 123.7, 123.5, 123.2, 121.5, 121.3, 120.0, 119.6, 118.6, 118.4, 114, 112.8, 112.6, 111.4, 111.2, 109.5, 54.3; HR-EIMS: m/z calcd for C31H22N6O2S [M]+ 542.1525, Found 542.1515.
4-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)benzene-1,2-diol (4)
Yield 89%, 1H-NMR (500 MHz, DMSO-d6): δ 10.90 (s, 2H, NH), 10.30 (s, 1H, NH), 10.10 (s, 1H, OH), 9.40 (s, 1H, OH) 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.56–7.50 (m, 4H, Ar), 7.26 (d, J = 7.0 Hz, 2H, Ar), 6.92 (d, J = 6.8 Hz, 1H, Ar), 6.81 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.63 (d, J = 7.0 Hz, 2H, Ar), 6.61 (d, J = 6.5 Hz, 1H, Ar), 6.54 (s, 1H, Ar), 6.24 (s, 1H, CH),13C-NMR (125 MHz, DMSO-d6): δ 174.3, 152.6, 148.6, 138.7, 137.3, 136.8, 136.4, 136.2, 130.3, 129.7, 129.3, 127.8, 127.6, 127.4, 127.2, 123.5, 123.3, 121.5, 121.3, 119.5, 119.2, 118.6, 118.3, 118.2, 114.5, 112.6, 112.4, 111.4, 111.2, 102.2, 54.3; HR-EIMS: m/z calcd for C31H23N5O2S [M]+ 529.1572, Found 529.1561.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(p-tolyl)-1,3,4-thiadiazol-2-amine (5)
Yield 83%, 1H-NMR (500 MHz, DMSO-d6): δ 11.65 (s, 2H, NH), 10.85 (s, 1H, NH), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.53 (d, J = 7.3 Hz, 2H, Ar), 7.31 (d, J = 7.2 Hz, 2H, Ar), 7.21 (d, J = 7.1 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.81–677 (m, 4H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.63 (d, J = 7.0 Hz, 2H, Ar), 6.31 (s, 1H, CH), 2.30 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ 174.3, 152.2, 138.2, 137.3, 136.2, 136.2, 131.5, 130.3, 129.5, 129.4, 129.2, 129.2, 127.1, 127.1, 127.0, 126.1, 123.4, 123.1, 121.5, 121.3, 120.5, 120.2, 119.6, 119.3, 118.6, 118.3, 112.4, 112.0, 111.6, 111.3, 54.4, 21.1, HR-EIMS: m/z calcd for C32H25N5S [M]+ 511.1831, Found 511.1816.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(o-tolyl)-1,3,4-thiadiazol-2-amine (6)
Yield 84%, 1H-NMR (500 MHz, DMSO-d6): δ 10.50 (s, 2H, NH), 9.71 (s, 1H, NH), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.48 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.17 (dd, J = 8.1 3.2 Hz, 1H, Ar), 7.12 (d, J = 7.1 Hz, 1H, Ar), 7.10 (d, J = 6.9 Hz, 1H, Ar), 6.92 (dd, J = 7.8 2.5 Hz, 1H, Ar), 6.82–678 (m, 4H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.57 (d, J = 7.0 Hz, 2H, Ar), 6.20 (s, 1H, CH), 2.10 (s, 1H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ 174.4, 152.4, 142.3, 138.5, 136.2, 136.3, 131.3, 130.1, 129.8, 129.4, 129.0, 127.8, 127.6, 127.4, 127.2, 126.3, 123.8, 123.5, 123.3, 123.1, 121.4, 121.1, 119.6, 119.4, 118.4, 118.2, 112.4, 112.2, 111.5, 111.3, 54.3, 17.3; HR-EIMS: m/z calcd for C32H25N5S [M]+ 511.1831, Found 511.1816.
2-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)benzene-1,4-diol (7)
Yield 92%, 1H-NMR (500 MHz, DMSO-d6): δ 11.91 (s, 2H, NH), 10.30 (s, 1H, NH), 8.90 (s, 2H, OH), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.54 (d, J = 7.3 Hz, 2H, Ar), 7.24 (d, J = 7.0 Hz, 2H, Ar), 6.80–6.75 (m, 4H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.56 (d, J = 6.4 Hz, 2H, Ar), 6.51 (d, J = 7.0 Hz, 2H, Ar), 6.49 (s, 1H), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.6, 152.4, 151.2, 138.2, 137.3, 136.8, 136.6, 135.3, 130.2, 129.7, 129.3, 127.8, 127.6, 127.4, 127.2, 123.4, 123.2, 121.3, 121.0, 120.2, 119.0, 118.6, 118.4, 112.4, 112.2, 111.5, 111.3, 111.0, 107.0, 102.2, 54.3; HR-EIMS: m/z calcd for C31H23N5O2S [M]+ 529.1572, Found 529.1561.
3-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)benzene-1,2-diol (8)
Yield 81%, 1H-NMR (500 MHz, DMSO-d6): δ 10.50 (s, 2H, NH), 10.32 (s, 2H, OH), 9.76 (s, 1H, NH), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.84–6.78 (m, 4H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.58 (d, J = 7.0 Hz, 2H, Ar), 6.57 (dd, J = 7.1 2.5 Hz, 1H, Ar), 6.55 (d, J = 6.8 Hz, 1H, Ar), 6.50 (d, J = 6.7 Hz, 1H, Ar), 6.32 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.0, 152.5, 148.3, 138.0, 136.8, 136.2, 135.3, 133.4, 130.2, 129.4, 129.0, 127.8, 127.5, 127.2, 127.1, 123.2, 123.0, 122.4, 121.5, 121.2, 119.4, 119.2, 118.5, 118.1, 112.4, 112.0, 111.4, 111.2, 107.1, 105.2, 54.2; HR-EIMS: m/z calcd for C31H23N5O2S [M]+ 529.1572, Found 529.1561.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(4-fluorophenyl)-1,3,4-thiadiazol-2-amine (9)
Yield 90%, 1H-NMR (500 MHz, DMSO-d6): δ 12.18 (s, 2H, NH), 11.48 (s, 1H, NH), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.53 (d, J = 7.3 Hz, 2H, Ar), 7.40 (d, J = 7.4 Hz, 2H, Ar), 7.30 (d, J = 7.1 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.83–6.79 (m, 4H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.63 (d, J = 7.0 Hz, 2H, Ar), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.3, 157.1, 152.3, 138.3, 136.7, 136.3, 136.0, 130.2, 129.3, 129.1, 127.8, 127.6, 127.3, 127.1, 123.3, 123.1, 121.5, 121.2, 120.4, 120.2, 119.4, 119.1, 118.4, 118.2, 116.5, 116.1, 112.6, 112.4, 111.3, 111.0, 54.4; HR-EIMS: m/z calcd for C31H22FN5S [M]+ 515.1580, Found 515.1566.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(pyridin-3-yl)-1,3,4-thiadiazol-2-amine (10)
Yield 83%, 1H-NMR (500 MHz, DMSO-d6): δ 11.60 (s, 2H, NH), 9.20 (s, 1H, NH), 8.02 (s, 1H, Ar), 7.90 (d, J = 7.5 Hz, 1H, Ar), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.48 (d, J = 7.3 Hz, 2H, Ar), 7.33 (dd, J = 8.1 2.4 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.12 (d, J = 7.2 Hz, 1H, Ar), 6.80 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.63–655 (m, 4H, Ar), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.4, 152.4, 138.6, 138.3, 137.3, 136.2, 136.0, 133.5, 130.3, 129.2, 129.1, 127.7, 127.5, 127.3, 127.2, 124.0, 123.3, 123.1, 122.4, 121.4, 121.2, 119.5, 119.2, 118.4, 118.2, 112.4, 112.2, 111.5, 111.3, 54.3; HR-EIMS: m/z calcd for C30H22N6S [M]+ 498.1627, Found 498.1612.
2-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)-5-methoxyphenol (11)
Yield 83%, 1H-NMR (500 MHz, DMSO-d6): δ 10.50 (s, 2H, NH), 9.77 (s, 1H, NH), 10.03 (s, 1H, OH), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.11 (d, J = 7.3 Hz, 1H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.78 (s, 1H, Ar), 6.70–6.64 (m, 4H, Ar), 6.67 (d, J = 7.0 Hz, 1H, Ar), 6.62 (d, J = 7.0 Hz, 2H, Ar), 6.22 (s, 1H, CH), 3.72 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ 174.3, 152.5, 150.3, 138.0, 136.4, 136.2, 134.1, 130.4, 130.2, 129.4, 129.1, 127.7, 127.5, 127.3, 127.1, 123.4, 123.2, 121.7, 121.5, 121.1, 119.4, 119.1, 118.4, 118.2, 117.1, 112.5, 112.3, 112.0, 111.4, 111.2, 55.4, 54.2, 17.5; HR-EIMS: m/z calcd for C32H25N5O2S [M]+ 543.1729, Found 543.1717.
2-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)phenol (12)
Yield 81%, 1H-NMR (500 MHz, DMSO-d6): δ 11.50 (s, 2H, NH), 9.77 (s, 1H, NH), 9.93 (s, 1H, OH), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.10 (dd, J = 8.3, 3.3 Hz, 1H, Ar), 7.00 (d, J = 7.1 Hz, 1H, Ar), 6.82 (dd, J = 7.8, 3.5 Hz, 1H, Ar), 6.81 (d, J = 7.1 Hz, 1H, Ar), 6.80 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70–6.65 (m, 4H, Ar), 6.60 (d, J = 7.0 Hz, 2H, Ar), 6.20 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.0, 152.5, 144.2, 138.0, 136.3, 136.1, 134.0, 130.2, 129.3, 129.4, 127.2, 127.1, 126.8, 126.4, 122.9, 122.7, 122.0, 121.5, 121.3, 120.0, 119.6, 119.4, 118.5, 118.3, 116.4, 112.2, 112.0, 111.9, 111.4, 111.1, 54.4; HR-EIMS: m/z calcd for C31H23N5OS [M]+ 513.1623, Found 513.1609.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(4-nitrophenyl)-1,3,4-thiadiazol-2-amine (13)
Yield 88%, 1H-NMR (500 MHz, DMSO-d6): δ 12.60 (s, 2H, NH), 12.24 (s, 1H, NH), 8.00 (d, J = 7.7 Hz, 2H, Ar), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.54 (d, J = 7.3 Hz, 2H, Ar), 7.42 (d, J = 7.2 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.82 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.57–652 (d, J = 7.0 Hz, 4H, Ar), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.0, 152.4, 146.4, 138.0, 137.7, 136.8, 136.3, 130.4, 129.7, 129.3, 127.6, 127.3, 127.9, 127.6, 124.4, 124.2, 123.4, 123.1, 121.5, 121.2, 119.6, 119.4, 119.2, 119.0, 118.6, 118.3, 112.6, 112.3, 111.5, 111.2, 54.3; HR-EIMS: m/z calcd for C31H22N6O2S [M]+ 542.1525, Found 542.1515.
5-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)benzene-1,3-diol (14)
Yield 77%, 1H-NMR (500 MHz, DMSO-d6): δ 12.60 (s, 2H, NH), 10.30 (s, 1H, NH), 9.33 (s, 2H, OH), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.20–716 (m, 5H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.68–6.64 (m, 4H, Ar), 6.58 (d, J = 7.0 Hz, 2H, Ar), 6.23 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.0, 160.5, 160.5, 152.8, 145.0, 138.0, 136.3, 136.1, 130.7, 129.8, 129.3, 127.9, 127.7, 127.5, 127.2, 123.5, 123.1, 121.6, 121.2, 119.4, 119.0, 118.8, 118.2, 113.6, 112.3, 111.8, 111.4, 95.6, 95.3, 93.4, 54.2; HR-EIMS: m/z calcd for C31H23N5O2S [M]+ 529.1572, Found 529.1561.
4-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)phenol (15)
Yield 90%, 1H-NMR (500 MHz, DMSO-d6): δ 11.60 (s, 2H, NH), 10.30 (s, 1H, NH), 9.58 (s, 1H, OH), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.61 (d, J = 7.0 Hz, 2H, Ar), 7.55 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.94 (d, J = 6.7 Hz, 2H, Ar), 6.84 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70–6.65 (m, 4H, Ar), 6.64 (d, J = 7.0 Hz, 2H, Ar), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.7, 174.4, 158.3, 137.9, 136.8, 136.3, 130.2, 129.7, 129.3, 128.9, 128.3, 127.1, 127.0, 126.7, 126.4, 126.2, 123.5, 123.1, 121.6, 121.4, 119.5, 119.2, 118.6, 118.2, 116.2, 116.1, 112.5, 112.2, 111.7, 111.2, 54.3; HR-EIMS: m/z calcd for C31H23N5OS [M]+ 513.1623, Found 513.1609.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(pyridin-2-yl)-1,3,4-thiadiazol-2-amine (16)
Yield 79%, 1H-NMR (500 MHz, DMSO-d6): δ 12.70 (s, 2H, NH), 10.30 (s, 1H, NH), 8.56 (d, J = 8.1 Hz, 1H, Ar), 8.08 (d, J = 7.8 Hz, 1H, Ar), 7.82 (dd, J = 8.1, 3.5 Hz, 1H, Ar), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.37 (dd, J = 7.3 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.82 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.63–6.59 (m, 4H, Ar), 6.19 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.4, 174.1, 157.0, 149.8, 138.3, 137.8, 136.7, 136.6, 130.4, 129.3, 129.1, 127.9, 127.6, 127.4, 127.1, 124.5, 123.3, 123.1, 122.8, 121.4, 121.2, 119.6, 119.4, 118.5, 118.2, 112.4, 112.0, 111.3, 111.2, 54.4; HR-EIMS: m/z calcd for C30H22N6S [M]+ 498.1627, Found 498.1612.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(2-fluorophenyl)-1,3,4-thiadiazol-2-amine (17)
Yield 71%, 1H-NMR (500 MHz, DMSO-d6): δ 12.63 (s, 2H, NH), 11.81 (s, 1H, NH), 8.38 (s, 1H, Ar), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.71 (d, J = 7.5 Hz, 1H, Ar), 7.70 (dd, J = 7.9, 3, 5 Hz, 1H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.46 (d, J = 7.7 Hz, 1H, Ar), 7.25 (dd, J = 7.4 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.63–6.58 (m, 3H, Ar), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.4, 174.2, 158.1, 138.5, 136.7, 136.2, 130.4, 130.1, 129.9, 129.5, 129.3, 127.5, 127.4, 127.2, 127.0, 125.1, 123.6, 123.1, 122.6, 121.5, 121.0, 119.4, 119.0, 118.2, 118.1, 114.5, 112.0, 111.8, 111.4, 110.4, 54.3; HR-EIMS: m/z calcd for C31H22FN5S [M]+ 515.1580, Found 515.1566.
5-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)-2-methoxyphenol (18)
Yield 78%, 1H-NMR (500 MHz, DMSO-d6): δ 12.10 (s, 2H, NH), 10.30 (s, 1H, NH), 10.08 (s, 1H, OH), 7.73 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.30 (d, J = 7.3 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.12 (s, 1H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.80 (d, J = 6.8 Hz, 1H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.63–6.58 (m, 4H, Ar), 6.23 (s, 1H, CH), 3.84 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ 174.6, 174.3, 147.9, 147.7, 138.0, 136.4, 136.2, 130.9, 129.2, 129.0, 127.9, 127.7, 127.6, 127.3, 127.0, 123.6, 123.3, 121.8, 121.5, 121.2, 119.6, 119.3, 118.6, 118.2, 113.6, 112.5, 112.2, 111.2, 111.7, 110.0, 56.0, 54.3; HR-EIMS: m/z calcd for C32H25N5O2S [M]+ 543.1729, Found 543.1717.
N-(3-Chlorophenyl)-5-(4-(di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-amine (19)
Yield 70%, 1H-NMR (500 MHz, DMSO-d6): δ 12.50 (s, 2H, NH), 11.92 (s, 1H, NH), 8.32 (s, 2H, Ar), 7.94 (s, 1H, Ar), 7.90 (d, J = 7.7 Hz, 1H, Ar), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.45 (d, J = 7.5 Hz, 1H, Ar), 7.46 (d, J = 7.3 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.64 (d, J = 7.0 Hz, 2H, Ar), 6.12 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.6, 174.2, 138.4, 136.2, 136.0, 134.7, 134.3, 130.2, 129.9, 129.7, 129.4, 129.2, 128.5, 127.8, 127.5, 127.2, 127.0, 126.4, 124.0, 123.6, 122.7, 121.4, 120.8, 119.3, 118.3, 118.0, 113.1, 112.4, 112.8, 111.4, 54.3; HR-EIMS: m/z calcd for C31H22ClN5S [M]+ 531.1284, Found 531.1270.
2-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)-4-methoxyphenol (20)
Yield 81%, 1H-NMR (500 MHz, DMSO-d6): δ 12.20 (s, 2H, NH), 10.64 (s, 1H, NH), 10.25 (s, 1H, OH), 7.73 (d, J = 7.5 Hz, 2H, Ar), 7.55 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.17 (s, 1H, Ar), 6.81 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.68 (d, J = 6.8 Hz, 1H, Ar), 6.66 (d, J = 6.7 Hz, 1H, Ar), 6.61–6.56 (m, 4H, Ar), 6.12 (s, 1H, CH), 3.84 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ 174.7, 174.3, 153.5, 147.2, 138.0, 137.5, 136.3, 130.2, 129.1, 128.5, 128.8, 127.6, 127.3, 127.1, 123.7, 123.5, 123.1, 122.7, 122.3, 119.4, 119.2, 118.6, 118.1, 117.2, 115.3, 113.7, 113.1, 112.5, 111.6, 111.4, 55.5, 54.3; HR-EIMS: m/z calcd for C32H25N5O2S [M]+ 543.1729, Found 543.1717.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(2-nitrophenyl)-1,3,4-thiadiazol-2-amine (21)
Yield 83%, 1H-NMR (500 MHz, DMSO-d6): δ 12.75 (s, 2H, NH), 12.25 (s, 1H, NH), 8.16 (d, J = 8.0 Hz, 1H, Ar), 8.04 (d, J = 7.9 Hz, 1H, Ar), 7.85 (dd, J = 8.0, 3.4 Hz, 1H, Ar), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.70 (d, J = 7.1 Hz, 1H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.82 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.64–659 (m, 4H, Ar), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 175.1, 174.0, 147.4, 139.1, 137.2, 136.2, 135.0, 131.2, 130.8, 129.4, 129.2, 128.5, 127.4, 127.2, 126.8, 126.4, 126.0, 124.8, 123.6, 123.3, 121.5, 121.2, 119.5, 119.1, 118.4, 118.0, 113.1, 112.7, 111.6, 111.3, 54.3; HR-EIMS: m/z calcd for C31H22N6O2S [M]+ 542.1525, Found 542.1515.
4-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)benzene-1,3-diol (22)
Yield 83%, 1H-NMR (500 MHz, DMSO-d6): δ 12.40 (s, 2H, NH), 11.20 (s, 1H, NH), 10.40 (s, 2H, OH), 7.76 (d, J = 7.0 Hz, 2H, Ar), 7.52 (d, J = 7.5 Hz, 2H, Ar), 7.22 (d, J = 7.0 Hz, 2H, Ar), 7.13 (d, J = 7.5 Hz, 1H, Ar), 6.80 (d, J = 8.0 Hz, 2H, Ar), 6.78 (s, 1H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.64 (d, J = 7.5 Hz, 1H, Ar), 6.60–6.55 (m, 4H, Ar), 3.72 (s, 3H, CH3), 6.32 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.6, 152.4, 150.2, 138.1, 136.5, 136.0, 134.2, 130.3, 130.2, 129.4, 129.0, 127.7, 127.5, 127.2, 127.1, 123.3, 123.2, 121.6, 121.5, 121.0, 119.3, 119.0, 118.4, 118.2, 117.1, 112.5, 112.5, 112.0, 111.4, 111.3, 55.4, 54.2; HR-EIMS: m/z calcd for C31H23N5O2S [M]+ 529.1572, Found 529.1561.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(m-tolyl)-1,3,4-thiadiazol-2-amine (23)
Yield 81%, 1H-NMR (500 MHz, DMSO-d6): δ 12.40 (s, 2H, NH), 11.84 (s, 1H, NH), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.53 (d, J = 7.3 Hz, 1H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.43 (s, 1H, Ar), 7.22 (dd, J = 8.4, 3.3 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.80 (d, J = 7.6 Hz, 1H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.66–6.62 (m, 2H, Ar), 6.20 (s, 1H, CH), 2.30 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ 175.1, 152.3, 142.1, 139.0, 138.4, 136.1, 136.2, 130.1, 129.9, 129.5, 129.1, 128.4, 128.0, 127.7, 127.1, 123.8, 123.3, 121.9, 121.6, 121.4, 119.4, 119.1, 119.0, 118.6, 118.4, 114.2, 112.4, 112.2, 111.6, 111.2, 54.3, 21.0; HR-EIMS: m/z calcd for C32H25N5S [M]+ 511.1831, Found 511.1816.
N-(4-Chlorophenyl)-5-(4-(di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-amine (24)
Yield 81%, 1H-NMR (500 MHz, DMSO-d6): δ 11.70 (s, 2H, NH), 10.92 (s, 1H, NH), 7.75 (d, J = 7.5 Hz, 2H, Ar), 7.63 (d, J = 7.6 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.25 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.81 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.64–6.59 (m, 4H, Ar), 6.25 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.4, 152.2, 138.2, 138.0, 136.8, 136.2, 130.2, 129.8, 129.4, 129.2, 129.0, 127.4, 127.2, 127.0, 126.8, 126.4, 123.6, 123.3, 122.6, 122.2, 121.4, 121.1, 119.2, 119.0, 118.6, 118.2, 112.7, 112.3, 111.8, 111.4, 54.3; HR-EIMS: m/z calcd for C31H22ClN5S [M]+ 531.1284, Found 531.1270.
N-(2-Chlorophenyl)-5-(4-(di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-amine (25)
Yield 93%, 1H-NMR (500 MHz, DMSO-d6): δ 12.80 (s, 2H, NH), 12.04 (s, 1H, NH), 8.17 (d, J = 7.9 Hz, 1H, Ar), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.54 (d, J = 7.5 Hz, 1H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.40 (dd, J = 8.3, 3.3 Hz, 1H, Ar), 7.24 (dd, J = 7.8, 2.7 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.83 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.72–6.68 (m, 4H, Ar), 6.63 (d, J = 7.0 Hz, 2H, Ar), 6.21 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.7, 152.2, 138.0, 136.8, 136.3, 136.0, 130.4, 130.1, 129.8, 129.2, 128.6, 128.0, 127.7, 127.3, 127.0, 125.7, 123.7, 123.3, 122.5, 122.0, 121.3, 121.0, 120.4, 119.4, 118.2, 118.0, 112.6, 112.2, 111.6, 111.3, 54.3; HR-EIMS: m/z calcd for C31H22ClN5S [M]+ 531.1284, Found 531.1270.
5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-N-(3-fluorophenyl)-1,3,4-thiadiazol-2-amine (26)
Yield 88%, 1H-NMR (500 MHz, DMSO-d6): δ 11.70 (s, 2H, NH), 10.10 (s, 1H, NH), 7.72 (d, J = 7.5 Hz, 2H, Ar), 7.70 (s, 1H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.42 (d, J = 7.5 Hz, 1H, Ar), 7.31 (dd, J = 8.3, 3.3 Hz, 1H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 6.82 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.73–6.68 (m, 3H, Ar), 6.70 (dd, J = 7.9, 3.2 Hz, 2H, Ar), 6.64 (d, J = 7.0 Hz, 2H, Ar), 6.22 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 175.0, 163.3, 152.1, 144.4, 138.4, 136.8, 136.3, 131.4, 130.2, 129.8, 129.2, 128.4, 128.0, 127.8, 127.3, 123.5, 123.1, 121.5, 121.2, 119.4, 119.2, 118.3, 118.0, 113.1, 112.8, 112.2, 111.4, 111.0, 110.0, 104.3, 54.3; HR-EIMS: m/z calcd for C31H22FN5S [M]+ 515.1580, Found 515.1566.
3-((5-(4-(Di(1H-indol-3-yl)methyl)phenyl)-1,3,4-thiadiazol-2-yl)amino)phenol (27)
Yield 90%, 1H-NMR (500 MHz, DMSO-d6): δ 9.90 (s, 2H, NH), 9.54 (s, 1H, NH), 8.40 (s, 1H, OH), 7.74 (d, J = 7.5 Hz, 2H, Ar), 7.50 (d, J = 7.3 Hz, 2H, Ar), 7.20 (d, J = 7.0 Hz, 2H, Ar), 7.18 (d, J = 7.5 Hz, 1H, Ar), 7.08 (d, J = 7.2 Hz, 1H, Ar), 6.82 (dd, J = 8.2, 2.5 Hz, 2H, Ar), 6.70–6.65 (m, 4H, Ar), 6.62 (s, 1H, Ar), 6.56 (d, J = 6.7 Hz, 1H, Ar), 6.48 (d, J = 7.0 Hz, 2H, Ar), 6.24 (s, 1H, CH); 13C-NMR (125 MHz, DMSO-d6): δ 174.8, 159.0, 152.3, 143.2, 138.3, 136.1, 136.0, 130.5, 130.2, 129.7, 129.3, 128.4, 126.4, 127.8, 127.2, 123.6, 123.2, 121.4, 121.1, 119.4, 119.2, 118.4, 118.0, 112.8, 112.4, 111.8, 111.1, 110.2, 109.3, 102.2, 54.1; HR-EIMS: m/z calcd for C31H23N5OS [M]+ 513.1623, Found 513.1609.
In vitro leishmaniasis assay
The assay was carried out according to Seifert and Croft [38]. Briefly, THP-1 cells (ATCC) were cultured in RPMI-1640 (R5886 Sigma) supplemented with 1% l-glutamine and 10% HIFBS (complete medium) before harvested at 1.0 × 106 cells/mL. Cells were diluted to 2.0 × 105 cells/mL with the complete medium, seeded in 16-well Lab Tek tissue culture chamber slide (Fisher Scientific) at a seeding density of 5.0 × 104 macrophage/well (100 μL) and allowed to adhere by the addition of PMA (Phorbol-12 myristate Acetate P8139 Sigma) for 3 days at 37 °C in a 5% CO2–95% air mixture. Macrophages were then infected with long-slender (stationary stage) of Leishmania major promastigote (JISH118) obtained from The London School of Hygiene and Tropical Medicine (LSHTM) United Kingdom, which were cultured at 26 °C in
Schneiders Drosophila medium (S0146 Sigma), at a macrophage-promastigote ratio of 1:5. Infected macrophages were maintained at 34 °C in a 5% CO2–95% air mixture. After 48 h, extracellular parasites were removed by substituting the overlay with new fresh RPMI-1640 medium supplemented with 1% l-glutamine. Fresh Pentamidine and test compounds with various concentrations were added and drug or compound activity was determined from the percentage of infected cells in drug-treated cultures in relation to non-treated cultures using GraphPad Prism after methanol fixation and Giemsa staining. All testing was triplicated.
Availability of data and materials
Data and materials are available.
Abbreviations
- IC50 :
-
the IC50 is the concentration of an inhibitor where the response (or binding) is reduced by half
- µM:
-
micromolar
- mM:
-
millimolar
- 1HNMR:
-
proton nuclear magnetic resonance
- 13CNMR:
-
13carbon nuclear magnetic resonance
- HR-EIMS:
-
high-resolution electron ionization mass spectrometry
- H:
-
hours
- SAR:
-
structure activity relationship
- SEM:
-
standard error mean
- Fig:
-
figure
- PTR:
-
pteridine reductase active
- Gly:
-
glycine
- Ana:
-
asparagine
- Phe:
-
phenylalanine
- Met:
-
methionine
- Leu:
-
leucine
- EIMS:
-
electron impact mass spectra
- TLC:
-
thin layer chromatography
- MTX:
-
methotrexate
- SP:
-
standards precision
- XP:
-
extra precision
- Tyr:
-
tyrosine
References
WHO (2010) Control of the leishmaniasis: report of a meeting of the WHO expert committee on the control of leishmaniases. WHO, Geneva
Singh N, Kumar M, Singh RK (2012) Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pac J Trop Med 5:485–497
Nagle AS, Khare S, Kumar AB, Supek F, Buchynskyy A, Mathison CJ, Chennamaneni NK, Pendem N, Buckner FS, Gelb MH, Molteni V (2014) Recent developments in drug discovery for leishmaniasis and human African trypanosomiasis. Chem Rev 114:11305–11347
Cagnoni PJ (2002) Liposomal amphotericin B versus conventional amphotericin B in theempirical treatment of persistently febrile neutropenic patients. J Antimicrob Chemother 49:81–86
Berman JD (1997) Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis 24:684–703
Grenfell RFQ, Marques-da-Silva EA, Souza-Testasicca MC, Coelho EAF, Fernandes AP, Afonso LCC, Rezende SA (2010) Antigenic extracts of Leishmania braziliensis and Leishmania amazonensis associated with saponin partially protects BALB/c mice against Leishmania chagasi infection by suppressing IL-10 and IL-4 production. Mem Inst Oswaldo Cruz 105:818–822
Noazina S, Modabberb F, Khamesipourc A, Smithd PG, Moultone LH, Nasserif K, Sharifig I, Khalilh EAG, Bernali IDV, Antunes CM, Kienya FMP, Tannerk M (2008) First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine 26:6759–6767
Colotti G, Ilari L (2011) Polyamine metabolism in Leishmania: from arginine to trypanothione. Amino Acids 40:269–285
Castañon A, Tristram A, Mesher D, Powell N, Beer H, Ashman S, Rieck G, Fielder H, Fiander A, Sasieni P (2012) Effect of diindolylmethane supplementation on low-grade cervical cytological abnormalities: double-blind, randomised, controlled trial. Br J Cancer 106:45–52
Sharma DK, Rah B, Lambu MR, Hussain A, Yousuf SK, Tripathi AK, Singh B, Jamwal G, Ahmed Z, Chanauria N, Nargotra A, Goswami A, Mukherjee D (2012) Design and synthesis of novel N,N′-glycoside derivatives of 3,3′-diindolylmethanes as potential antiproliferative agents. Med Chem Commun 3:1082–1091
Kamal A, Srikanth YV, Ramaiah MJ, Khan MN, Reddy MK, Ashraf M, Lavanya A, Pushpavalli SN, Pal-Bhadra M (2012) Synthesis, anticancer activity and apoptosis inducing ability of bisindole linked pyrrolo[2,1-c][1,4]benzodiazepine conjugates. Bioorg Med Chem Lett 22:571–578
Sreenivasulu R, Durgesh R, Jadav SS, Sujitha P, Kumar CG, Raju RR (2018) Synthesis, anticancer evaluation and molecular docking studies of Bis(indolyl)triazinones. Nortopsentin analogues. Chem Pap 72:1369–1378
Durgesh R, Sreenivasulu R, Raju RR (2018) Synthesis and antitumor evaluation of indole-substituted indole fused keto hydrazide-hydrazones. J Pharm Res 12:42–46
Hatti I, Sreenivasulu R, Jadav SS, Ahsan MJ, Raju RR (2015) Synthesis and biological evaluation of 1,3,4-oxadiazole-linked bisindole derivatives as anticancer agents. Monatsh Chem 146:1699–1705
Oh KB, Mar W, Kim S, Kim JY, Lee TH, Kin JG, Shin D, Sim CJ, Shin J (2006) Antimicrobial activity and cytotoxicity of bis(indole) alkaloids from the sponge Spongosorites sp. Biol Pharm Bull 29:570–573
Mansouri MD, Opperman TJ, Williams JD, Stager C, Darouiche RO (2012) In vitro potency and in vivo efficacy of a novel bis-indole antimicrobial compound in reducing catheter colonization. Antimicrob Agents Chemother 56:2201–2204
Mitchell SS, Lam KS, Grodberg J, Potts, BC, Tsueng G, White DJ, Reed KA (2008) Bis-indole pyrroles useful as antimicrobials agents. United States patent US7375129, Nereus Pharmaceuticals, Inc
Gunasekera SP, McCarthy PJ, Kelly-Borges M (1994) Hamacanthins A and B, new antifungal bis indole alkaloids from the deep-water marine sponge, Hamacantha sp. J Nat Prod 57:1437–1441
Martinez R, Espinosa A, Tarraga A, Molina P (2008) Bis(indolyl)methane derivatives as highly selective colourimetric and ratiometric fluorescent molecular chemosensors for Cu+2 cations. Tetrahedron 64:2184–2191
Mishra BB, Kale RR, Singh RK, Tiwari VK (2009) Alkaloids: future prospective to combat leishmaniasis. Fitoterapia 80:81–90
Singh GS, Al-kahraman YM, Mpadi D, Yasinzai M (2012) Synthesis of N-(1-methyl-1H-indol-3-yl) methyleneamines and 3,3-diaryl-4-(1-methyl-1Hindol-3-yl)azetidin-2-ones as potential antileishmanial agents. Bioorg Med Chem Lett 22:5704–5706
Staerk D, Lemmich E, Christensen J, Kharazmil A, Olsen CE, Jaroszewski JW (2000) Leishmanicidal, antiplasmodial and cytotoxic activity of indole alkaloids from Corynanthe pachyceras. Planta Med 66:531–536
Delorenzi JC, Attias M, Gattass CR, Abdrade M, Rezende C, Cunha-Pinto A, Henrique AT, Bou-Habib DC, Saraiva EM (2001) Antileishmanial activity of an indole alkaloid from Peschiera australis. Antimicrob Agents Chemother 45:1349–1354
Tanaka JCA, Da Silva CC, Ferreira ICP, Machado GMC, Leon LL, De Oliveira AJB (2007) Antileishmanial activity of indole alkaloids from Aspidosperma ramiflorum. Phytomedicine 14:377–380
Munoz V, Moretii C, Sauvain M, Caron C, Prozel A, Massiot G, Richard B, Le Men-Olivier L (1994) Isolation of bis-indole alkaloids with antileishmanial and antibacterial activities from Peschiera van heurkii (Syn. Tabernaemontana van heurkii). Planta Med 60:455–459
Taha M, Ismail NH, Jamil W, Khan KM, Salar U, Kashif SM, Rahim F, Latif Y (2015) Synthesis and evaluation of unsymmetrical heterocyclic thioureas as potent β-glucuronidase inhibitors. Med Chem Res 24:3166–3173
Taha M, Ismail NH, Jamil W, Rashwan H, Kashif SM, Sain AA, Adenan MI, Anouar EH, Ali M, Rahim F, Khan KM (2014) Synthesis of novel derivatives of 4-methylbenzimidazole and evaluation of theirbiological activities. Eur J Med Chem 84:731–738
Saify ZS, Kamil A, Akhtar S, Taha M, Khan A, Khan KM, Jahan S, Rahim F, Perveen S, Choudhary MI (2014) 2-(2-Pyridyl) benzimidazole derivatives and their urease inhibitory activity. Med Chem Res 23:4447–4454
Abdullah NKNZ, Taha M, Ahmat N, Wadood A, Ismail NH, Rahim F, Ali M, Abdullah N, Khan KM (2015) Novel 2,5-disubtituted-1,3,4-oxadiazoles with benzimidazole backbone: a new class of β-glucuronidase inhibitors and insilico studies. Bioorg Med Chem 23:3119–3125
Taha M, Ismail NH, Khan A, Shah SAA, Anwar A, Halim SA, Fatmi MQ, Imran S, Rahim F, Khan KM (2015) Synthesis of novel derivatives of oxindole, their urease inhibition and molecular dockingstudies. Bioorg Med Chem Lett 25:3285–3289
Taha M, Ismail NH, Imran S, Selvaraj M, Rashwan H, Farhanah FU, Rahim F, Selvarajan KK, Ali M (2015) Synthesis of benzimidazole derivatives as potent β-glucuronidase inhibitors. Bioorg Chem 61:36–44
Taha M, Shah SAA, Khan A, Arshad F, Ismail NH, Afifi M, Imran S, Choudhary MI (2015) Synthesis of 3,4,5-trihydroxybenzohydrazone and evaluation of their urease inhibition potential. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.06.036
Imran S, Taha M, Ismail NH, Kashif SM, Rahim F, Jamil W, Wahab H, Khan KM (2016) Synthesis, In vitro and docking studies of new flavone ethers as α-glucosidase inhibitors. Chem Biol Drug Des. 87:361–373
Imran S, Taha M, Ismail NH, Kashif SM, Rahim F, Jamil W, Hariono M, Yusuf M, Wahab H (2015) Synthesis of novel flavone hydrazones: in vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. Eur J Med Chem 105:156–170
Imran S, Taha M, Ismail NH, Fayyaz S, Khan KM, Choudhary MI (2015) Synthesis, biological evaluation, and docking studies of novel thiourea derivatives of bisindolylmethane as carbonic anhydrase II inhibitor. Bioorg Chem 62:83–93
Taha M, Ismail NH, Javaid K, Imran S, Wadood A, Ali M, Khan KM, Saad SM, Rahim F, Choudhary MI (2015) Evaluation of 2-indolcarbohydrazones as potent α-glucosidase inhibitors, in silico studies and DFT based stereochemical predictions. Bioorg Chem. 63:24–35
Taha M, Ismail NH, Imran S, Anouar EH, Selvaraj M, Jamil W, Ali M, Kashif SM, Rahim F, Khan KM, Adenan MI (2017) Synthesis and molecular modelling studies of phenyl linked oxadiazole-phenylhydrazone hybrids as potent antileishmanial agents. Eur J Med Chem 126:1021–1033
Seifert K, Croft SL (2006) In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob Agents Chemother 50(1):73–79
Acknowledgements
The authors are thankful to IRMC, Imam Abdulrahman Bin Faisal University for providing lab facilities for the research.
Funding
There is no funding for this study.
Author information
Authors and Affiliations
Contributions
MT and IU conceived and designed the experiments; MG and NBA performed the experiments; FR and RKF analyzed the data; MN and MI wrote the paper; MAA and YAB authors read and approved the final manuscript; MS performed molecular docking studies. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1.
The file contained Proton NMR spectra.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Taha, M., Uddin, I., Gollapalli, M. et al. Synthesis, anti-leishmanial and molecular docking study of bis-indole derivatives. BMC Chemistry 13, 102 (2019). https://doi.org/10.1186/s13065-019-0617-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-019-0617-4