Abstract
Background
The SARS CoV-2 pandemic has resulted in more than 1.1 million deaths in the USA alone. Therapeutic options for critically ill patients with COVID-19 are limited. Prior studies showed that post-infection treatment of influenza A virus-infected mice with the liponucleotide CDP-choline, which is an essential precursor for de novo phosphatidylcholine synthesis, improved gas exchange and reduced pulmonary inflammation without altering viral replication. In unpublished studies, we found that treatment of SARS CoV-2-infected K18-hACE2-transgenic mice with CDP-choline prevented development of hypoxemia. We hypothesize that administration of citicoline (the pharmaceutical form of CDP-choline) will be safe in hospitalized SARS CoV-2-infected patients with hypoxemic acute respiratory failure (HARF) and that we will obtain preliminary evidence of clinical benefit to support a larger Phase 3 trial using one or more citicoline doses.
Methods
We will conduct a single-site, double-blinded, placebo-controlled, and randomized Phase 1/2 dose-ranging and safety study of Somazina® citicoline solution for injection in consented adults of any sex, gender, age, or ethnicity hospitalized for SARS CoV-2-associated HARF. The trial is named “SCARLET” (Supplemental Citicoline Administration to Reduce Lung injury Efficacy Trial). We hypothesize that SCARLET will show that i.v. citicoline is safe at one or more of three doses (0.5, 2.5, or 5 mg/kg, every 12 h for 5 days) in hospitalized SARS CoV-2-infected patients with HARF (20 per dose) and provide preliminary evidence that i.v. citicoline improves pulmonary outcomes in this population. The primary efficacy outcome will be the SpO2:FiO2 ratio on study day 3. Exploratory outcomes include Sequential Organ Failure Assessment (SOFA) scores, dead space ventilation index, and lung compliance. Citicoline effects on a panel of COVID-relevant lung and blood biomarkers will also be determined.
Discussion
Citicoline has many characteristics that would be advantageous to any candidate COVID-19 therapeutic, including safety, low-cost, favorable chemical characteristics, and potentially pathogen-agnostic efficacy. Successful demonstration that citicoline is beneficial in severely ill patients with SARS CoV-2-induced HARF could transform management of severely ill COVID patients.
Trial registration
The trial was registered at www.clinicaltrials.gov on 5/31/2023 (NCT05881135).
Trial status
Currently enrolling.
Similar content being viewed by others
Background
To date, there have been more than 100 million SARS CoV-2 coronavirus infections in the USA, resulting in more than 1.1 million deaths and counting. Effective vaccines are now available [1], but the virus continues to mutate and circulate within the population, and there is an ongoing risk of future severe pandemic waves.
Symptomatic SARS CoV-2 infection can progress to a clinical syndrome termed coronavirus disease of 2019 (COVID-19). Severe COVID-19 is characterized by acute respiratory failure and development of the acute respiratory distress syndrome (ARDS). COVID-19 may be complicated by coagulopathy, acute cardiac injury, renal injury, and other systemic manifestations [2,3,4,5]. Approximately 20% of all hospitalized patients [6] and up to 40% of those admitted to the intensive care unit (ICU) die [7]. Moreover, around 15% of survivors [8] develop “long COVID” [9], a disabling condition which is more likely after severe acute illness [10].
Treatment options for severely ill patients with COVID-19 are limited. Remdesivir, immunomodulators such as tocilizumab and baricitinib, and corticosteroids have been shown to be of some benefit and are currently recommended for use, but supportive ICU care remains central to managing these patients. Specifically, administration of high concentrations of supplemental O2 and mechanical ventilation are often required [11, 12]. Supportive care is resource-intensive, and interventions such as O2 therapy and mechanical ventilation can themselves be injurious to the lung [13, 14]. Additional therapeutics to manage patients with COVID-19 and reduce the need for ICU care are therefore urgently needed.
Approximately 50% of the epithelial cells lining the alveoli in the distal lung are small cuboidal ATII cells [15]. ATII cells regulate the depth of the alveolar lining fluid by alveolar fluid clearance [16]. They also synthesize, secrete, and recycle pulmonary surfactant proteins and lipids (including phospholipids), which help to maintain low alveolar surface tension [17], reducing dynamic alveolar collapse and preventing gas exchange impairment during ventilation. Surfactant phospholipids also have anti-inflammatory properties, and surfactant proteins play an important role in host defense against pathogens [18]. ATII cells are thus essential to normal lung function and host defense and play a central role in ARDS pathogenesis.
ATII cell dysfunction and death in ARDS results in reduced surfactant production and function, leading to poor lung compliance, predisposition to ventilator-induced lung injury (VILI), impaired pathogen clearance, and enhanced inflammation [19,20,21,22,23,24]. ARDS also results in impaired alveolar fluid clearance [25], which is associated with a poor prognosis [11, 26]. Importantly, human ATII cells express the receptor for CoV-2 (ACE2) [27] and the protease cofactor TMPRSS2 [28] and SARS CoV-2 antigens have been detected in ATII cells from COVID-19 autopsy samples [29,30,31,32,33]. SARS CoV-2 has also been shown to replicate in ATII cells in vitro [34,35,36]. In addition to inducing release of inflammatory mediators [37], SARS CoV-2 infection of ATII cells may result in disruption of surfactant synthesis and alveolar fluid clearance, both of which may contribute significantly to development of COVID-19 [38, 39].
The phospholipid dipalmitoyl-phosphatidylcholine (16:0/16:0) is the largest component of pulmonary surfactant [40]. Dipalmitoyl-phosphatidylcholine is synthesized by the Kennedy pathway [41]. Synthesis of the liponucleotide cytidine 5’-diphospho (CDP)-choline from choline phosphate and CTP by the enzyme CTP-phosphocholine cytidylyltransferase-α (CCT-α) is rate limiting for this pathway. We showed that murine ATII cell CDP-choline synthesis is rapidly and completely inhibited by IAV infection in vivo [42]. Although we have not yet shown that SARS CoV-2 also inhibits ATII cell CDP-choline synthesis, multiple groups have reported that plasma or serum from COVID-19 patients contains reduced phosphatidylcholine [43,44,45,46,47,48], suggesting that SARS CoV-2 disrupts this pathway. Moreover, some of the beneficial effects of dexamethasone in COVID-19 patients could be because it stimulates CCT-α activity [49,50,51,52,53,54,55,56], as does CDP-choline [57].
We hypothesize that impaired ATII cell CDP-choline synthesis is a common feature of viral ARDS, which contributes significantly to its pathogenesis. Hence, we propose that the de novo phospholipid synthesis pathway is a promising target for host-directed therapy for viral ARDS. In support of this, we have shown that CDP-choline strongly attenuates IAV-induced ARDS and pulmonary inflammation [58] and prevents hypoxemia in SARS CoV-2-infected male K18-hACE2-Tg mice (unpublished data). Hence, we propose that this compound will be effective in COVID-19 patients. The pharmaceutical form of CDP-choline is citicoline.
SCARLET has two goals. Firstly, we will show that i.v. citicoline administration is safe over a range of doses in hospitalized SARS CoV-2-infected patients with hypoxemic acute respiratory failure (HARF). Secondly, we will obtain preliminary evidence that i.v. citicoline improves outcomes in this patient population relative to current standard of care and identify the recommended dose and appropriate clinical endpoints for larger Phase 3 efficacy trials. We will use a placebo control to represent current standard of care. We expect to find that citicoline is safe at all tested doses in COVID-19 patients. We also expect to find that citicoline has clinical benefit at one or more doses.
Methods
Participants, interventions, and outcomes
Study design and setting
SCARLET is a single-center, double‐blinded, placebo‐controlled, and randomized Phase 1/2 trial of i.v. citicoline in adult patients of any sex, gender, age, or ethnicity who are hospitalized with HARF following SARS CoV‐2 infection (Table 1). Patients will be enrolled at The Ohio State University (OSU) Wexner Medical Center (OSU WMC) and OSU WMC Hospital East (OSU East), both of which are in Columbus, Ohio, USA. The goals are to confirm citicoline safety over a range of doses and demonstrate potential for efficacy in this population for at least one citicoline dose. The trial will enroll 20 patients per dose for 3 citicoline doses (0.5, 2.5, and 5 mg/kg every 12 h for 5 days) along with 20 placebo‐treated controls (See Fig. 1).
Trial design. SCARLET is a single-center, double‐blinded, placebo‐controlled, and randomized Phase 1/2 trial of i.v. citicoline in adult patients of any sex, gender, age, or ethnicity who are hospitalized with HARF following SARS CoV‐2 infection. The trial will enroll 20 patients per dose for 3 citicoline doses (1, 5, and 10 mg/kg/day, split into 2 doses 12 h apart) along with 20 placebo (sterile saline)‐treated controls
Eligibility criteria
Adult patients (≥ 18 years) of both sexes and any race or ethnicity will be eligible for enrollment. Research personnel (physicians and research coordinators) will screen patients daily for eligibility daily under partial HIPAA waiver using the electronic medical record (EMR; Epic, Madison, WI, USA) to facilitate identification of SARS CoV-2-positive patients who may meet other eligibility criteria for the trial. Enrollment is feasible any time during the hospital stay; however, if hospital discharge is anticipated within 24 h the patient will not be enrolled. Patients previously screened and not meeting inclusion criteria or with exclusion criteria may be rescreened daily until eligibility expires (> 10 days post admission).
Initial screening will assess for evidence of HARF plus evidence of active SARS CoV-2 infection. Only patients positive for SARS CoV-2 using antigen or PCR test within the 10 days prior to randomization will be eligible. Patients must also have a C-reactive protein > 32 mg/dl. Assurance of adequate peripheral or central venous access is a requirement. Female subjects of childbearing potential must have a negative pregnancy test upon study entry.
Patients meeting inclusion criteria will be screened for exclusion criteria. Individuals who meet any of these criteria are not eligible for enrollment as study participants: (1) Prisoners; (2) Women who may be pregnant, are pregnant, or who are breast feeding; (3) Subjects who are unable or unwilling to give written informed consent or to comply with study protocol and who have no legal authorized representative (LAR) available to give consent on their behalf; (4) Individuals with a known allergy to citicoline; (5) Subjects that are taking medications that contain L‐Dopa, centrophenoxine, or meclofenoxate; (6) Individuals with hypertonia of the parasympathetic nervous system; (7) Subjects who, in the clinicians estimation, will be unlikely to survive the protocol duration due to imminent and unavoidable risk of death; (8) Individuals being treated with extracorporeal membrane oxygenation (ECMO); (9) Individuals with multiple organ failure; and/or (10) Subjects with past or current medical problems or findings from physical examination or laboratory testing that are not listed above, which, in the opinion of the principal investigator (PI; E.D.C.), may pose additional risks from participation in the study or that may impact the quality or interpretation of the data obtained from the study.
Critically ill children with SARS CoV-2 infection generally present with severe multisystem inflammatory syndrome (MIS-C) requiring vasoactive drugs and immunomodulators and are less likely to need ventilatory support than adults with severe COVID-19, who primarily develop progressive HARF which often leads to ARDS [59,60,61,62]. Hence, children will not be enrolled.
Following screening, eligible patients will be discussed with the patient’s primary care team. The ability to provide consent, directly or by a surrogate decision maker, will then be determined among patients who are deemed eligible. The patient or their LAR must be able to understand and provide informed consent. For subjects who are eligible for enrollment but are not recruited based on the clinician’s opinion that participation may not be in their best interests, the stated reason and name of the physician making the decision not to enroll will be recorded.
After approval from their care team, one of the study physicians or their official designee (a research coordinator) will approach the patient or their LAR if the patient is deemed unable to provide consent. The research study will be explained in lay terms in accordance with OSU standard operating procedures. Disclosures of potential conflicts of financial interest for the Institution and for I.C.D. (who will not be involved in patient interactions or treatment decisions) will be included in the final consent form. Once informed consent has been obtained, subjects will be enrolled into the study and randomized using random block permutations. Randomization will be done by OSU WMC Investigational Drug Services (IDS) and all clinical and research personnel will be blinded to allocation. Randomization must occur within 24 h from time of consent.
A participant will be deemed to have completed the study following administration of their final dose of test agent (10 doses over 5 days), unless discharged earlier. Study participation may be prematurely terminated for the following reasons: (1) The participant elects to withdraw consent from all future study activities, including follow‐up; (2) The participant dies; (3) The PI no longer believes participation is in the best interest of the participant; or (4) The PI believes that a serious adverse event (SAE) in the patient may be attributable to the investigational agent.
Participants who withdraw or are withdrawn will not be replaced if they have received at least one dose of the investigational agent. No follow-up will occur for participants who withdraw or are withdrawn from the trial.
Informed consent
The consent process will provide information about the study to a prospective participant and will allow adequate time for review and discussion prior to his/her/their decision. The PI will review the consent with the patient or their LAR and answer questions. The prospective participant will be told that being in the trial is voluntary and that they may withdraw from the study at any time, for any reason. All participants (or their LAR) will read, sign, and date a consent form before undergoing any study procedures. Consent materials will be presented in participants’ primary language. A copy of the signed consent form will be given to the participant.
The consent process will be ongoing. The consent form will be revised when important new safety information is available, the protocol is amended, and/or new information becomes available that may affect participation in the study.
Interventions
Intervention description
Patients will be randomized 1:1:1:1 to receive one of 3 doses (0.5, 2.5, or 5 mg/kg) of Somazina® citicoline solution for injection (Ferrer Internacional, S.A., Barcelona, Spain) diluted in sterile normal saline or placebo (sterile normal saline). Somazina® is approved by the European Medicines Agency for use with a prescription in Europe. Patients will be treated with study agent every 12 h for up to 5 days.
Citicoline will be provided in sterile, single-use, Type I neutral glass ampoules containing 1000 mg active pharmaceutical ingredient (citicoline) dissolved in 4 ml water (final concentration 250 mg/ml), with HCl added to normalize pH; no other excipients are present. Study ampoules will be shipped from Ferrer directly to the IDS at OSU WMC and stored at room temperature in the dark. The immediate package of this investigational new drug will be labeled with the statement “Caution: New Drug – Limited by Federal (or United States) law to investigational use.”
Study drugs will be prepared on the day of use by a research pharmacist in the IDS at OSU WMC. Citicoline solution will be diluted in USP-compliant sterile normal saline to a final volume of 20 ml containing the appropriate total daily dose for each patient (1, 5, or 10 mg/kg/day, based on their ID number). Doses will be rounded to the nearest 0.1 ml per standard rounding rules. Two 10-ml aliquots will then be prepared in sterile disposable syringes labeled with the patient ID number for administration 12 h apart and then kept refrigerated. Unused undiluted or diluted citicoline will be discarded by the IDS pharmacist and documented as such. Placebo controls will receive 10 ml USP-compliant sterile normal saline every 12 h for 5 days.
Each 10 ml dose of test agent will be delivered by nursing staff as a slow i.v. bolus over 3–5 min and immediately documented in the EMR. EMRs will be reviewed daily by the clinical trial coordinator to ensure test agent administration has occurred and been documented.
Criteria for discontinuing or modifying allocated interventions
No modifications to the test agent dose or frequency will be permitted during the study period. Study therapy may be prematurely discontinued for any participant for any of the following reasons: (1) SAEs occur that, in the view of the attending clinician, study investigator, or PI, may be attributable to citicoline; (2) The PI believes that the study treatment is no longer in the best interest of the participant; or (3) The Data Safety Monitoring Board (DSMB) will make a determination of futility or potential for harm to the patient(s).
Strategies to improve adherence to interventions
All patients enrolled in the study will be hospitalized and will therefore receive monitoring by their physicians, nurses, respiratory therapists, and ancillary staff as a part of routine clinical care. A drug‐dispensing log will be kept current for each participant. This log will contain the identification of each participant and the date and quantity of drug dispensed. Under Title 21 of the Code of Federal Regulations (21CFR §312.62), the PI will maintain adequate records of the disposition of the investigational agent, including the date and quantity of the drug received, to whom the drug was dispensed (participant‐by‐participant accounting), and a detailed accounting of any drug accidentally or deliberately destroyed. Records for receipt, storage, use, and disposition will be maintained by the IDS and will be available for inspection.
Relevant concomitant care permitted or prohibited during the trial
Enrolled participants will not receive open-label citicoline during the 5-day intervention period. All other treatment decisions will be made by treating clinicians without reference to the protocol. Treatment of the underlying SARS CoV-2 viral infection is left to the discretion of the treating team. Depending on disease severity, and as based on current recommendations from the NIH, treatment may include but is not limited to antiviral therapy with remdesivir and administration of anti-inflammatory agents such as dexamethasone or tocilizumab. Patients deemed high risk for venothromboembolic disease may be treated with either prophylactic or therapeutic anticoagulation as appropriate. Other common medications such as vasopressors, sedative agents, antimicrobials, and neuromuscular blockade will be used in accordance with local standard of care in accordance with best practices.
Serious adverse reactions to citicoline have not been reported in the medical literature and are considered unlikely. Nevertheless, between randomization and day 5, study personnel will review the EMR daily for potential medication interactions with citicoline. There are no rescue medications for citicoline per se; however, reported side effects include headache, nausea, and low blood pressure. Rescue therapies for these side effects include (1) Analgesics for headache (acetaminophen, ibuprofen, rarely narcotics); (2) Antiemetics (ondansetron, metoclopramide); and (3) Fluid bolus for low blood pressure. Should a patient develop evidence of a severe reaction, resuscitation and treatment will be at the discretion of the treating physicians. Specifically, study drug will be discontinued at the time of suspicion of an adverse reaction. If anaphylaxis is suspected, the patient will receive epinephrine, anti‐histamines, and corticosteroids as appropriate. Resuscitation for hypotension will occur with fluids or vasopressor agents.
Rescue medications themselves can cause adverse reactions. Dexamethasone often causes transient hyperglycemia and may increase susceptibility to secondary infection of the lungs. Remdesivir may predispose to reversible transaminase elevation and case reports of bradycardia have been seen in post-market analysis. Mild transaminase elevation has been seen after administration of tocilizumab, so liver function monitoring is recommended. Immune suppression is expected following corticosteroid and tocilizumab dosing, and patients will be monitored for secondary bacterial infections.
On-study monitoring
Peripheral arterial O2 saturation (SpO2) will be monitored continuously and recorded every 2 min. Arterial blood gases will be measured on study days 1, 3, 5, and 8, or as clinically necessary.
Venous blood will be drawn at Time 0 and then once daily on study days 1–5 and 8 and processed by a research coordinator. At each study timepoint, a sufficient volume of venous blood will be drawn into blood container tubes containing appropriate anticoagulants to complete all the following assays: (1) CBC/differential count; (2) Hematology panel; (3) Clinical chemistry panels for renal, cardiac, and hepatic function; (4) Prothrombin Time and Partial Thromboplastin Time; (5) D-dimer, ferritin, fibrinogen; and (6) Thromboelastography. These assays will be performed by the clinical lab at the hospital in which that subject is located. All OSU hospital clinical laboratories are Clinical Laboratory Improvement Amendment-approved by the Dept. of Health and Human Services and are accredited by the College of American Pathologists’ Laboratory Accreditation Program. All clinical laboratory testing for study participants will comply with 42 CFR part 493.2 and 493.3(b)(2).
An additional 10 ml heparinized venous blood will be collected from each subject at each study timepoint for research purposes. Venous blood collected for research use from patients at both OSU East and OSU Main will be transferred to Dr. Crouser’s laboratory in the Davis Heart and Lung Research Institute (part of OSU WMC and an approved research space). Plasma will be divided into 1-ml aliquots and stored in a locked − 80 °C freezer for batch analysis of biomarkers. Leukocytes will be resuspended in freezing media, aliquoted into cryovials, and stored at − 80 °C for downstream research studies.
Chest X-rays will be obtained on admission and subsequently when clinically indicated: for example, to confirm suspected secondary infections, etc. Chest X-ray is also routine after certain procedures (placing of central lines, intubation, thoracentesis, etc.). Subjective scoring by quadrants will be performed to determine whether this may be a useful secondary outcome measure for Phase 3 trials.
Biomarker analysis
Effects of citicoline on a variety of potential plasma biomarkers of ARDS and/or COVID-19 severity will be determined. Plasma IL-6, IL-8, IL-10, IL-18, and other cytokines and chemokines in plasma will be measured by Bio-Plex (48-Plex Human Cytokine Screening Panel). IFN-α, IFN-λ, Angiopoietin 2, C-reactive protein, lactate dehydrogenase, RAGE, and SP-D will be assayed using commercial ELISA kits. Plasma viremia, which has also been linked to prognosis [63, 64], will be quantified by qRT-PCR for SARS CoV-2 N gene using the CDC protocol. Batched assays will be performed in accordance with Good Laboratory Practice principles using validated commercial reagents. Residual samples will be retained in the − 80 °C freezer until conclusion of the study and analysis and then destroyed according to OSU protocol.
Outcomes
The primary goals of SCARLET are to establish the safety of citicoline in hospitalized SARS CoV-2-infected patients with HARF, to generate preliminary evidence of efficacy, and to identify an optimal citicoline dose for Phase 3 efficacy trials. There are therefore both safety- and efficacy-related primary outcome measures.
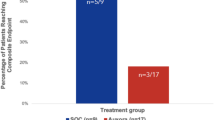
A particular dose of citicoline will be considered safe if the number of SAEs attributable to that citicoline dose (in the view of treating clinicians) remains below the threshold indicative of excessive toxicity once all 20 subjects have been treated (see Table 2).
The primary clinical outcome is a statistically significant difference in lowest recorded SpO2:FiO2 (S:F) ratio on study day 3, at P < 0.05. The lowest SpO2 for the day will be identified and used to generate the S:F ratio (based on the FiO2 at time of lowest SpO2 value).
Secondary/exploratory outcomes will be evaluated to identify statistically significant differences (P < 0.05) in (1) Lowest recorded S:F value on study days 1–2 and 4–8, or until extubation (whichever comes first); (2) Lowest Sequential Organ Failure Assessment (SOFA) score on study days 1, 3, 5, and 8; and (3) Highest COVID Ordinal Outcomes Scale score on study days 1, 3, 8, 15, and 29. On study day 29, we will also assess (1) Oxygen-free days through day 28; (2) Ventilator-free days through day 28; (3) ICU-free days through day 28; and (4) Hospital-free days through day 28.
Participant timeline
The timing of study procedures is based on the time at which consent was obtained, which is defined as “Time 0”. Chest X-ray will be performed and baseline Sequential Organ Failure Assessment (SOFA) and COVID Ordinal Outcomes Scale (HRS) scores will be calculated at Time 0. Study test agents will be administered by study personnel while the patient is hospitalized, with the first dose being administered within 4 h of Time 0. If the patient is discharged prior to completion of the study medication, no further study medication will be administered.
Individual patient schedules are shown below and in Fig. 2.
Individual patient schedule. Patients will receive test agent (citicoline or saline placebo) every 12 h for 5 days. Patients will be monitored out to day 29. ABGs—arterial blood gases; CBC/CHEM—complete blood count and chemistry panel; COOS—COVID Ordinal Outcomes Scale; CXR—chest X-ray; S:F—SaO2:FiO2 ratio; SOFA—Sequential Organ Failure Assessment
Day 0–1:
-
(1)
Informed consent will be obtained.
-
(2)
Order for study drug will be placed by the investigator.
-
(3)
Initial clinical data will be obtained by research staff using the EMR and recorded in the REDCap study database. Missing laboratory data will be ordered by the investigator.
-
(4)
Blood will be drawn from all patients prior to the first treatment dose for laboratory studies.
-
(5)
Study drug will be administered i.v. by the bedside nurse operating within their usual scope of practice. The timing of study drug administration may be adjusted up to 4 h on subsequent dosing to achieve standard dosing times.
-
(6)
Clinical information, including other drugs administered, will be recorded by the research team into the REDCap study database.
Days 1–5:
-
(1)
The patient will be assessed daily by study team for evidence of adverse drug reactions.
-
(2)
Dosing with the test agent will continue every 12 h until each subject has received 10 doses total.
-
(3)
Laboratory samples will be collected according to the study timeline.
-
(4)
Clinical information, including other drugs administered, will be recorded by the research team into the REDCap study database.
Days 6–8:
-
(1)
The patient will be assessed daily by study team for evidence of adverse drug reactions or clinical change.
-
(2)
Laboratory samples will be collected according to the study timeline.
Clinical information, including other drugs administered, will be recorded by the research team into the REDCap study database.
Days 9–29:
-
(1)
Patient will be assessed daily by study team for evidence of adverse drug reactions or clinical change.
-
(2)
Patients discharged home prior to day 29 will be called on day 29 to verify vital status and discharge location.
-
(3)
Patients who withdrew from the trial at any point prior to the cessation of test agent administration
Participant stopping rules and withdrawal criteria
Participants may be prematurely terminated from the study for the following reasons: (1) The participant elects to withdraw consent from all future study activities, including follow‐up; (2) The participant dies; (3) The Investigator no longer believes participation is in the best interest of the participant; or (4) The Investigator believes that an SAE in the patient may be attributable to the test agent.
Participants who withdraw or are withdrawn will not be replaced if they have received at least one dose of the investigational agent.
Patients wishing to withdrawal early from the study (i.e., before all 10 doses were administered) will be asked if they are agreeable to follow-up phone call on day #29. If approval is granted, they will be called on day 29 to verify vital status and discharge location. Patients opting complete withdrawal from the study will not be contacted further.
Protocol modifications
Any modifications to the approved clinical protocol will be developed in consultation with the DSMB and will be implemented only after approved by the OSU IRB and FDA. Once the protocol change is approved by the IRB, the DSMB and NIAID will be notified.
The PI will then schedule an educational session to review changes in the protocol with the participation of each team member recorded.
-
(1)
Sample size. Sample size calculations are based on differences in % carotid SpO2 between saline and CDP‐choline treatment groups in SARS CoV‐2-infected mice (unpublished data). Effect sizes post treatment were 1.32 in IAV and 1.4 in SARS CoV‐2 infected mice. With 24 subjects in the 2.5 and 5 mg/kg/dose treatment groups and 24 controls, and allowing 5% missing data at day 3 (80 participants total), there is 80% power (α = 0.05) to detect an effect size of 0.9 in S:F ratio between any individual arm and control (0.39 effect size overall), 32% below that in our preliminary data.
Safety events
Definitions
An adverse event (AE) will be defined as any untoward or unfavorable medical occurrence associated with the subject’s participation in SCARLET, whether or not considered related to the subject’s participation in the trial (modified from the definition of AEs in the 1996 International Conference on Harmonization E‐6 Guidelines for Good Clinical Practice). For this study, an AE will include any untoward or unfavorable medical occurrence associated with (1) The Study therapy regimen (any AE occurring after initial dosing of study drug until hospital discharge or study day 29 whichever is longer); or (2) Study-mandated procedures (any AE associated within 12 h of study-mandated phlebotomy).
Severity of AEs experienced by the study subjects will be graded according to the criteria set forth in the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. This manual provides a common language to describe levels of severity, to analyze and interpret data, and to articulate the clinical significance of all AEs. AEs will be graded Mild (Grade 1), Moderate (Grade 2), Severe but not immediately life threatening (Grade 3), or Severe with life‐threatening consequences requiring urgent intervention (Grade 4). An AE-related death is considered Grade 5. For grading an abnormal value or result of a clinical or laboratory evaluation, a treatment‐emergent AE is defined as an increase in grade from baseline or from the last post‐baseline value that does not meet grading criteria. Changes in grade from screening to baseline will also be recorded as AEs but are not treatment‐emergent. An abnormal result would also be considered an AE if changes in therapy or monitoring are implemented as a result of the event/result.
A suspected adverse reaction (SAR) will be defined as any AE for which there is a reasonable possibility that the investigational drug caused the AE. For the purposes of safety reporting, “reasonable possibility” means there is evidence to suggest a causal relationship between the drug and the AE. A suspected adverse reaction implies a lesser degree of certainty about causality than adverse reaction, which means any AE caused by a drug (21 CFR 312.32(a)).
An AE or serious adverse reaction (SAR) will be considered “unexpected” if it is not listed in the package insert. An AE or SAR is considered “serious” if, in the view of either the PI or DMSB, it results in any of the following outcomes (21 CFR 312.32(a)): (1) Death; (2) A life‐threatening event (an AE or SAR is considered “life‐threatening” if, in the view of the Investigator, the Medical Monitor, or the DMSB, its occurrence places the subject at immediate risk of death; it does not include an AE or SAR that, had it occurred in a more severe form, might have caused death); (3) Prolongation of existing hospitalization; (4) Persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions; (5) A congenital anomaly or birth defect; or (6) Any other important medical events that may not result in death, be life threatening, or require hospitalization but may be considered serious when, based upon appropriate medical judgment, they may jeopardize the subject and may require medical or surgical intervention to prevent one of the outcomes listed above.
Recording of AEs
AEs will be collected from the time of first dose of study drug, until a subject completes study participation (day 29) or until 30 days after they prematurely withdraw (without withdrawing consent) or is withdrawn from the study. AEs (including SAEs) may be discovered through any of these methods: (1) Observing the subject; (2) Interviewing the subject; or (3) Receiving an unsolicited complaint from the subject. In addition, an abnormal value or result from a clinical or laboratory evaluation can also indicate an AE.
Throughout the study, the PI and his team will record Grade 2 or higher AEs on the appropriate AE/SAE or electronic case report form (eCRF) regardless of the relationship to study therapy regimen or study procedure. Once recorded, an AE will be followed until it resolves with or without sequelae, or until the end of study participation, or until 30 days after the subject prematurely withdraws (without withdrawing consent)/or is withdrawn from the study, whichever occurs first.
The relationship, or attribution, of an AE to the study therapy regimen or study procedure(s) will initially be determined by the PI and recorded on the appropriate AE (AE/SAE or eCRF). Final determination of attribution for safety reporting will be determined by the Medical Monitor, DSMB, PI, and NIAID.
Reporting of AEs and SAEs
The PI shall report any suspected adverse reaction that is both serious and unexpected, as described below. The PI shall report an AE as a suspected adverse reaction only if there is evidence to suggest a causal relationship between the study drug and the AE, such as (1) a single occurrence of an event that is uncommon and known to be strongly associated with drug exposure (e.g., angioedema, hepatic injury, or Stevens‐Johnson Syndrome); (2) one or more occurrences of an event that are not commonly associated with drug exposure, but are otherwise uncommon in the population exposed to the drug (e.g., tendon rupture); (3) an aggregate analysis of specific events observed in a clinical trial (such as known consequences of the underlying disease or condition under investigation or other events that commonly occur in the study population independent of drug therapy) that indicates those events occur more frequently in the drug treatment group than in a concurrent or historical control group.
Worsening respiratory function, including but not limited to need for increased oxygen or mechanical ventilation, occurs commonly in this study population and will not be categorized as an SAE if deemed by treating team to be consistent with progression of known COVID-19, unless there is evidence to suggest a causal relationship to citicoline. These events will be captured in the study database but will not be reported as expedited Safety Reports:
Timely reporting of AEs is required by 21 CFR and ICH E6 guidelines. The PI will report all SAEs regardless of relationship or expectedness within 24 h of discovering the event. Other AEs, including expedited reports, will be reported in a timely fashion to the OSU IRB in accordance with applicable regulations and guidelines. The DSMB Annual Study Report to health authorities will include all AEs classified as serious, expected, or suspected. All Safety Reports to the FDA shall be distributed by the DSMB or designee for submission to the OSU IRB.
For SAEs, all requested information on the AE/SAE eCRF will be provided. However, unavailable details of the event will not delay submission of the known information. As additional details become available, the AE/SAE eCRF will be updated and submitted.
The sponsor shall notify the FDA and all participating investigators of Expedited Safety Reports within 15 calendar days; unexpected fatal or immediately life‐threatening suspected adverse reaction(s) shall be reported as soon as possible or within 7 calendar days to appropriate health authorities. The sponsor shall also report any findings from other epidemiological studies, analyses of AEs within the current study or pooled analysis across clinical studies or animal or in vitro testing (e.g., mutagenicity, teratogenicity, carcinogenicity) that suggest a significant risk in humans exposed to the drug that would result in a safety‐related change in the protocol, informed consent, or other aspects of the overall conduct of the study.
Interim safety analysis
After enrollment of 8 subjects per group, an interim safety analysis will be performed based on a continuous safety monitoring rule to guide accrual suspension decisions based on unacceptable toxicity and SAEs. The number of patients with an SAE that would warrant temporary suspension of accrual in a given group corresponds to a high posterior probability that the true SAE probability is greater than an acceptable level (i.e., Pr(\({p}_{i}\) > 0.25 | data) > 0.85), where the posterior probability is determined from a Beta-Binomial distribution with Beta (1, 1) as the prior on \({p}_{i}\). This is illustrated in Table 2.
For example, if 9 patients have been treated and 4 or more patients have an SAE, then accrual will be suspended while data are reviewed more closely and shared with the DSMB so that consensus regarding temporary suspension of accrual, protocol modifications, or decision to close the study early is made. Administration of the blinded study drug may be stopped temporarily or permanently for SAEs attributable to citicoline (in the view of the attending clinician) and in the event of clinical deterioration. In the latter case, the primary treating team is empowered to decide whether to stop the study drug and unblind group assignment. SAEs and unblinding will be recorded and reported to the DSMB at DSMB reviews and interim analyses.
Care provisions for patients experiencing harm
In the unlikely event a participating subject experiences an injury from participating in this study, the cost for any required treatment will be billed to that patient or their medical or hospital insurance. The Ohio State University has no funds set aside for the payment of health care expenses for this study.
Randomization and blinding
Eligible participants who have provided informed consent will be randomized 1:1:1:1 to 0.5, 2.5, or 5 mg/kg/dose citicoline or placebo. Randomization will be completed in permuted blocks of variable size. Each subject will receive a computer-generated randomization ID number, which will be provided to the research pharmacy, where the appropriate test agent will be prepared. Test agent will be labeled with the ID number and date only.
Patients, treating clinicians, trial personnel, and outcome assessors will be blinded to group assignment until after the database is locked and blinded analysis is completed.
Data management
Data collection plan
Data will be collected and managed using REDCap (Research Electronic Data Capture), which is a secure, web‐based electronic system for data entry, integration, processing, and reporting(144, 145). In addition to generating and tracking on‐line case report forms, REDCap allows data validation (through checks on variable formatting and range and logic checks), query generation and tracking, and reporting. REDCap is easy to navigate and has been historically vetted for security and HIPAA compliance. REDCap also has functionality to store source documents, enabling staff and PI access to key study documents and improving operational workflows. Data access will be defined and tightly controlled according to each person’s role (blinded, unblinded). Advanced data filtering functions will ensure that staff have access to appropriate data while reducing the likelihood of unblinding. Data and data labels can be downloaded selectively (for interim progress reports) or in their entirety (at end of study) directly from REDCap in SAS or Excel format.
Data will be collected prospectively by study staff using the EMR. Following verification, data will be locked. Data access will be defined and tightly controlled according to each person’s role (blinded, unblinded).
Quality assurance and quality control
The clinical research manager will perform internal quality management of study conduct, data and biological specimen collection, documentation, and completion. Quality control procedures will be implemented beginning with the data entry system and data quality control checks that will be run on the database will be generated. Any missing data or data anomalies will be communicated to the PI for clarification/resolution.
Following written Standard Operating Procedures, study monitors will verify that the clinical trial is conducted, data are generated, and biological specimens are collected, documented (recorded), and reported in compliance with the protocol, International Conference on Harmonisation Good Clinical Practice, and applicable regulatory requirements (e.g., Good Laboratory Practices and Good Manufacturing Practices).
Access to source data
Documentation of source data is necessary for the reconstruction, evaluation and validation of clinical findings, observations, and other activities during a clinical trial. The original documentation where subject information, visits consultations, examinations, and other information are recorded are all considered source documents and source data. The PI and site staff will make all source documents, data, and reports available for inspection to the NIAID, as well as to relevant local and regulatory authorities for the purpose of monitoring and auditing. All representatives of these entities are bound to maintain the strict confidentiality of medical and research information that may be linked to identified individuals.
Statistical analysis
All analysis will be based on intention to treat guidelines and reporting will follow CONSORT guidelines [65]. Data will be analyzed using STAT software (SAS; Cary, NC). The primary clinical outcome (S:F ratio on day 3) will be analyzed using either analysis of variance or a non-parametric Kruskal–Wallis test if analysis of variance assumptions (normality, homoscedasticity) are violated. Follow-up contrasts comparing each dose to control will be performed at α = 0.05/3 if the global test of differences between groups is rejected. If subject dropout due to mortality or discharge is present prior to day 3, the survivor average causal effect will employ a potential outcomes approach to account for potential survival bias and estimate the average causal effect of the intervention on S:F ratio among participants who would survive regardless of their intervention status, e.g., the always-survivors [66, 67]. The recommended citicoline dose for Phase 3 trials will be the dose achieving the greatest statistically significant improvement in day 3 S:F ratios, with consideration of key secondary endpoints (SOFA scores, etc.). Standardized mean differences will assess balance on baseline covariates across treatment arms. Competing risks analysis will assess differences between groups for competing time-to-event outcomes (e.g., hospital mortality, time to recovery, time off ventilator, discharge) [68].
Differences in SOFA scores between groups will be evaluated using non-parametric tests. A multinomial ordinal proportional odds model will estimate the odds ratio between study groups for COVID Ordinal Outcomes Scale. For outcomes with repeated assessments, generalized mixed models will evaluate differences between groups with a subject-level random effect to account for repeated measures. The mixed model accounts for data that are missing at random, when including baseline covariates associated with missing status. To account for potential non-ignorable dropout (e.g., due to mortality), a joint modeling approach will incorporate a shared random effect to simultaneously model the time-to-event process and longitudinal process [69].
Oversight and monitoring
Composition of the data monitoring committee, its role and reporting structure
The Ohio State University Center for Clinical and Translational Science (CCTS) established an external DSMB according to NIAID policies. The DSMB is responsible for safeguarding the interests of study participants, assessing the safety and efficacy of study procedures, ensuring data quality, and for monitoring the overall conduct of the study and is independent from the sponsor and any competing interests. The DSMB is asked to make recommendations, as appropriate, to the NIAID about: (1) Efficacy of the study intervention; (2) Benefit/risk ratio of procedures and participant burden; (3) Selection, recruitment, and retention of participants; (4) Adherence to protocol requirements; (5) Completeness, quality, and analysis of measurements; (6) The data and statistical analysis plan; (7) Amendments to the study protocol and consent forms, including whether any new data from other sources affect the equipoise of the study being monitored; (8) Performance of core labs; (9) Participant safety, including review of consent forms; (10) Notification of and referral for abnormal findings; and (11) Participant safety and parent study burden of proposed ancillary studies, including whether the total burden of ancillary studies might compromise the parent study.
The members of the DSMB include independent content experts in pulmonary and critical care medicine, biostatistics, clinical trials, and ethics (some overlapping). The DSMB convened to review the final protocol and DSMB Charter before study initiation and will continue to meet periodically, and not less frequently than annually. The DSMB chairperson will lead each scheduled board meeting and review the prepared board meeting minutes. At each meeting, they will determine whether study progress, data integrity, and safety monitoring warrant continuation of the study and will recommend the study to continue or be terminated, accordingly. The DSMB also approve the data safety plan before the study begins.
It is expected that all DSMB members will attend every meeting and conference call; however, it is recognized that this may not always be possible. A quorum for voting is half of the standing members plus one. A quorum of this DSMB is considered 3 of the 4 standing members, of which the chairperson must be included.
Potential motions of the board meeting include recommendations for either of the following: (1) Study continuation; no change required; (2) Study continuation with stipulation to be formally addressed and approved by the DSMB chair; (3) Study suspension with stipulation to be formally addressed and approved by the DSMB prior to resumption; and (4) Study termination.
The DSMB’s summary report will be reviewed and finalized by the chairperson. The report will then be communicated to the NIAID who will communicate recommendations to the Principal Investigators. DSMB recommendations will be communicated to the IRB in the context of the continuing review process. It is the responsibility of the Principal Investigator to ensure that the IRB is notified by motions requiring termination or suspension of the trial.
An independent Medical Monitor (P.D.) has also been appointed. The Medical Monitor is also independent from the sponsor and any competing interests. Their role is to ensure the safety of trial participants throughout the study and to serve as a point of reference for study team members as they evaluate safety events.
Adverse event reporting and harms
The DSMB and Medical Monitor shall receive monthly reports from the PI compiling new and accumulating information on AEs and SAEs recorded on appropriate eCRFs or paper CRFs. In addition, the Medical Monitor shall review and make decisions on the disposition of any SAE received by the PI.
Frequency and plans for auditing trial conduct
The DSMB will review all safety data after the first 5 patients and at least yearly thereafter during planned DSMB Data Review Meetings. Data for the planned safety reviews will include, at a minimum, a listing of all reported AEs and SAEs, but access to primary data sources (e.g., the RedCAP database) will also be possible. Such data may be provided blinded by treatment group, with the understanding that unblinding may need to occur in a closed session of the DSMB. In addition, the DSMB will be informed of an Expedited Safety Report in a timely manner (within 14 days).
In addition to the pre‐scheduled data reviews and planned safety monitoring, the DSMB may be called upon for ad hoc reviews. The DSMB will review any event that potentially impacts safety at the request of the PI. In addition, the following events will trigger an ad hoc comprehensive DSMB Safety Review: (1) Any death that occurs in the study, which is possibly or definitely related to the study treatment regimen (to be reported within 24 h to DSMB); (2) SAEs that are possibly, probably, or definitely related to the study treatment regimen (reported within 24 h to DSMB); Any common trends in unexpected AEs (reported to DSMB within 5 business days). A temporary halt in study recruitment/randomization will be implemented if an ad hoc DSMB safety review is required. Subjects currently in study and tolerating therapy will be allowed to complete their 5-day course. After review of the data, the DSMB will make recommendations regarding study conduct and/or continuation.
Procedure for unblinding
Unblinding must be approved by the study Medical Monitor (P.D.) unless an immediate life-threatening condition has developed, and the Medical Monitor is not accessible. The care team clinician will notify the PI (E.D.C.) and the study statistician of the unblinding event on the next business day. The emergency unblinding will also be reported to the DSMB. A full account of the event will be recorded, including the date and time of the unblinding, the reason for the decision to unblind, and the name of the individual who made the decision and the names of the Medical Monitor and others who were notified. The reasons for unblinding of a participant’s treatment will be included in the final study report.
Any unblinding the study due to an approved interim analysis, final analysis, or study termination will require written approval from NIAID.
Dissemination plans
All data will be deposited to the Dataverse that is supported by Harvard University under waivers providing for public availability of data repository starting 12 months after the trial begins and will be deposited every 6 months thereafter. This repository provides metadata, persistent unique identifiers, and long-term access for at least 10 years. Data will be findable for the research community under a unique study identifier through the Dataverse repository. All publications will also be deposited in the Dataverse repository. The study will be assigned a digital object identifier (DOI). This data DOI will be referenced in the publication to allow the research community easy access to the exact data used in the publication.
All data will be stored in common and open formats, such as JPEG, CSV, TXT, or PDF. Information needed to make use of this data (e.g., the meaning of variable names, codes, information about missing data, other metadata) along with references to the sources of those standardized names and metadata items will be included wherever applicable. Project-level metadata will be provided at the time of data deposit using Dryad’s web-based deposit form, which conforms to the DataCite metadata schema, a general standard for describing scientific data. No specialized tools, software, and/or code will be needed to access or manipulate shared scientific data.
Data will be made available as soon as possible or at the time of associated publication or end of the performance period. The research community will have access to data at the end of the grant award or when a publication has been submitted. To request access to the data, researchers will use the standard Dataverse processes. The only controls will be those applied by the repository as per the guidelines for authorized access by the public to maintain data safety and integrity.
The researchers’ intention for scientific data management and sharing will be part of the informed consent process. To protect research participants’ privacy and confidentiality, data submitted to the repository will not include personally identifiable information such as names or addresses. Additional protections, such as the approach for managing Health Insurance Portability and Accountability Act identifiers, will be used for de-identification or to provide a limited data set to minimize the risk of participant reidentification. The study datasets will be collected with Health/Medical/Biomedical informed consent. Consequently, the dataset can only be used for studying health, medical, or biomedical conditions and not for the study of population origins or ancestry.
Intermediate and final data from the SCARLET trial will be disseminated via scientific presentations at appropriate local, regional, national, and international Conferences.
The Ohio State University’s policy on the publication of study results will apply to this trial. Intermediate and final study data will also be submitted for publication in appropriate peer-reviewed journals. Biomarker data may also be submitted to appropriate peer-reviewed journals. Eligibility for authorship for all publications will be based on recommended criteria from the International Committee of Medical Journal Editors [70].
If findings from the SCARLET trial are highly significant, The Ohio State University Media Relations Team (a unit of University Communications) will assist with generation of press articles that will facilitate further dissemination of results to the general public. A website may be established to report progress.
Ethical considerations and compliance with good clinical practice
This clinical study will be conducted using good clinical practice, as delineated in Guidance for Industry: E6 Good Clinical Practice Consolidated Guidance, and according to the criteria specified in this study protocol.
As this study is being performed under a research IND, the PI (E.D.C.) is the sponsor. Both the sponsor and the funding agency (the National Institutes of Health) played a role in trial design. The sponsor will oversee and has ultimate authority over collection, management, analysis, and interpretation of data and writing the final study report. The sponsor will work with the co-PI (I.C.D.) to decide where and when to publish the final study report, which will be done regardless of the trial outcome. A steering committee composed of members of the trial team and representatives from NIAID has been established. This committee meets monthly to discuss progress of the trial and any potential concerns or anticipated changes in trial design and conduct.
Before study initiation, the protocol and the informed consent documents were reviewed and approved by the OSU Conflicts Advisory Committee, the OSU Institutional Biosafety Committee, and the OSU Institutional Review Board (IRB). Any amendments to the protocol or to the consent materials will be approved by the IRB before they are implemented.
Each participant’s privacy and confidentiality will be respected throughout the study. Each participant will be assigned a unique identification number and these numbers rather than names will be used to collect, store, and report participant information. Site personnel will not transmit documents containing personal health identifiers to the study sponsors or their representatives.
Discussion
Citicoline has many characteristics that would be advantageous for any candidate COVID-19 or ARDS therapeutic. Firstly, it has been widely evaluated in both normal and sick human subjects and has an exceptional safety profile. Indeed, food-grade citicoline is classified as GRAS (Generally Regarded as Safe) by the FDA and as safe by the European Food Safety Authority [71]. Citicoline is well tolerated in humans at doses > 1 g/kg/day (below the highest dose proposed for the SCARLET study) and adverse effects are very rare (< 1:10,000) [71,72,73]. Occasionally, initial digestive intolerance, self-limiting headache, tingling sensation, numbness, and excitability or restlessness have been reported following oral citicoline administration [74,75,76]. No clinically significant ECG and EEG abnormalities have ever been registered after citicoline administration. Moreover, there is extensive clinical data on citicoline safety. Twenty-four clinical trials of citicoline have been completed to date (see clinicaltrials.gov), primarily for neurologic indications. This includes three trials in which drug was administered i.v.. Frequency of AEs in the largest such trial (ICTUS), which involved 1149 patients on drug, was no higher than in placebo controls [77]. However, citicoline is contra-indicated in patients taking medications that contain L-Dopa, centrophenoxine, or meclofenoxate, and in patients with hypertonia of the parasympathetic nervous system, hence their exclusion from SCARLET.
A large amount of published PK/PD and ADME data is available for citicoline [71, 78]. The pharmacokinetics of oral and intravenous citicoline are almost indistinguishable [79]. In healthy adults, > 99% of a single oral dose of 300 mg of 14C-labelled citicoline was absorbed, which resulted in two peaks in plasma radioactivity at 1 h and 24 h post-dose, the second peak being larger [80]. After 5 days, 16% of the administered dose had been recovered, suggesting that the remainder had been incorporated into tissues or was available for biosynthetic and biodegradative pathways. The elimination half-life for CDP-choline that is not incorporated into phospholipids is rapid (56 h for CO2 and 71 h for urinary excretion). Hence, it is unlikely to alter the function of cells in which phospholipid synthesis is not impaired [80, 81].
Citicoline is also a simple small molecule with high aqueous solubility and methods for its synthesis are well defined and straightforward [82, 83]. Citicoline is stable in aqueous solution at room temperature for at least 3 years. Hence, it is amenable to pre-pandemic stockpiling and new stocks can be rapidly generated as existing stockpiles become depleted due to expiration or usage. Citicoline’s stability would also facilitate usage in developing countries that lack the resources to maintain cold chains. Moreover, because citicoline targets the host, it is likely to be effective against newly emerging SARS CoV-2 mutants and, unlike vaccines and antivirals, is highly unlikely to constitute a selection pressure for resistance. For the same reason, citicoline will provide a longer window for delayed therapy initiation than current antiviral drugs which are generally only effective early in infection [84, 85].
Importantly for a pulmonary drug, citicoline has excellent bioavailability when administered parenterally [86] so does not need to be instilled directly into the lungs. Hence, issues commonly associated with intra-pulmonary drug administration in ARDS, such as heterogeneity of distribution in the injured lung [87] and induction of transient hypoxemia [88] can be avoided. Moreover, i.v. administration will result in distribution to other affected organs in COVID-19 patients, potentially with beneficial effects.
Finally, citicoline is profoundly anti-inflammatory [58, 89,90,91,92]. The apparent beneficial effects of dexamethasone and tocilizumab in COVID-19 [93, 94] suggest that other anti-inflammatory drugs (such as citicoline) may likewise be useful. Given its minimal side effects, citicoline may be superior to corticosteroids, which are harmful in COVID-19 patients not requiring O2 [95], increase risk for invasive fungal infections [96,97,98], and are contra-indicated for other severe respiratory viral infections such as influenza, SARS, and MERS [99].
There are some limitations to the SCARLET study. COVID-19 has a broad spectrum of clinical severity, so designing a pilot trial with statistical power to detect a meaningful difference in ICU-free days or mortality as primary outcome would require an unfeasibly large sample size and could miss significant beneficial effects of citicoline in hospitalized patients. Likewise, the COVID Ordinal Outcomes Scale score [100] would be underpowered to assess efficacy in a small study. As most COVID-19 morbidity relates to HARF and the greatest experimental effect of citicoline is on SpO2 in mice infected with both IAV [58] and SARS CoV-2 (unpublished observations), we chose to use the lowest S:F ratio value on study day 3 as our primary efficacy readout. P:F and S:F ratios are closely correlated and essentially interchangeable [101,102,103]. P:F ratios can also be imputed from SpO2 values [102, 104, 105]. Changes in both are highly and comparably predictive of ICU mortality in ARDS [104]. Lower S:F ratios are also highly predictive of ICU mortality in COVID-19 patients [106, 107].
As a secondary efficacy readout, we will also evaluate the impact of citicoline treatment on SOFA scores in enrolled patients. The SOFA score comprises 0 to 4 points assigned to each of 6 organ systems, based on P:F or S:F ratio, Glasgow Coma Scale score, mean arterial pressure, serum creatinine, serum bilirubin, and platelet count [108]. Higher SOFA scores indicate worse organ function. Although originally designed for sepsis patients, and not universally accepted as being of prognostic value in COVID-19 [109], multiple studies have shown associations between elevated SOFA scores and poor outcomes in this population [110,111,112,113,114,115]. Moreover, the multisystem nature of the SOFA score may allow us to identify beneficial effects of citicoline on other organs.
Synthesis of CDP-choline is both essential and rate limiting for de novo phosphatidylcholine synthesis and this pathway is highly conserved across all mammals [116]. We propose that ATII cell dysfunction secondary to impaired de novo phospholipid synthesis is a central player in the pathogenesis of ARDS from multiple causes, including infection other RNA viruses with pandemic potential, such as IAV and other coronaviruses (SARS and MERS). We also propose that this host ATII cell dysfunction is readily reversed by treatment with citicoline. Given its broad efficacy, safety, favorable chemical characteristics, and potentially pathogen-agnostic efficacy, successful demonstration that citicoline is beneficial in severely ill patients with SARS CoV-2-induced HARF could transform management of severely ill COVID patients.
Trial status
Protocol version: 4.81, December 5th, 2023.
Date of first enrollment: 14th July, 2023.
Approximate date for completion of recruitment: March 2025.
Availability of data and materials
Our ethical and legal responsibility to respect participants’ rights to privacy and to protect their identity will inform our policy for sharing clinical data once the trial has been completed. We will gain informed consent for publication of the dataset from participants at the point of enrollment in the trial. Clinical datasets will not contain any direct or indirect identifiers and participants will remain fully anonymous.
Abbreviations
- AE:
-
Adverse event
- ARDS:
-
Acute respiratory distress syndrome
- ATII cell:
-
Alveolar type II respiratory epithelial cell
- CCT-α:
-
CTP-phosphocholine cytidylyltransferase-α
- CDP-choline:
-
Cytidine 5’diphosphocholine
- COVID-19:
-
Coronavirus disease 2019
- DSMB:
-
Data Safety Monitoring Board
- eCRF:
-
Electronic case report form
- EMR:
-
Electronic medical record
- FiO2 :
-
Fraction of inspired oxygen, %
- HARF:
-
Hypoxemic acute respiratory failure
- ICU:
-
Intensive care unit
- IDS:
-
Investigational Drug Service
- IRB:
-
Institutional Review Board
- LAR:
-
Legally authorized representative
- OSU:
-
The Ohio State University
- PaO2 :
-
Arterial partial pressure of oxygen (index of hypoxemia), mmHg
- P:F:
-
PaO2:FiO2 ratio, mmHg
- REDCap:
-
Research Electronic Data Capture
- SAE:
-
Serious adverse event
- SAR:
-
Serious adverse reaction
- SARS CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- SCARLET:
-
Supplemental Citicoline Administration for Resolution of Lung injury Efficacy Trial
- S:F:
-
SpO2:FiO2 ratio
- SOFA:
-
Sequential Organ Failure Assessment
- SpO2 :
-
Peripheral oxygen saturation (%)
- WMC:
-
Wexner Medical Center
References
Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - Eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):495–500.
Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24(1):198.
Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–300.
Tsolaki V, Zakynthinos GE. Are COVID-19 patients dying of or with cardiac injury? Am J Respir Crit Care Med. 2020;202:300–1.
Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–91.
Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058.
Di Fusco M, Shea KM, Lin J, Nguyen JL, Angulo FJ, Benigno M, et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–17.
Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021.
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15.
Iqbal A, Iqbal K, Arshad Ali S, Azim D, Farid E, Baig MD, et al. The COVID-19 sequelae: A cross-sectional evaluation of post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus. 2021;13(2):e13080.
Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–40.
Hough CL, Herridge MS. Long-term outcome after acute lung injury. Curr Opin Crit Care. 2012;18(1):8–15.
Dhanireddy S, Altemeier WA, Matute-Bello G, O’Mahony DS, Glenny RW, Martin TR, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest. 2006;86(8):790–9.
Bates JHT, Smith BJ. Ventilator-induced lung injury and lung mechanics. Ann Transl Med. 2018;6(19):378.
Beers MF, Moodley Y. When is an alveolar type 2 cell an alveolar type 2 cell? A conundrum for lung stem cell biology and regenerative medicine. Am J Respir Cell Mol Biol. 2017;57(1):18–27.
Davis IC, Matalon S. Epithelial sodium channels in the adult lung–important modulators of pulmonary health and disease. Adv Exp Med Biol. 2007;618:127–40.
Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Ann Rev Med. 2010;61(1):105–19.
Han S, Mallampalli RK. The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc. 2015;12(5):765–74.
Lamonica G, Amigoni M, Vedovelli L, Zambelli V, Scanziani M, Bellani G, et al. Pulmonary surfactant synthesis after unilateral lung injury in mice. J Appl Physiol. 2014;116(2):210–5.
Dushianthan A, Cusack R, Grocott MPW, Postle AD. Abnormal liver phosphatidylcholine synthesis revealed in patients with acute respiratory distress syndrome. J Lipid Res. 2018;59(6):1034–45.
Dushianthan A, Goss V, Cusack R, Grocott M, Postle A. Altered molecular specificity of surfactant phosphatidycholine synthesis in patients with acute respiratory distress syndrome. Respir Res. 2014;15(1):128.
Schmidt R, Markart P, Ruppert C, Wygrecka M, Kuchenbuch T, Walmrath D, et al. Time-dependent changes in pulmonary surfactant function and composition in acute respiratory distress syndrome due to pneumonia or aspiration. Respir Res. 2007;8(1):55.
Schmidt R, Meier U, Yabut-Perez M, Walmrath D, Grimminger F, Seeger W, et al. Alteration of fatty acid profiles in different pulmonary surfactant phospholipids in acute respiratory distress syndrome and severe pneumonia. Am J Respir Crit Care Med. 2001;163(1):95–100.
Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016;37(4):633–46.
Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1376–83.
Cardinal-Fernández P, Lorente JA, Ballén-Barragán A, Matute-Bello G. Acute respiratory distress syndrome and diffuse aveolar damage. New insights on a complex relationship. Ann Am Thorac Soc. 2017;14(6):844–50.
Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry pepends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-80.e8.
Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. New Engl J Med. 2020.
Adachi T, Chong JM, Nakajima N, Sano M, Yamazaki J, Miyamoto I, et al. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19. Japan Emerg Infect Dis. 2020;26(9):2157–61.
Damiani S, Fiorentino M, De Palma A, Foschini MP, Lazzarotto T, Gabrielli L, et al. Pathological post mortem findings in lungs infected with SARS-CoV 2. J Pathol. 2020;253(1):31–40.
Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156–68.
Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429-46.e14.
Hekman RM, Hume AJ, Goel RK, Abo KM, Huang J, Blum BC, et al. Actionable cytopathogenic host responses of human alveolar type 2 cells to SARS-CoV-2. Mol Cell. 2020;80(6):1104-22.e9.
Pei R, Feng J, Zhang Y, Sun H, Li L, Yang X, et al. Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection. Protein Cell. 2020:1–17.
Youk J, Kim T, Evans KV, Jeong YI, Hur Y, Hong SP, et al. Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell. 2020;27(6):905-19.e10.
Aboudounya MM, Heads RJ. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediators Inflamm. 2021;2021:8874339.
Islam A, Khan MA. Lung transcriptome of a COVID-19 patient and systems biology predictions suggest impaired surfactant production which may be druggable by surfactant therapy. Sci Rep. 2020;10(1):19395.
Gentzsch M, Rossier BC. A pathophysiological model for COVID-19: Critical importance of transepithelial sodium transport upon airway infection. Function (Oxf). 2020;1(2):zqaa024.
Fessler MB, Summer RS. Surfactant lipids at the host-environment interface. Metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am J Respir Cell Mol Biol. 2016;54(5):624–35.
Fagone P, Jackowski S. Phosphatidylcholine and the CDP-choline cycle. Biochim Biophys Acta. 2013;1831(3):523–32.
Woods PS, Doolittle LM, Rosas LE, Joseph LM, Calomeni EP, Davis IC. Lethal H1N1 influenza A virus infection alters the murine alveolar type II cell surfactant lipidome. Am J Physiol Lung Cell Mol Physiol. 2016;311(6):L1160–9.
Barberis E, Timo S, Amede E, Vanella VV, Puricelli C, Cappellano G, et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int J Mol Sci. 2020;21(22):8623.
Overmyer KA, Shishkova E, Miller IJ, Balnis J, Bernstein MN, Peters-Clarke TM, et al. Large-Sscale multi-omic analysis of COVID-19 severity. Cell Syst. 2021;12(1):23-40.e7.
Schwarz B, Sharma L, Roberts L, Peng X, Bermejo S, Leighton I, et al. Cutting Edge: Severe SARS-CoV-2 infection in humans is defined by a shift in the serum lipidome, resulting in dysregulation of eicosanoid immune mediators. J Immunol. 2021;206(2):329–34.
Sindelar M, Stancliffe E, Schwaiger-Haber M, Anbukumar DS, Albrecht RA, Liu WC, et al. Longitudinal metabolomics of human plasma reveals robust prognostic markers of COVID-19 disease severity. medRxiv. 2021.
Song JW, Lam SM, Fan X, Cao WJ, Wang SY, Tian H, et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 2020;32(2):188-202.e5.
Wu J, Cyr A, Gruen D, Lovelace T, Benos P, Chen T, et al. Lipidomic signatures align with inflammatory patterns and outcomes in critical illness. Res Sq. 2021.
Post M, Barsoumian A, Smith BT. The cellular mechanism of glucocorticoid acceleration of fetal lung maturation. Fibroblast-pneumonocyte factor stimulates choline-phosphate cytidylyltransferase activity. J Biol Chem. 1986;261(5):2179–84.
Spragg RG, Li J. Effect of phosphocholine cytidylyltransferase overexpression on phosphatidylcholine synthesis in alveolar type II cells and related cell lines. Am J Respir Cell Mol Biol. 2000;22(1):116–24.
Caesar PA, McElroy MC, Kelly FJ, Normand IC, Postle AD. Mechanisms of phosphatidylcholine acyl remodeling by human fetal lung. Am J Respir Cell Mol Biol. 1991;5(4):363–70.
Post M. Maternal administration of dexamethasone stimulates choline-phosphate cytidylyltransferase in fetal type II cells. Biochem J. 1987;241(1):291–6.
Rooney SA, Smart DA, Weinhold PA, Feldman DA. Dexamethasone increases the activity but not the amount of choline-phosphate cytidylyltransferase in fetal rat lung. Biochim Biophys Acta. 1990;1044(3):385–9.
Rooney SA, Dynia DW, Smart DA, Chu AJ, Ingleson LD, Wilson CM, et al. Glucocorticoid stimulation of choline-phosphate cytidylyltransferase activity in fetal rat lung: receptor-response relationships. Biochim Biophys Acta. 1986;888(2):208–16.
Viscardi RM, Weinhold PA, Beals TM, Simon RH. Cholinephosphate cytidylyltransferase in fetal rat lung cells: activity and subcellular distribution in response to dexamethasone, triiodothyronine, and fibroblast-conditioned medium. Exp Lung Res. 1989;15(2):223–37.
Schousboe P, Wiese L, Heiring C, Verder H, Poorisrisak P, Verder P, et al. Assessment of pulmonary surfactant in COVID-19 patients. Crit Care. 2020;24(1):552.
Giménez R, Soler S, Aguilar J. Cytidine diphosphate choline administration activates brain cytidine triphosphate: phosphocholine cytidylytransferase in aged rats. Neurosci Lett. 1999;273(3):163–6.
Rosas LE, Doolittle LM, Joseph LM, El-Musa H, Novotny MV, Hickman-Davis JM, et al. Post-exposure liponucleotide prophylaxis and treatment attenuates ARDS in influenza-infected mice. Am J Respir Cell Mol Biol. 2021.
Girona-Alarcon M, Bobillo-Perez S, Sole-Ribalta A, Hernandez L, Guitart C, Suarez R, et al. The different manifestations of COVID-19 in adults and children: a cohort study in an intensive care unit. BMC Infect Dis. 2021;21(1):87.
García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, Balcells Ramírez J, Slöcker Barrio M, Leóz Gordillo I, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care. 2020;24(1):666.
Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249.
Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–87.
Colagrossi L, Antonello M, Renica S, Merli M, Matarazzo E, Travi G, et al. SARS-CoV-2 RNA in plasma samples of COVID-19 affected individuals: a cross-sectional proof-of-concept study. BMC Infect Dis. 2021;21(1):184.
Järhult JD, Hultström M, Bergqvist A, Frithiof R, Lipcsey M. The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Sci Rep. 2021;11(1):7163.
Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18.
Frangakis CE, Rubin DB. Principal stratification in causal inference. Biometrics. 2002;58(1):21–9.
Frangakis CE, Rubin DB, An MW, MacKenzie E. Principal stratification designs to estimate input data missing due to death. Biometrics. 2007;63(3):641–9.
Brock GN, Barnes C, Ramirez JA, Myers J. How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med Res Methodol. 2011;11:144.
Rizopoulos D. JM: An R package for the joint modelling of longitudinal and time-to-event data. J Stat Software. 2010;35.
Defining the role of authors and contributors 2024. Available from: https://icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html.
EFSA Panel on Dietetic Products NaAN. Scientific opinion on the safety of "citicoline" as a novel food ingredient. EFSA J. 2013;11(10).
Romero A, Grau T, Sacristan A, Ortiz JA. CDP-choline: 6-month study on toxicity in dogs. Arzneimittelforschung. 1983;33(7a):1038–42.
Secades JJ. Citicoline: pharmacological and clinical review, 2016 update. Rev Neurol. 2016;63(S03):S1–73.
Lozano Fernández R. Efficacy and safety of oral CDP-choline Drug surveillance study in 2817 cases. Arzneimittelforschung. 1983;33(7a):1073–80.
Cho HJ, Kim YJ. Efficacy and safety of oral citicoline in acute ischemic stroke: drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol. 2009;31(3):171–6.
Dinsdale JR, Griffiths GK, Castelló J, Maddock J, Ortiz JA, Aylward M. CDP-choline: repeated oral dose tolerance studies in adult healthy volunteers. Arzneimittelforschung. 1983;33(7a):1061–5.
Dávalos A, Alvarez-Sabín J, Castillo J, Díez-Tejedor E, Ferro J, Martínez-Vila E, et al. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet. 2012;380(9839):349–57.
Grieb P. Neuroprotective properties of citicoline: Facts, doubts and unresolved issues. CNS Drugs. 2014;28(3):185–93.
Agut J, Font E, Sacristán A, Ortiz JA. Bioavailability of methyl-14C CDP-choline by oral route. Arzneimittelforschung. 1983;33(7a):1045–7.
Dinsdale JR, Griffiths GK, Rowlands C, Castelló J, Ortiz JA, Maddock J, et al. Pharmacokinetics of 14C CDP-choline. Arzneimittelforschung. 1983;33(7a):1066–70.
Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC. The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives. Clin Interv Aging. 2015;10:1421–9.
Ghezal S, Thomasson MS, Lefebvre-Tournier I, Périgaud C, Macnaughtan MA, Roy B. CDP-ethanolamine and CDP-choline: one-pot synthesis and 31P NMR study. Tetrahedron Lett. 2014;55(38):5306–10.
Ren Y, Liu Q, Liu H, Zhou X, Zhang Y, Cai M. Engineering substrate and energy metabolism for living cell production of cytidine-5’-diphosphocholine. Biotechnol Bioeng. 2020;117(5):1426–35.
Dimitrov NB, Goll S, Hupert N, Pourbohloul B, Meyers LA. Optimizing tactics for use of the U.S. antiviral strategic national stockpile for pandemic influenza. PLoS ONE. 2011;6(1):e16094.
Team CNIR. Does oseltamivir really reduce complications of influenza? Clin Infect Dis. 2011;53(12):1302–3.
D’Orlando KJ, Sandage BW Jr. Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury. Neurol Res. 1995;17(4):281–4.
Ueda T, Ikegami M, Rider ED, Jobe AH. Distribution of surfactant and ventilation in surfactant-treated preterm lambs. J Appl Physiol. 1994;76(1):45–55.
Dushianthan A, Cusack R, Goss V, Postle A, Grocott M. Clinical review: Exogenous surfactant therapy for acute lung injury/acute respiratory distress syndrome - where do we go from here? Crit Care. 2012;16(6):238.
Yilmaz Z, Ilcol YO, Torun S, Ulus IH. Intravenous administration of choline or cdp-choline improves platelet count and platelet closure times in endotoxin-treated dogs. Shock. 2006;25(1):73–9.
Feng X, Chen Y, Zhang M, Fang M, Xiao C, Chen J. Protective effect of citicoline on random flap survival in a rat mode. Int Immunopharmacol. 2020;83:106448.
Cetinkaya M, Cansev M, Cekmez F, Tayman C, Canpolat FE, Kafa IM, et al. CDP-choline reduces severity of intestinal injury in a neonatal rat model of necrotizing enterocolitis. J Surg Res. 2013;183(1):119–28.
Cetinkaya M, Cansev M, Kafa IM, Tayman C, Cekmez F, Canpolat FE, et al. Cytidine 5’-diphosphocholine ameliorates hyperoxic lung injury in a neonatal rat model. Pediatr Res. 2013;74(1):26–33.
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693–704.
Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021.
Pasin L, Navalesi P, Zangrillo A, Kuzovlev A, Likhvantsev V, Hajjar LA, et al. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: A meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2021;35(2):578–84.
White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020.
Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: A case report. Eur J Ophthalmol. 2021:11206721211009450.
Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021:1–6.
Mattos-Silva P, Felix NS, Silva PL, Robba C, Battaglini D, Pelosi P, et al. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol. 2020;280:103492.
Dodd LE, Follmann D, Wang J, Koenig F, Korn LL, Schoergenhofer C, et al. Endpoints for randomized controlled clinical trials for COVID-19 treatments. Clin Trials. 2020;17(5):472–82.
Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–7.
Pandharipande PP, Shintani AK, Hagerman HE, St Jacques PJ, Rice TW, Sanders NW, et al. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med. 2009;37(4):1317–21.
Fukuda Y, Tanaka A, Homma T, Kaneko K, Uno T, Fujiwara A, et al. Utility of SpO2/FiO2 ratio for acute hypoxemic respiratory failure with bilateral opacities in the ICU. PLoS ONE. 2021;16(1):e0245927.
Chen W, Janz DR, Shaver CM, Bernard GR, Bastarache JA, Ware LB. Clinical characteristics and outcomes are similar in ARDS diagnosed by oxygen saturation/FiO2 ratio compared with PaO2/FiO2 ratio. Chest. 2015;148(6):1477–83.
Brown SM, Duggal A, Hou PC, Tidswell M, Khan A, Exline M, et al. Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among mechanically ventilated patients in the ICU: A prospective, observational study. Crit Care Med. 2017;45(8):1317–24.
Choi KJ, Hong HL, Kim EJ. The association between mortality and the oxygen saturation and fraction of inhaled oxygen in patients requiring oxygen therapy due to COVID-19-associated pneumonia. Tuberc Respir Dis (Seoul). 2021;84(2):125–33.
Lu X, Jiang L, Chen T, Wang Y, Zhang B, Hong Y, et al. Continuously available ratio of SpO2/FiO2 serves as a noninvasive prognostic marker for intensive care patients with COVID-19. Respir Res. 2020;21(1):194.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Raschke RA, Agarwal S, Rangan P, Heise CW, Curry SC. Discriminant accuracy of the SOFA score for determining the probable mortality of patients with COVID-19 pneumonia requiring mechanical ventilation. JAMA. 2021;325(14):1469–70.
Bels JLM, van Kuijk SMJ, Ghossein-Doha C, Tijssen FH, van Gassel RJJ, Tas J, et al. Decreased serial scores of severe organ failure assessments are associated with survival in mechanically ventilated patients; the prospective Maastricht Intensive Care COVID cohort. J Crit Care. 2021;62:38–45.
Gamberini L, Tonetti T, Spadaro S, Zani G, Mazzoli CA, Capozzi C, et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicenter observational study in fifteen Italian ICUs. J Intensive Care. 2020;8(1):80.
Herrmann J, Adam EH, Notz Q, Helmer P, Sonntagbauer M, Ungemach-Papenberg P, et al. COVID-19 induced acute respiratory distress syndrome-A multicenter observational study. Front Med (Lausanne). 2020;7:599533.
Tu Y, Yang P, Zhou Y, Wen X, Li Q, Zhou J, et al. Risk factors for mortality of critically ill patients with COVID-19 receiving invasive ventilation. Int J Med Sci. 2021;18(5):1198–206.
Liu S, Yao N, Qiu Y, He C. Predictive performance of SOFA and qSOFA for in-hospital mortality in severe novel coronavirus disease. Am J Emerg Med. 2020;38(10):2074–80.
Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1–12.
Agassandian M, Mallampalli RK. Surfactant phospholipid metabolism. Biochim Biophys Acta. 2013;1831(3):612–25.
Acknowledgements
The authors would like to thank Dr. Jean Schelhorn for her assistance with technology development, the members of the DSMB for volunteering their valuable time, and all staff involved in the conduct of this clinical trial. The authors would also like to thank all those patients and their families who have already participated in or will participate in this trial in the future.
Funding
This work is supported by The National Institute of Allergy and Infectious Diseases at the National Institutes of Health (U01-AI167784). Reviewers at the National Institutes of Health played a role in trial design by suggesting refinements to the clinical trial protocol that were subsequently adopted.
Author information
Authors and Affiliations
Contributions
M.C.E., G.B., A.B., I.C.D., and E.D.C. developed the clinical trial protocol. M.C.E., S.P., J.S.B., J.A.E., A.B., Q.W., and E.D.C. are responsible for conduct and oversight of the trial. P.D. is the Medical Monitor for the trial. All authors were involved in drafting, editing, and final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The SCARLET protocol was reviewed and approved by The OSU Institutional Review Board (protocol number 2022H0451). Written, informed consent to participate will be obtained from all participants or their legally authorized representative if incapacitated and deemed unable to provide consent.
Consent for publication
Not applicable.
Competing interests
I.C.D. is the sole inventor on a patent (US 10,874,684) covering use of citicoline as an ARDS therapeutic, which is assigned to the OSU Innovation Foundation. As such, there is the potential that both could profit from the trial should it result in out-licensing of the technology. However, Dr. Davis is not involved in patient enrollment or data analysis and the OSU Innovation Foundation has played no role in developing or conducting the trial.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13063_2024_8155_MOESM1_ESM.docx
Supplementary file 1. The Ohio State University Combined Consent to Participate in Research and HIPAA Research Authorization.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pannu, S., Exline, M.C., Bednash, J.S. et al. SCARLET (Supplemental Citicoline Administration to Reduce Lung injury Efficacy Trial): study protocol for a single-site, double-blinded, placebo-controlled, and randomized Phase 1/2 trial of i.v. citicoline (CDP-choline) in hospitalized SARS CoV-2-infected patients with hypoxemic acute respiratory failure. Trials 25, 328 (2024). https://doi.org/10.1186/s13063-024-08155-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-024-08155-0