Abstract
Background
Patients with a history of cardiac disease are prone to develop cardiovascular adverse events such as hypotension, hypertension, and tachycardia during anesthesia induction. Therefore, hemodynamic stability is one of the most important concerns for induction of anesthesia in patients undergoing cardiac surgery. Remimazolam tosilate is a new, ultra-short-acting benzodiazepine agent, with the advantages of rapid onset, rapid offset, and minimal cardiorespiratory depression. We aim to compare the effect of remimazolam tosilate and etomidate on hemodynamics during anesthesia induction in patients undergoing valve replacement surgery.
Methods/design
The trial is a prospective, randomized, double-blinded, controlled, single-center trial to compare the effect of remimazolam tosilate and etomidate on hemodynamics in patients undergoing valve replacement surgery. One hundred seventeen patients undergoing selective valve replacement surgery between January 1, 2022, and December 31, 2023, will be enrolled and randomly allocated into one of three groups: low-dose remimazolam group (Group LR), high-dose remimazolam group (Group HR), or etomidate group (Group E). The primary outcome is hemodynamic fluctuations during anesthesia induction (the difference between mean arterial pressure [MAP] to baseline, ▴MAP; and the difference between maximum or minimum heart rate [HR] and baseline, ▴HR). Secondary outcomes include the incidence of adverse cardiovascular events (hypotension, severe bradycardia, hypertension, tachycardia, and arrhythmia), the cumulative doses of vasoactive drugs used per patient, incidence and degree of injection pain and myoclonus, blood glucose values, and vital signs at different time points.
Discussion
This research will determine the effectiveness and safety of remimazolam tosilate induction on hemodynamics in patients undergoing valve replacement surgery.
Trial registration
www.chictr.org.cn identifier ChiCTR2100052535. Registered on 17th Dec 2021, http://www.chictr.org.cn/).
Similar content being viewed by others
Background
Patients undergoing cardiac surgery are at risk of developing hemodynamic instability, including hypotension and arrhythmia, especially during the induction of anesthesia. Etomidate is a non-barbiturate hypnotic agent that exerts a sedative and anesthetic effect through γ-aminobutyric acid subtype A (GABAA) receptors in the central nervous system [1]. Previous studies have shown that etomidate has minimal side effects on the cardiovascular and respiratory systems [2, 3]. Due to the low risk of hypotension, etomidate is considered to be a relatively safer agent for the induction of anesthesia in patients undergoing cardiac surgery [4, 5]. However, etomidate may result in adrenal cortex function inhibition [6, 7], nausea and vomitin g[8], injection pain [9, 10], and myoclonic movement [11, 12], which have limited its clinical application to a certain extent. Remimazolam tosilate is a new-type benzodiazepine agent, acting on the GABAA receptor, which has been reported to have the advantages of rapid onset, short maintenance and recovery times, and less cardiorespiratory depression [13,14,15]. Therefore, we hypothesized that remimazolam tosilate may be a relatively safer agent for anesthesia induction in patients undergoing cardiac surgery. Currently, research on the comparison of remimazolam tosilate and etomidate on hemodynamics during anesthesia induction in patients undergoing cardiac surgery is lacking. The aim of this study is to compare remimazolam tosilate and etomidate on the hemodynamics in patients undergoing valve replacement surgery.
Methods
Trial design
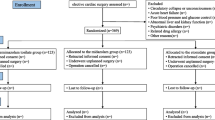
The trial is a prospective, randomized, controlled, double-blinded, single-center trial designed to evaluate the effect of remimazolam anesthesia induction on hemodynamics in patients undergoing valve replacement surgery. Patients will be enrolled and randomly allocated into one of the three groups: low-dose remimazolam group (Group LR), high-dose remimazolam group (Group HR), or etomidate group (Group E). The trial scheme is illustrated in Fig. 1.
Ethics
This study protocol has been approved by the Chinese Ethics Committee of Registering Clinical Trials (Ref: ChiECRCT20210524) and registered on www.chictr.org.cn (ChiCTR2100052535). This trial will be performed according to the principles of the Declaration of Helsinki (Edinburgh 2000 version). Written informed consent will be obtained from each patient before enrollment.
Sequence generation
All eligible participants will be randomized to one of the three groups (Group LR, Group HR, or Group E ) using computer-generated random numbers and a 1:1:1 allocation ratio. The randomization assignment will be generated by one independent investigator using a computer-generated random number sequence.
Allocation concealment mechanism
Numbered-Opaque-sealed envelopes will be used to conceal the allocation information. The nurse who is blinded to the trial will open the opaque-sealed envelopes after the participant’s arrival to the operation room. The opaque-sealed envelopes will be stored in a secure place throughout the study. The operator, anesthesiologists, and outcome investigators will be blind to the allocation information.
Blinding
The patients, attending anesthesiologist, and nurse, as well as the researchers, will not be aware of the randomization and allocation concealment throughout the study. All syringes containing the experimental medications will be covered with masking tape to conceal the anesthetic information. Data will be analyzed and collected by an investigator who is blinded to the patient’s group allocation.
Participants
Study population
Patients who are scheduled to undergo elective valve replacement surgery aged 18–65 years old will be recruited from the Affiliated Hospital of Guizhou Medical University. The trial will be ongoing from January 2022 to December 2023.
Inclusion criteria
-
1.
American Society of Anesthesiologists (ASA) Physical Status I–III.
-
2.
Patient aged between 18 and 65 years old.
Exclusion criteria
-
1.
Patients who are allergic to or possess contraindications to benzodiazepines, etomidate, opioids, or their components;
-
2.
Patients who participated in other clinical trials within 4 weeks of operation;
-
3.
Severe cardiac insufficiency (acute heart failure, myocardial infarction, etc) or hemodynamic instability;
-
4.
Patients with uncontrolled or poorly controlled hypertension;
-
5.
Abnormal liver and kidney function: ALT and/or exceed 1.5 times of the upper limit of medical reference value; creatinine exceeds the upper limit of medical reference value;
-
6.
Patients with myasthenia gravis or psychiatric disorders (schizophrenia, mania, bipolar disorder, cognitive dysfunction, etc.);
-
7.
Patients with a history of alcohol abuse or addicted to opioids or benzodiazepine; and
-
8.
Patients with expected difficult airway (e.g., thyromental distance ≤4 cm or Mallampati scores of 3 or 4).
Drop-out criteria
1. Participant’s request to withdraw the study;
2. Occurrence of serious complications or adverse events during the anesthesia;
3. Difficult intubation; and
4. Other reasons that enable the principal researcher to decide to terminate the study.
Implementation
After all the participants have finished the preoperative evaluation and screening, participants will be informed of their randomized allocation. Eligibility evaluation and enrolment will be conducted by one independent investigator, while randomization will be finished by another investigator.
Interventions
All patients will routinely fast for 8h before surgery. After the patient enters the operating room, standard anesthesia monitoring including electrocardiogram (ECG), noninvasive blood pressure (NIBP), peripheral oxygen saturation (SPO2), and bispectral index (BIS) will be routinely monitored. A 20-gauge intravenous cannula will be placed for Ringer lactate (10 ml/kg) infusion. Invasive arterial blood pressure (IBP) will be continuously monitored via insertion of a catheter into the left radial artery under local infiltration anesthesia with lidocaine. After preoxygenation, patients in Group E will be induced with etomidate (0.3 mg/kg), Group LR will be induced with remimazolam (0.2 mg/kg), and Group HR will be induced with remimazolam (0.3 mg/kg). Then, sufentanil (0.5 μg/kg) will be injected in all groups. After that, all patients will be injected with rocuronium (0.6 mg/kg) for muscle relaxation when loss of consciousness. Tracheal intubation will be performed two minutes after rocuronium administration.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) will be measured and recorded at baseline (T0), 1 min after the induction of anesthesia (T1), 3 min after the induction of anesthesia (T2), immediately at tracheal intubation (T3), 1 min after tracheal intubation (T4), and 5 min after tracheal intubation (T5). Laryngoscopy and anesthesia administration will be performed by an anesthesiologist who is blinded to the study groups. The duration of laryngoscopy and intubation are also recorded for each patient.
Hypotension is considered when MAP falls below 60 mmHg or decreases to less than 20% of baseline during induction, and 50 μg norepinephrine will be given intravenously as needed. When HR decreases below 45 beats per min (bpm), it is considered severe bradycardia and 0.25 mg atropine or more will be administrated by intravenous injection. If the MAP rises above 120 mmHg, nitroglycerin will be given, and once HR rises above 120 bpm, esmolol will be given. The incidence of adverse cardiovascular events (hypotension, severe bradycardia, hypertension, tachycardia, and arrhythmia) and the cumulative doses of vasoactive drugs will be recorded.
Outcomes
Primary outcome
The primary outcome of this study are the resultant changes in hemodynamic parameters: a) ▴MAP, defined as the difference of maximum or minimum MAP to baseline; b)▴HR, defined as the difference of maximum or minimum HR to baseline.
Secondary outcomes
The secondary outcomes include the following:
-
1.
The incidence of adverse cardiovascular events (hypotension, severe bradycardia, hypertension, tachycardia, and arrhythmia).
-
2.
The cumulative doses of vasoactive drugs used per patient.
-
3.
Hemodynamic parameters (HR, MAP, SBP, DBP), BIS index, lactic acid, and glucose values
-
4.
Incidence and degree of injection pain is measured using a 4-point graded scale: 0 = no pain, 1 = verbal complaint of pain, 2 = withdrawal of the arm, 3 = both verbal complaint and withdrawal of the arm.
-
5.
Incidence and degree of myoclonus is recorded as follows: 0 = no myoclonic movements, 1 = minor myoclonic movements, 2 = moderate myoclonic movements, 3 = major myoclonic movements [16].
Quality control
The principal investigator, study coordinator, and Office of Scientific Research at the Affiliated Hospital of Guizhou Medical University are jointly responsible for all aspects of the study protocol and amendments. Data collection and follow-up will be performed by two dedicated research residents. Designated trial monitors will periodically review all investigational data for accuracy and completeness to ensure protocol compliance.
Data collection
Each participant’s characteristics, including age, sex, and body weight, as well as ASA grade, type and duration of surgery, vital signs, cumulative doses of vasoactive drugs, and the incidence of adverse events during induction, will be recorded in a designed data form by an investigator who is blinded to this study.
Sample size
The sample size calculation was based on the primary outcome, the fluctuations in hemodynamic parameters (▴MAP). Based on our pilot data and relevant literature, assuming a type I error protection of 0.05 and a power of 0.90, 31 patients in each group are required in this study. Considering an estimated 20% dropout rate, 39 patients in each group will be included in this study.
Statistical analysis
Statistical analysis will be performed by SPSS 22.0 software (SPSS Inc, Chicago, IL, USA). Data distribution will be tested for normality using the Shapiro-Wilk test. Continuous data will be presented as mean [standard deviation (SD)] when normally distributed or median (interquartile range [IQR]) when not normally distributed; Numerical variables will be compared using one-way analysis of variance (ANOVA) or repeated-measures ANOVA or Kruskal-Wallis tests after checking the normal distribution. Post hoc tests will be performed using a Bonferroni method. Categorical variables will be compared using a Fisher’s exact test or chi-square test, as appropriate. A p value < 0.05 is considered statistically significant.
Auditing
In order to review and verify the development of the study throughout the trial period, the research team will hold a weekly meeting and submit a progress report every month.
Protocol amendments
If necessary, decisions on protocol amendment will be made by the research team and authorized by the ethics committee.
Dissemination
The database and related materials will be available accessible for the study staff and researchers of the project. Results will be published in open-access peer-reviewed journals within 12 months of completion of the trial.
Discussion
Patients with cardiac disease often have different degrees of cardiac functional impairment and almost all anesthetic drugs possess capacity to suppress the myocardium and induce vasodilatation, therefore, these patients are prone to developing hypotension during anesthesia induction [17]. Post-induction hypotension is a threatening complication and is associated with increased morbidity and mortality. Considering the importance and impact of hemodynamic changes on complications and outcomes, anesthesiologists should comprehensively consider the effectiveness and safety when selecting anesthetics agents. An ideal induction agent would be one that is easy to administer, has rapid onset and rapid offset, and few adverse effects. However, such an agent does not exist.
Etomidate is a well-known hypnotic agent routinely used for induction of anesthesia [18]. It was previously considered to be superior compared to other anesthetic agents (such as propofol, midazolam, thiopental) for patients with hemodynamic instability owing to its lower inhibition effect on the cardiovascular system [19,20,21]. However, etomidate may lead to adrenal cortical function inhibition, nausea and vomiting, injection pain, and myoclonic movement, which are some undesirable adverse complications [22, 23]. Therefore, there is a need for anesthetic agents that have a rapid onset of action, short recovery time, and stable cardiovascular profile.
Remimazolam tosilate is an ultra-short-acting GABA-A receptor agonist [24]; a growing number of studies have shown that remimazolam display stable peri-induction hemodynamics, rapid onset, and rapid elimination [25,26,27], indicating that remimazolam may be a safe agent for patients with unstable pre-operative hemodynamics. A recent study showed that remimazolam was superior to propofol during anesthesia induction in patients undergoing valve replacement surgery [28]. Furthermore, Saito et al. [29] also reported that remimazolam could be used safely in induction and maintenance of anesthesia for cardiac surgery with cardiopulmonary bypass (CPB). Considering the advantages of remimazolam as mentioned above, remimazolam has the potential to be a better choice during induction and maintenance of anesthesia for cardiac surgery. Currently, there are few studies on the effect of remimazolam on hemodynamics during anesthesia induction in cardiac surgery. To the best of our knowledge, this is the first study comparing remimazolam tosilate and etomidate on hemodynamics during anesthesia induction in patients with cardiac disease. Thus, the aim of the study is to compare the effect of remimazolam tosilate and etomidate for anesthesia induction on hemodynamics in patients undergoing valve replacement surgery. This study will provide new clinical practice data for evaluating the effectiveness and disadvantages of remimazolam in the field of cardiac anesthesia.
Public and patient involvement
There was no patient or public involvement in the design of the protocol. However, a patient and public involvement group has been assembled and will be consulted during dissemination of the findings.
Trial status
The trial is currently in the recruitment phase. Trial completion is expected by December 2023.
Availability of data and materials
The database and related materials will be available accessible for the study staff and researchers of the project.
Abbreviations
- MAP:
-
Mean arterial pressure
- HR:
-
Heart rate
- GABAA :
-
γ-Aminobutyric acid subtype A
- ASA:
-
American Society of Anesthesiologists
- NIBP:
-
Noninvasive blood pressure
- SPO2 :
-
Peripheral oxygen saturation
- BIS:
-
Bispectral index
- IBP:
-
Invasive arterial blood pressure
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- CPB:
-
Cardiopulmonary bypass
References
Song JC, Lu ZJ, Jiao YF, Yang B, Gao H, Zhang J, et al. Etomidate anesthesia during ERCP caused more stable haemodynamic responses compared with propofol: a randomized clinical trial. Int J Med Sci. 2015;12:559–65.
Yao YT, He LX, Fang NX, Ma J. Anesthetic induction with etomidate in cardiac surgical patients: A PRISMA-compliant systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2021;35:1073–85.
Aggarwal S, Goyal VK, Chaturvedi SK, Mathur V, Baj B, Kumar A. A comparative study between propofol and etomidate in patients under general anesthesia. Braz J Anesthesiol. 2016;66:237–41.
Hannam JA, Mitchell SJ, Cumin D, Frampton C, Merry AF, Moore MR, et al. Haemodynamic profiles of etomidate vs propofol for induction of anaesthesia: a randomised controlled trial in patients undergoing cardiac surgery. Br J Anaesth. 2019;122:198–205.
Dai ZL, Cai XT, Gao WL, Lin M, Lin J, Jiang YX, et al. Etomidate vs propofol in coronary heart disease patients undergoing major noncardiac surgery: a randomized clinical trial. World J Clin Cases. 2021;9:1293–303.
Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Med. 2011;37:901–10.
Kaushal RP, Vatal A, Pathak R. Effect of etomidate and propofol induction on hemodynamic and endocrine response in patients undergoing coronary artery bypass grafting/mitral valve and aortic valve replacement surgery on cardiopulmonary bypass. Ann Card Anaesth. 2015;18:172–8.
Ruth WJ, Burton JH, Bock AJ. Intravenous etomidate for procedural sedation in emergency department patients. Acad Emerg Med. 2001;8:13–8.
Chen L, Liang X, Tan X, Wen H, Jiang J, Li Y. Safety and efficacy of combined use of propofol and etomidate for sedation during gastroscopy: systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e15712.
Komatsu R, You J, Mascha EJ, Sessler DI, Kasuya Y, Turan A. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013;117:1329–37.
Falk J, Zed PJ. Etomidate for procedural sedation in the emergency department. Ann Pharmacother. 2004;38:1272–7.
Miner JR, Danahy M, Moch A, Biros M. Randomized clinical trial of etomidate versus propofol for procedural sedation in the emergency department. Ann Emerg Med. 2007;49:15–22.
Chen X, Sang N, Song K, Zhong W, Wang H, Jiang J, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42:614–24.
Chen S, Wang J, Xu X, Huang Y, Xue S, Wu A, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. 2020;12:4594–603.
Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. Bmc Anesthesiol. 2021;21:156.
Miao S, Zou L, Wang G, Wang X, Liu S, Shi M. Effect of dexmedetomidine on etomidate-induced myoclonus: a randomized, double-blind controlled trial. Drug Des Devel Ther. 2019;13:1803–8.
Baradari AG, Alipour A, Habibi MR, Rashidaei S, Emami ZA. A randomized clinical trial comparing hemodynamic responses to ketamine-propofol combination (ketofol) versus etomidate during anesthesia induction in patients with left ventricular dysfunction undergoing coronary artery bypass graft surgery. Arch Med Sci. 2017;13:1102–10.
Iribarren JL, Jimenez JJ, Hernandez D, Lorenzo L, Brouard M, Milena A, et al. Relative adrenal insufficiency and hemodynamic status in cardiopulmonary bypass surgery patients. A prospective cohort study. J Cardiothorac Surg. 2010;5:26.
Morel J, Salard M, Castelain C, Bayon MC, Lambert P, Vola M, et al. Haemodynamic consequences of etomidate administration in elective cardiac surgery: a randomized double-blinded study. Br J Anaesth. 2011;107:503–9.
Sarkar M, Laussen PC, Zurakowski D, Shukla A, Kussman B, Odegard KC. Hemodynamic responses to etomidate on induction of anesthesia in pediatric patients. Anesth Analg. 2005;101:645–50.
Kim TK, Park IS. Comparative study of brain protection effect between thiopental and etomidate using bispectral index during temporary arterial occlusion. J Korean Neurosurg Soc. 2011;50:497–502.
Nyman Y, Von Hofsten K, Palm C, Eksborg S, Lonnqvist PA. Etomidate-Lipuro is associated with considerably less injection pain in children compared with propofol with added lidocaine. Br J Anaesth. 2006;97:536–9.
Nyman Y, von Hofsten K, Ritzmo C, Eksborg S, Lonnqvist PA. Effect of a small priming dose on myoclonic movements after intravenous anaesthesia induction with Etomidate-Lipuro in children. Br J Anaesth. 2011;107:225–8.
Keam SJ. Remimazolam: First Approval. Drugs. 2020;80:625–33.
Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. 2021;127:41–55.
Rex DK, Bhandari R, Lorch DG, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy: A randomized trial. Dig Liver Dis. 2021;53:94–101.
Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34:491-501.
Liu T, Lai T, Chen J, Lu Y, He F, Chen Y, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: A randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9:e851.
Saito K, Ohno S, Maeda M, Hirata N, Yamakage M. Remimazolam anesthesia for cardiac surgery with cardiopulmonary bypass: a case report. JA Clin Rep. 2021;7:21.
Acknowledgements
We would like to thank all the participants willing to participate in this study and the researchers involved in the study design, recruitment, treatment, and data collection.
Ancillary and post-trial care
If serious adverse events occur during anesthesia induction, the anesthesiologists may suspend the study and take corresponding treatment measures until a stable situation is reached. If the adverse events are related to the anesthesia intervention after being confirmed by the professional committee, we will provide corresponding economic compensation, psychological comfort, and follow-up treatment.
Funding
No external funding.
Author information
Authors and Affiliations
Contributions
BH conceived the trial, prepared the initial protocol, conducted the study, and helped draft the manuscript. HZ participated in the study design and data collection. XZ participated in the study design and manuscript editing. LZ helped to revise the manuscript; LT, TS, and YL are the principal investigator of this trial and participated in its design, data analysis, and manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was approved by the Chinese Ethics Committee of Registering Clinical Trials (Ref: ChiECRCT20210524) and registered on www.chictr.org.cn (ChiCTR2100052535). Written informed consent will be obtained from each patient before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, B., Zhou, H., Zou, X. et al. Effect of remimazolam tosilate versus etomidate on hemodynamics in patients undergoing valve replacement surgery: study protocol for a randomized controlled trial. Trials 23, 992 (2022). https://doi.org/10.1186/s13063-022-06962-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06962-x