Abstract
Background
Major depressive disorder (MDD) is a leading cause of disability worldwide. The current treatments are ineffective in approximately one-third of patients, resulting in a large economic burden and reduced quality of life for a significant proportion of the global population. There is considerable evidence that increased inflammation may distinguish a sub-type of MDD, and there are no validated diagnostic tools or treatments for neuroinflammation in MDD patients. The current study aims to explore the potential role of low-dose naltrexone (LDN), a drug with purported anti-inflammatory properties in the central nervous system, as an adjunctive treatment in patients with MDD.
Methods/design
This double-blind placebo-controlled hybrid parallel arm study enables the exploration of peripheral and central inflammatory markers with LDN as an approach to investigate inflammation as a pathophysiological contributor to MDD. Eligible participants with MDD (n = 48) will be stratified into the high and low inflammatory groups according to the levels of high-sensitivity C-reactive protein (hs-CRP) and then randomized to receive LDN or placebo for an initial 12 weeks, followed by a further 12 weeks during which all participants will receive LDN. The primary outcome measure will be the Montgomery-Åsberg Depression Rating Scale (MADRS) administered at baseline, 2 weeks, 4 weeks, 8 weeks, 12 weeks, 14 weeks, 16 weeks, 20 weeks, and 24 weeks, to assess the effectiveness of the anti-depressant response. The secondary outcomes include the use of MRI techniques including quantitative magnetization transfer (qMT), echo-planar spectroscopic imaging (EPSI), and diffusion-weighted imaging (DWI) to help to elucidate the neurobiological mechanism of LDN, and the inflammatory mechanisms in action in MDD. Electroencephalography, blood samples, cognitive tasks, and additional questionnaires will also be used to determine if there is a specific profile of symptoms in individuals with inflammatory MDD. Healthy participants (n = 24) will be recruited for baseline outcome measures only, to enable comparison with patients with MDD.
Discussion
This trial contributes to the literature on inflammation in MDD, including the understanding of the pathophysiology and efficacy of anti-inflammatory treatments. The investigation of inflammatory mechanisms in MDD is an important first step in the development of biomarkers to classify patient sub-groups, increase the accuracy of diagnosis, and tailor the approach to patients in clinical practice. This study may provide evidence of the benefit of LDN for the groups in whom conventional anti-depressants are ineffective and lead the way for translation into clinical practice.
Trial registration
Australian New Zealand Clinical Trials Registry ACTRN12622000881730. Registered on 21 June 2022
Similar content being viewed by others
Background
Approximately 280 million individuals suffer from depression worldwide, and depression is now the leading cause of disability [1]. Major depressive disorder (MDD), described as a persistent low mood for more than 2 weeks by the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), can severely impact an individual’s ability to function [2]. Current treatments for MDD are ineffective in approximately one-third of patients resulting in poor outcomes, significant economic burden, and reduced quality of life for a significant proportion of the global population [3].
The current pharmacological therapies for MDD primarily target the monoaminergic systems, based on the theory that depression is due to a reduction in monoaminergic neurotransmission [4]. Conventional treatments, such as selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, and selective noradrenaline reuptake inhibitors (SNRIs), all target the monoaminergic system by various mechanisms. Recent research demonstrates that depression is influenced by factors beyond monoamines, such as neuroinflammation [5, 6].
Depression is a heterogenous disorder with varying pathophysiology, and substantial evidence suggests increased inflammation may distinguish a sub-type of MDD, present in 30% of total MDD cases [7]. Evidence of increased inflammation is demonstrated in MDD by (a) elevated peripheral levels of cytokines, chemokines, and circulating immune cells [5]; (b) post-mortem and microarray studies [8]; (c) that depression is a common comorbid disorder in neurodegenerative conditions with a known inflammatory component [9]; (d) sickness behavior, characterized by fatigue, anhedonia, and loss of appetite, is observed in both MDD and in cases of infection or inflammation [10, 11]; (e) MDD is often associated with factors which increase inflammatory markers such as stress, reduced sleep, and obesity [12]; and (f) medications that modulate the immune system, such as interferon, appear to affect mood [6]. Despite considerable evidence implicating inflammatory processes in MDD, there are no validated diagnostic tools or treatments for neuroinflammation in MDD.
Measurement of neuroinflammation in living patients is currently limited to two options. Lumbar puncture for the acquisition of cerebrospinal fluid is invasive and unable to identify the site of neuroinflammation [8]. Positron imaging tomography (PET) scanning utilizes ionizing radiation and is expensive, so it is not ideal for routine clinical use. Magnetic resonance imaging (MRI) is non-invasive, comparatively affordable, and more easily accessible. Advances in MRI techniques offer the possibility of measurement of neuroinflammation in people with MDD to better understand the pathophysiology of depression.
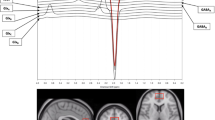
Quantitative magnetization transfer (qMT) and echo-planar spectroscopic imaging (EPSI) demonstrate sensitivity to changes in markers associated with inflammatory activity in the brain, such as water content of tissue [13] and brain temperature, respectively. qMT measures the exchange of magnetization between immobile protons bound to macromolecules, such as in myelin or membrane lipids, and the mobile protons in free water in intra- and extra-cellular tissue. qMT parameters, such as the forward exchange rate, which quantifies the efficiency of the magnetization transfer, are sensitive to the effects of neuroinflammation [14].
EPSI is a technique used for brain thermometry by measuring the chemical shift of the temperature-dependent water resonance frequency compared to a temperature-independent metabolite such as creatine [15, 16]. Brain temperature is expected to increase during neuroinflammation due to microglial activation, which increases metabolic demands leading to the release of excess heat. In a study of patients with chronic fatigue/myalgic encephalomyelitis, a condition believed to represent chronic low-level neuroinflammation, elevations of regional brain temperature between 0.28 and 0.50 °C were observed compared to control participants [17].
Diffusion-weighted imaging (DWI) techniques are used to investigate microstructural abnormalities in white matter. Recent advances in diffusion techniques show potential in detecting neuroinflammatory components such as astrogliosis and demyelination [18]. A novel diffusion technique named diffusion kurtosis imaging (DKI) has been used to indicate neuroinflammation in conditions such as traumatic brain injury [19], stroke [20], and multiple sclerosis [21]. qMT, EPSI, and DWI demonstrate promise for measurement of neuroinflammation in patients with MDD and will be used to (1) investigate potential biomarkers of brain inflammation in participants with MDD compared to control participants and (2) to monitor the neurobiological response to anti-inflammatory adjunctive treatment.
There is evidence to suggest that anti-inflammatory treatments have anti-depressant effects. However, the research does little to elucidate the mechanisms by which the anti-depressant effects occur [22]. Such studies have focused on the therapeutic efficacy of anti-inflammatory and immunomodulatory medications without clearly understanding the mechanism in action, thus leading to mixed results. Traditional non-steroidal anti-inflammatory drugs (NSAIDs), cytokine inhibitors, omega-3 fatty acids, and N-acetylcysteine are among the medications trialed for an anti-depressant response [23, 24]. There are critical limitations of these medications, however, including that they do not deeply penetrate the central nervous system tissue, are associated with adverse effects such as the increased risk of immunosuppression and stroke, and evidence suggests COX-2 selective NSAIDs may increase glial cell activation and neuroinflammation contrary to the anti-inflammatory hypothesis [25].
Low-dose naltrexone (LDN) is an atypical opioid antagonist with purported immunomodulatory and central anti-inflammatory effects [26]. Naltrexone is most often used for the treatment of opioid and alcohol addiction. However, the use of daily low doses (4.5 mg/day, 1/10th of the dose for addiction) appears to have anti-inflammatory effects in conditions such as Crohn’s disease [27, 28] and multiple sclerosis [28,29,30]. In addition to its role as a competitive opioid receptor antagonist, naltrexone also has an antagonist effect on non-opioid receptors, including Toll-like receptor-4 (TLR-4) found on microglia [31, 32]. Microglia activation results in sickness behaviors due to the release of inflammatory factors. By blocking TLR-4, the microglia may be prevented from assuming an inflammatory state, thus stopping the release of pro-inflammatory cytokines and neurotoxic superoxides [33]. Furthermore, naltrexone causes a continuous blockade of the opioid growth factor receptor axis (OGFr), resulting in the proliferation of immune cells [34, 35]. Though at the most common low dose of naltrexone, 4.5 mg/day, there is reduced proliferation of T and B cells due to an intermittent blockade.
In a small pilot trial of individuals with fibromyalgia, a significant reduction in several pro-inflammatory cytokines as well as improved mood was observed following treatment with LDN [36]. Furthermore, in a small trial of 12 individuals with MDD, LDN augmentation in addition to dopamine-enhancing agents was associated with reduced depressive symptomology [37]. LDN may be an effective adjunctive anti-inflammatory treatment for depressive symptoms in MDD.
The current study explores the potential role of LDN, a drug with purported anti-inflammatory properties in the central nervous system, as an adjunctive treatment in people with MDD. Moreover, using MRI techniques including qMT, EPSI, and DWI will help elucidate the neurobiological mechanism of LDN and the inflammatory mechanisms in action in MDD. This double-blind placebo-controlled hybrid parallel arm study enables the exploration of peripheral and central inflammatory markers with LDN as an approach investigating inflammation as a pathophysiological contributor to MDD.
Methods/design
Participants
The participants (n = 48) will be adults aged 18 to 55 years with moderate MDD currently receiving antidepressant medication. Healthy participants (n = 24) will be recruited to complete the baseline outcome measures to enable comparison with individuals without MDD. The complete inclusion criteria are outlined in Table 1. Strict exclusion criteria will be applied to limit the heterogeneity of the sample. The exclusion criteria are outlined in Table 2.
Participant recruitment
Participants will be recruited by the members of the study team from general practices within the greater Auckland area and via advertisements placed in local newspapers, noticeboards, and online using social media, allowing potential participants to make initial contact. Participants may also be recruited from an ethics-approved database of individuals who previously expressed interest in participating in studies on MDD (Auckland Health Research Ethics Committee number AH23223). Participants will be checked for eligibility at a screening visit and approved for inclusion in the trial by the study psychiatrist. The participant information sheet and informed consent form will be provided to participants prior to screening, allowing time for participants to seek independent advice. The participant information sheet and informed consent form provide details on the study including the type of trial, proposed involvement of participants, possible side effects, and risks of participation. Participants will have the opportunity to ask questions of the study investigators prior to and during the screening visit. If individuals choose to participate, verbal understanding of the information and written informed consent will be given to members of the study team at the screening.
Study design
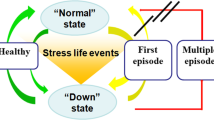
A randomized, double-blind, placebo-controlled, hybrid parallel-arm, superiority study (Fig. 1) will be used to test the potential of LDN as an adjunctive treatment for MDD. Participants with MDD will be allocated into parallel groups of high and low inflammatory status in blocks of 12 in a 1:1 ratio. The study will take place primarily at the Clinical Research Centre at the University of Auckland. After an initial screening, participants with MDD who meet the inclusion criteria will be prospectively stratified into low/high inflammatory status (n = 24 per group) based on the levels of high-sensitivity C-reactive protein (hs-CRP). Hs-CRP is a pro-inflammatory acute phase protein. Serum chemistry and hematology will also be conducted at the initial screening, including measurements of complete blood count, liver function tests, hs-CRP, and a human chorionic gonadotrophin (hCG) pregnancy test. The laboratory variable stated normal ranges will be used to categorize the results, and an additional interpretation by the study’s clinicians will deem abnormal values as either clinically or not clinically significant. A second blood sample, measuring only hs-CRP, will be taken at least 1 week later and be used to confirm inflammatory status. A high inflammatory state will be characterized by hs-CRP ≥ 3 mg/L, and a low inflammatory state will be characterized by hs-CRP ≤ 1 mg/L. Once the inflammatory status is confirmed, participants will complete a urine drug test and their baseline assessments and be randomly assigned to receive either placebo or LDN.
The primary, secondary, and tertiary endpoints of the study will be measured at baseline, following 12 weeks of LDN or placebo and following a further 12 weeks of LDN only. Previous studies using LDN indicate that a minimum of 12 weeks is required to observe anti-inflammatory effects [26, 39]. The anti-depressant effects of LDN will be measured using the Montgomery-Åsberg Depression Rating Scale (MADRS) which assesses the severity of depressive symptoms [40].
The primary outcome for this trial is change in MADRS scores at 12 weeks relative to baseline. The secondary outcomes include change in MADRS scores at 12 weeks in the high inflammatory group versus the low inflammatory group, detection of central inflammation in MRI scans, and measurement of peripheral inflammatory markers.
MRI scans will be conducted using the 3-T Siemens Vida Fit scanner at the Centre for Advanced MRI at the University of Auckland. A T1-weighted image will be acquired for segmentation and anatomical reference using a magnetization prepared rapid gradient echo (MPRAGE) sequence: repetition time (TR) = 2000 ms; echo time (TE)= 2.85 ms; flip angle= 8o; 208 slices; slice thickness= 1.00 mm, field of view (FOV) = 256 × 256 mm, matrix = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm, and acquisition time (TA) = 4 min 56 s.
The qMT technique will be used to measure the magnetization exchange rate. To acquire the qMT data, a series of MT-weighted 3D fast low-angle shot (FLASH) sequences are applied: echo time (TE) = 3.78 ms, repetition time (TR) = 35 ms, voxel size = 2 × 2 × 5 mm, varying flip angles for T1 mapping, and acquisition time (TA) = 10 min. Four pulse powers of 1000 degrees, 900 degrees, 800 degrees, and 400 degrees are applied to acquire 2, 3, 1, and 2 measurements, respectively. Finally, FABBER CEST in FSL is used to model the MT parameters.
Brain temperature will be calculated from the whole-brain spectroscopy data obtained using the EPSI sequence: TR1 = 1710 ms, TR2 = 591 ms, TE = 17.6 ms, voxel size = 5.6 × 5.6 × 10 mm, and TA = 17 min. The Metabolite Imaging and Data Analysis System (MIDAS) package will be used to analyze the data. The formula Tbrain = − 102.61 × ∆water − CRE + 206.1°C will be used to calculate the absolute brain temperatures according to the distance between the creatine (CRE) and water peaks.
DWI scans will use a three-shell protocol to acquire 18 non-diffusion-weighted images (b = 0 s/mm2) and 180 diffusion-weighted images (30 at b = 500 s/mm2; 60 at b = 1000 s/mm2; 90 at b = 2000 s/mm2) using non-collinear diffusion-weighting directions. The following are the other imaging parameters: TE/TR = 78/12,500 ms, slice thickness 2 mm, FOV = 224 × 224 mm, matrix = 112 × 112, resulting in 2 mm3 isotropic voxels, and TA = 15 min.
Blood samples will be acquired to measure peripheral inflammatory markers: hs-CRP, interleukin (IL)-1β, IL-2, IL-6, and IL-8. IL-10, IL-12p70, tumor-necrosis factor (TNF)-α, interferon-inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, vascular endothelial growth factor (VEGF), regulated upon activation, normal T cell expressed, secreted (RANTES), erythrocyte sedimentation rate (ESR), and glycoprotein nonmetastatic melanoma protein B (GPNMB) will be analyzed using multiplexed bead-based immunoassays. The blood samples will be analyzed for ESR 1 h after collection. Prior to analysis of the remaining peripheral inflammatory markers, the blood samples will be centrifuged, and the plasma will be frozen at − 80 °C.
Tertiary outcomes include electroencephalography (EEG) assessments and questionnaires compared between baseline, at 12 weeks, and at 24 weeks and the change in MADRS score between 12 and 24 weeks. Questionnaires for tertiary outcomes include the Beck Depression Inventory (BDI-II; [41]), Behavioural Activation for Depression Scale (BADS; [42]), Profile of Mood States (POMS; [43]), Lifetime Stress and Adversity Inventory (STRAIN; [44]), the SF-36 health survey [45], and the Sickness Questionnaire (SicknessQ; [46]). These questionnaires will enable a thorough understanding of each participant’s symptoms, stressors, and quality of life. The National Institute of Health (NIH) Toolbox will be used to assess the aspects of cognition including attention, executive function, psychomotor speed, and memory [47]. Resting-state and task-based electroencephalography (EEG) scans will be conducted with tasks including doors [48], long-term potentiation (LTP, [49]), and the attention network task (ANT; [50]) to assess responsiveness to positive and negative feedback, memory, and attention. In combination, these outcomes will be used to determine if a specific profile of symptoms is associated with inflammatory MDD.
Finally, the Generic Assessment of Side Effects (GASE, [51]) questionnaire will be used to monitor the adverse effects of LDN, and treatment expectancy effects will be measured using the Stanford Expectations of Treatment Scale (SETS, [52]). Pregnancy status will be reassessed at 12 weeks with a human chorionic gonadotropin (hCG) urine test.
A complete list of outcome measures is summarized in the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) figure (Fig. 2), and the SPIRIT checklist is provided as Additional file 1.
Randomization, blinding, and code-breaking
Following stratification of the eligible participants into high inflammatory and low inflammatory status, participants will be randomly allocated to parallel LDN or placebo groups in blocks of 12 in a 1:1 ratio. Computer-generated randomization will be performed by one member of the research team. LDN or placebo will be prescribed by the study psychiatrist(s) and delivered to the participant by the study pharmacist(s). The randomizer and pharmacist will be the only members of the research team unblinded to the identity of the medication. To maintain triple-blind conditions, the participant and study team members conducting the measurements of primary and secondary outcomes will not be aware of the identity of the medication.
During patient debriefing, participants will be asked to identify the medication they think they received. When all participants in their randomization block have completed the trial, participants and study team members will be informed of the correct medication identity. If the participant experiences a reaction or acute deterioration of health during the study and requires medical intervention, the identity of the medication may be revealed. An on-call member of the study team will be available 24/7 to break blinding. While this member is blinded, the randomizer will have prepared code-break envelope which may be broken in the event unblinding is required.
Drug preparation and administration
Naltrexone will be supplied to CompoundLabs by a licensed pharmaceutical material supplier who will confirm the identity and potency of the naltrexone. LDN capsules will be prepared by blending 1.5 mg naltrexone hydrochloride with microcrystalline cellulose (MCC) and filling the powder into a gelatin capsule shell. Placebo will be MCC only in a gelatin capsule shell. An unblinded pharmacist independent of the study team will store and dispense the interventions to participants, in accordance with Medicine Regulations 1984 [53]. The intervention will be dispensed to participants once per month. Participants in the durability arm will take one capsule orally once daily at night for 1 week, then 2 capsules at night for 1 week, then 3 capsules (4.5 mg) at night for 10 weeks. This dosing regimen will be repeated at 12 weeks, at the beginning of the 12-week open-label extension. If participants experience adverse effects, the dose may be titrated down to either 2 capsules daily or 1 capsule twice daily.
Alterations to allocated interventions
There will be no special criteria for modifying or discontinuing the allocated medication.
Strategies to improve adherence
Participants will be contacted by the study team after the day of medication delivery at 1 day, 3 days, 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, 10 weeks, 12 weeks, 13 weeks, 14 weeks, 16 weeks, and 20 weeks. The study team may contact participants via email, phone call, or text message to remind participants to adhere to study protocols (e.g., take medication daily) and to check for any adverse effects. At 4, 8, 16, and 20 weeks, participants will return to the University of Auckland for a treatment refill. At 12 and 24 weeks, participants will return to the University of Auckland for follow-up measurements of all outcomes. Participants will return their capsule bottles at the end of each 4-week period, and the number of capsules remaining in the bottle will be used to calculate intervention compliance.
Relevant concomitant care and post-trial care
There will not be any modification to the usual access to care pathways while participants are enrolled in the study. If a participant becomes ineligible for the study while enrolled, the participant may be discharged from the trial. If a participant is discharged due to physical health or psychiatric reasons, the participant will be referred to appropriate medical services. Upon completion of the study, participants will be reimbursed for their time in grocery vouchers. Individuals who complete initial blood tests but do not meet the inclusion criteria will be given a $20 grocery voucher. Healthy control participants who complete a single visit will be given $80 in grocery vouchers. Individuals with MDD who participate in the 24-week trial will receive a total of $320 in grocery vouchers: $80 at each study visit and $20 at each collection of their next treatment supply. There is no expectation of harm to participants caused by the current trial.
Statistical analyses and power calculations
Linear mixed effects models will be used to assess the primary outcome (change in MADRS scores at 12 weeks). Time (baseline and at 12 weeks) and drug (naltrexone and placebo) will be treated as fixed effects, and participants will be treated as a random effect. The primary estimand of interest will be the time × drug interaction coefficient with associated confidence intervals. Satterthwaite’s method (two-tailed) will be used to obtain p-values with an alpha set at p < .05.
To inform the power calculations for the primary outcome, a sensitivity analysis of the primary outcome was conducted for the fixed sample size of 48 (12 participants per group). Monte Carlo simulations were conducted using the mixed effects model described above with 10,000 simulations per run. Data were simulated on each iteration using the following parameters: n = 48, 4 dropouts with data missing at random, α = 0.05, (1−β) = 0.8, baseline MADRS scores of 30, random effect variance = 6.32, and error variance = 4.89. Variance estimates were obtained from linear mixed effects models fit to data from a previous antidepressant trial [54]. The present study is sensitive to detect changes of ~ 6 MADRS points according to the Monte Carlo simulations. The previous pilot study of 12 participants with MDD showed an 18-point decrease in MADRS scores with LDN compared to an 8-point drop with placebo [37]. The current study of 48 participants is considerably better powered for the primary outcome measure.
Sub-group data analyses and missing data
Sub-group analyses, such as a comparison of the hs-CRP level between sexes, may be conducted if a significant effect on hs-CRP is observed. A modified intention-to-treat scheme will be used including all participants who received at least one dose of intervention. All missing data will be classified as “missing at random” or “not missing at random” prior to unblinding. Data imputation techniques will be used where necessary.
Adverse event reporting
The GASE scale, a 36-item scale that assesses the most frequent side effects in clinical trials of medicines [51], will be used to evaluate the side effects at monthly visits. Any adverse events that occur during the trial will be recorded, and any serious adverse events will be reported within 24 h to the Centre for Adverse Reactions Monitoring consistent with the guidelines provided by the New Zealand Medicines and Medical Devices Safety Authority (“MedSafe”).
Data and safety monitoring committee
The study will be conducted to International Conference on Harmonisation (ICH) Good Clinical Practice (GCP) clinical trial standards. Two independent consultant psychiatrists and a biostatistician will form the Data and Safety Monitoring Committee for the trial. The study is considered low risk given the comprehensive inclusion/exclusion criteria; however, in the unlikely event of a serious adverse event, the Safety Monitoring Committee may decide to suspend the study or request suspension until study protocols are appropriately revised.
Data management and record keeping
Separate paper-based files will be kept for each participant; however, the majority of data will be captured by the online Research Electronic Data Capture (REDCap) tools hosted at the University of Auckland [55]. REDCap is a secure web-based platform designed to capture data and manage databases online. Demographics, medical history, height, weight, current medications, MADRS, and GASE scores will be entered directly into REDCap. Data from MRI scans and blood sample analysis (including hs-CRP and peripheral cytokine analysis) will also be stored electronically on secure University of Auckland servers on password-protected files.
During the study, participants will be identified by a unique study number and/or code. The name and any other identifying detail will not be included in any trial data electronic file. On all study-specific documents, other than the signed consent form, the participant will be referred to by their unique number/code. All data will be held for a period of 10 years from the completion of the study.
Dissemination policy
The results from the present study will be published in selected academic journals and presented at academic conferences. The results may also be distributed through social media, community forums, or news outlets. Participants may request a summary of their individual results at the end of the trial.
Discussion
The present study will investigate a novel anti-inflammatory drug as an adjunctive treatment for patients with MDD. LDN is already used off-label as an emerging therapy but is currently not prescribed in standard clinical practice. This study may provide evidence of the benefit of naltrexone for patient groups in whom conventional anti-depressants are ineffective and lead the way for translation into clinical practice.
The study’s unique design whereby participants are stratified into high/low inflammatory status may significantly contribute to the understanding of the pathophysiology of MDD. Despite evidence of inflammatory mechanisms in 30% of participants with MDD, diagnostic tools, and treatments for these patients are so far unclear: previous studies of anti-inflammatories have relied on heterogeneous groups of participants that led to mixed results. The present study will provide evidence on the efficacy of an adjunctive anti-inflammatory treatment specifically for patients with MDD associated with inflammation and enable comparison with a placebo treatment with patients of low-inflammatory status and with patients without MDD.
Finally, exploring potential blood-based and imaging biomarkers of peripheral and central inflammation may enable the development of more accurate diagnostic tools for patients with MDD and other disorders associated with inflammatory mechanisms. Inflammation is observed in various neuropsychiatric and neurological disorders, but the role of the inflammatory mechanisms in these conditions is unclear. Investigating inflammatory mechanisms in MDD is a critical first step in identifying biomarkers to classify patient sub-groups, increase the accuracy of diagnosis, and tailor the approach to patients in clinical practice.
Trial status
The study will advertise for enrollment in September 2022. Enrollment is expected to be completed in July 2024.
Availability of data and materials
The datasets analyzed during the current study and statistical code are available from the corresponding author on reasonable request, as is the full protocol.
Abbreviations
- ANOVA:
-
Analysis of variance
- ANT:
-
Attention Network Task
- BADS:
-
Behavioral Activation for Depression Scale
- BDI-II:
-
Beck Depression Inventory II
- CRE:
-
Creatine
- Hs-CRP:
-
High-sensitivity C-reactive protein
- EEG:
-
Electroencephalography
- EPSI:
-
Echo planar spectroscopic imaging
- ESR:
-
Erythrocyte sedimentation rate
- DBSI:
-
Diffusion basis spectrum imaging
- DWI:
-
Diffusion-weighted imaging
- GASE:
-
General assessment of side effects
- GCP:
-
Good Clinical Practice
- GPNMB:
-
Glycoprotein nonmetastatic melanoma protein B
- HCG:
-
Human chorionic gonadotropin
- ICH:
-
International Conference on Harmonisation
- IL:
-
Interleukin
- IP-10:
-
Interferon-inducible protein 10
- LDN:
-
Low-dose naltrexone
- LTP:
-
Long-term potentiation
- MADRS:
-
Montgomery-Åsberg Depression Scale
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDD:
-
Major depressive disorder
- MIDAS:
-
Metabolite Imaging and Data Analysis System
- MRI:
-
Magnetic resonance imaging
- MT:
-
Magnetization transfer
- NIH:
-
National Institute of Health
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- POMS:
-
Profile of Mood States
- qMT:
-
Quantitative magnetization transfer
- RANTES:
-
Regulated upon activation, normal T cell expressed, and secreted
- REDCap:
-
Research Electronic Data Capture
- SETS:
-
Stanford Expectations of Treatment Scale (SETS)
- SNRI:
-
Selective noradrenaline reuptake inhibitor
- SSRI:
-
Selective serotonin reuptake inhibitor
- STRAIN:
-
Lifetime Stress and Adversity Inventory
- TA:
-
Acquisition time
- TE:
-
Echo time
- TR:
-
Repetition time
- TLR-4:
-
Toll-like receptor-4
- TNF-α:
-
Tumor necrosis factor-α
- VEGF:
-
Vascular endothelial growth factor
References
World Health Organization: Depression. (2021). https://www.who.int/en/news-room/fact-sheets/detail/depression. Accessed 7 Sept 2022.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
The Royal Australian and New Zealand College of Psychiatrists. Global measures of prevalence of serious mental illness and associated premature mortality - a ‘top-down’ approach to estimating costs. In: The economic cost of serious mental illness and comorbidities in Australia and New Zealand: RANZCP; 2016. https://www.ranzcp.org/files/resources/reports/ranzcp-serious-mental-illness.aspx. Accessed 7 Sept 2022.
Hindmarch I. Expanding the horizons of depression: beyond the monoamine hypothesis. Hum Psychopharmacol. 2001;16(3):203–18. https://doi.org/10.1002/hup.288.
Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31.
Bullmore E. The art of medicine: inflamed depression. Lancet. 2018;392(10154):1189–90. https://doi.org/10.1016/S0140-6736(18)32356-0.
Strawbridge R, Hodsoll J, Powell TR, Hotopf M, Hatch SL, Breen G, et al. Inflammatory profiles of severe treatment-resistant depression. J Affect Disord. 2019;246:42–51.
Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun. 2019;81:24–40.
Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases. Mol Med Rep. 2016;13(4):3391–6.
Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66(5):415–22.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56.
Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32(1):7–24 PMID: 21407167.
Grossman RI, Gomori JM, Ramer KN, Lexa FJ, Schnall MD. Magnetization transfer: theory and clinical applications in neuroradiology. Radiographics. 1994;14(2):279–90.
Harrison NA, Cooper E, Dowell NG, Keramida G, Voon V, Critchley HD, et al. Quantitative magnetization transfer imaging as a biomarker for effects of systemic inflammation on the brain. Biol Psychiatry. 2015;78(1):49–57.
Ebel A, Maudsley AA. Improved spectral quality for 3D MR spectroscopic imaging using a high spatial resolution acquisition strategy. Magn Reson Imaging. 2003;21(2):113–20.
Mulkern RV, Panych LP. Echo planar spectroscopic imaging. Concepts Magn Reson. 2001;13(4):213–37.
Mueller C, Lin JC, Sheriff S, Maudsley AA, Younger JW. Evidence of widespread metabolite abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2020;14(2):562–72.
Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage. 2012;59(1):467–77.
Stokum JA, Sours C, Zhuo J, Kane R, Shanmuganathan K, Gullapalli RP. A longitudinal evaluation of diffusion kurtosis imaging in patients with mild traumatic brain injury. Brain Injury. 2015;29(1):47–57.
Rudrapatna SU, Wieloch T, Beirup K, Ruscher K, Mol W, Yanev P, et al. Can diffusion kurtosis imaging improve the sensitivity and specificity of detecting microstructural alterations in brain tissue chronically after experimental stroke? Comparisons with diffusion tensor imaging and histology. Neuroimage. 2014;97:363–73.
de Kouchkovsky I, Fieremans E, Fleysher L, Herbert J, Grossman RI, Inglese M. Quantification of normal-appearing white matter tract integrity in multiple sclerosis: a diffusion kurtosis imaging study. J Neurol. 2016;263(6):1146–55.
Eyre HA, Air T, Proctor S, Rositano S, Baune BT. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2015;57:11–6.
Köhler O, Petersen L, Mors O, Gasse C. Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain Behav. 2015;5(8):e00338.
Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–91.
Resnick RB, Volavka J, Freedman AM, Thomas M. Studies of EN-1639A (naltrexone): a new narcotic antagonist. Am J Psychiatry. 1974;131(6):646–50.
Smith JP, Bingaman SI, Ruggiero F, Mauger DT, Mukherjee A, McGovern CO, et al. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial. Dig Dis Sci. 2011;56(7):2088–97.
Smith JP, Stock H, Bingaman S, Mauger D, Rogosnitzky M, Zagon IS. Low-dose naltrexone therapy improves active Crohn’s disease. Am J Gastroenterol. 2007;102(4):820–8.
Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33(4):451–9.
Sharafaddinzadeh N, Moghtaderi A, Kashipazha D, Majdinasab N, Shalbafan B. The effect of low-dose naltrexone on quality of life of patients with multiple sclerosis: a randomized placebo-controlled trial. Mult Scler. 2010;16(8):964–9.
Cree BA, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol. 2010;68(2):145–50.
Toljan K, Vrooman B. Low-dose naltrexone (LDN)-review of therapeutic utilization. Med Sci (Basel). 2018;6(4):82. https://doi.org/10.3390/medsci6040082 PMID: 30248938; PMCID: PMC6313374.
Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, et al. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). Eur J Neurosci. 2008;28(1):20–9.
Liu B, Du L, Hong JS. Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther. 2000;293(2):607–17 PMID: 10773035.
Hammer LA, Waldner H, Zagon IS, McLaughlin PJ. Opioid growth factor and low-dose naltrexone impair central nervous system infiltration by CD4+ T lymphocytes in established experimental autoimmune encephalomyelitis, a model of multiple sclerosis. Exp Biol Med. 2016;241(1):71–8.
Donahue RN, McLaughlin PJ, Zagon IS. Low-dose naltrexone targets the opioid growth factor–opioid growth factor receptor pathway to inhibit cell proliferation: mechanistic evidence from a tissue culture model. Exp Biol Med. 2011;236(9):1036–50.
Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65(2):529–38.
Mischoulon D, Hylek L, Yeung AS, Clain AJ, Baer L, Cusin C, et al. Randomized, proof-of-concept trial of low dose naltrexone for patients with breakthrough symptoms of major depressive disorder on antidepressants. J Affect Disord. 2017;208:6–14.
Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(Suppl 13):23–9 PMID: 9402916.
Parkitny L, Younger J. Reduced pro-inflammatory cytokines after eight weeks of low-dose naltrexone for fibromyalgia. Biomedicines. 2017;5(2):16.
Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52–8.
Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996.
Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The Behavioral Activation for Depression Scale (BADS): psychometric properties and factor structure. J Psychopathol Behav Assess. 2007;29(3):191–202.
McNair D, Lorr M, Droppleman L. Manual for the profile of mood states (POMS). San Diego: Educational and Industrial Testing Service; 1971.
Slavich GM, Shields GS. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): an overview and initial validation. Psychosom Med. 2018;80(1):17.
Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83 PMID: 1593914.
Andreasson A, Wicksell RK, Lodin K, Karshikoff B, Axelsson J, Lekander M. A global measure of sickness behaviour: development of the Sickness Questionnaire. J Health Psychol. 2018;23(11):1452–63.
Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Supplement 3):S54–64.
Proudfit GH. The reward positivity: from basic research on reward to a biomarker for depression. Psychophysiology. 2015;52(4):449–59.
Kirk IJ, Spriggs MJ, Sumner RL. Human EEG and the mechanisms of memory: investigating long-term potentiation (LTP) in sensory-evoked potentials. J R Soc N Z. 2021;51(1):24–40.
Balter LJ, Bosch JA, Aldred S, Drayson MT, van Zanten JJ, Higgs S, et al. Selective effects of acute low-grade inflammation on human visual attention. NeuroImage. 2019;202:116098.
Rief W, Barsky AJ, Glombiewski JA, Nestoriuc Y, Glaesmer H, Braehler E. Assessing general side effects in clinical trials: reference data from the general population. Pharmacoepidemiol Drug Saf. 2011;20(4):405–15. https://doi.org/10.1002/pds.2067 Epub 2010 Nov 8. PMID: 21442687.
Younger J, Gandhi V, Hubbard E, Mackey S. Development of the Stanford Expectations of Treatment Scale (SETS): a tool for measuring patient outcome expectancy in clinical trials. Clin Trials. 2012;9(6):767–76.
Beattie D. Part 7: prescription. In: Medicines regulations 1984: Parliamentary Counsel Office; 2022. https://www.legislation.govt.nz/regulation/public/1984/0143/latest/DLM95668.html. Accessed 17 Aug 2022.
Sumner RL, McMillan R, Spriggs MJ, Campbell D, Malpas G, Maxwell E, et al. Ketamine enhances visual sensory evoked potential long-term potentiation in patients with major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(1):45–55.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Trial sponsor and role of the sponsor
The University of Auckland is the trial sponsor. The sponsor played no part in the study design; collection, management, analysis, and interpretation of the data; writing of the report; and the decision to submit the report for publication.
Funding
The study is funded by the Health Research Council of New Zealand. The funders have not played a role in the study design, conduct, data analysis, or writing of this manuscript.
Author information
Authors and Affiliations
Contributions
JP: writing—original draft. SG: investigation and methodology. BM: investigation and methodology. NH: conceptualization. FS: conceptualization. RS: methodology and conceptualization. SM: methodology, conceptualization, resources, and supervision. JL: conceptualization, methodology, funding acquisition, and supervision. All authors contributed to the review and editing of the manuscript. There were no professional writers involved. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial protocol (version 2 dated 19 May 2022) has been approved by the Southern Health and Disability Ethics Committee on 14 June 2022, approval number 12781. Written informed consent will be received from each study participant by the study team prior to admittance into the study.
Consent for publication
Participants provided informed consent for the use of their de-identified data in publications.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SPIRIT Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Plank, J.R., Glover, S.C., Moloney, B.D. et al. A randomized, double-blind, placebo-controlled, hybrid parallel-arm study of low-dose naltrexone as an adjunctive anti-inflammatory treatment for major depressive disorder. Trials 23, 822 (2022). https://doi.org/10.1186/s13063-022-06738-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06738-3