Abstract
Background
Bacterial infection is a major cause of mortality in patients with cirrhosis. Spontaneous bacterial peritonitis (SBP) is a serious and common infection in patients with cirrhosis and ascites. Secondary prophylactic antibiotic therapy has been shown to improve outcomes after an episode of SBP but primary prophylaxis to prevent the first episode of SBP remains contentious. The aim of this trial is to assess whether primary antibiotic prophylaxis with co-trimoxazole improves overall survival compared to placebo in adults with cirrhosis and ascites.
Methods
The ASEPTIC trial is a multicentre, placebo-controlled, double-blinded, randomised controlled trial (RCT) in England, Scotland, and Wales. Patients aged 18 years and older with cirrhosis and ascites requiring diuretic treatment or paracentesis, and no current or previous episodes of SBP, are eligible, subject to exclusion criteria. The trial aims to recruit 432 patients from at least 30 sites. Patients will be randomised in a 1:1 ratio to receive either oral co-trimoxazole 960 mg or an identical placebo once daily for 18 months, with 6 monthly follow-up visits thereafter (with a maximum possible follow-up period of 48 months, and a minimum of 18 months). The primary outcome is overall survival. Secondary outcomes include the time to the first incidence of SBP, hospital admission rates, incidence of other infections (including Clostridium difficile) and antimicrobial resistance, patients’ health-related quality of life, health and social care resource use, incidence of cirrhosis-related decompensation events, liver transplantation, and treatment-related serious adverse events.
Discussion
This trial will investigate the efficacy, safety, and cost-effectiveness of co-trimoxazole for patients with liver cirrhosis and ascites to determine whether this strategy improves clinical outcomes. Given there are no treatments that improve survival in decompensated cirrhosis outside of liver transplant, if the trial has a positive outcome, we anticipate widespread adoption of primary antibiotic prophylaxis.
Trial registration
ClinicalTrials.gov NCT043955365. Registered on 18 April 2020. Research ethical approval was granted by the Research Ethics Committee (South Central – Oxford B; REC 19/SC/0311) and the Medicines and Healthcare products Regulatory Agency (MHRA).
Similar content being viewed by others
Administrative information
Note: The numbers in curly brackets in this protocol refer to the SPIRIT checklist item numbers [1]. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
Title {1} | ASEPTIC: Primary Antibiotic prophylaxis using co-trimoxazole to prevent SpontanEous bacterial PeritoniTIs in Cirrhosis—study protocol for an interventional randomised controlled trial |
|---|---|
Trial registration {2a and 2b} | EudraCT # 2019–000,581-38 IRAS: 262,176 REC # 19/SC/0311 |
Protocol version {3} | Version 5.0, 20th August 2021 |
Funding {4} | National Institute of Health and Care Research (NIHR) HTA grant number 17/67/01. No other external funding. |
Author details {5a} | Dominic Crocombe (UCL Institute of Liver and Digestive Health; Sheila Sherlock Liver Centre, Royal Free London NHS Foundation Trust), Norin Ahmed (University College London Comprehensive Clinical Trials Unit), Indran Balakrishnan (Royal Free London NHS Foundation Trust; University College London), Ekaterina Bordea (University College London Comprehensive Clinical Trials Unit), Marisa Chau (University College London Comprehensive Clinical Trials Unit), Louise China (UCL Institute of Liver and Digestive Health; Sheila Sherlock Liver Centre, Royal Free London NHS Foundation Trust), Lynsey Corless (Hull University Teaching Hospitals NHS Trust), Victoria Danquah (University College London Comprehensive Clinical Trials Unit), Hakim-Moulay Dehbi (University College London Comprehensive Clinical Trials Unit), John F Dillon (Division of Molecular and Clinical Medicine, School of Medicine, University of Dundee), Ewan H Forrest (Gastroenterology Unit, Glasgow Royal Infirmary; University of Glasgow), Nick Freemantle (University College London Comprehensive Clinical Trials Unit), David Peter Gear (Cancer Research UK and UCL Cancer Trials Centre), Coral Hollywood (Gloucestershire Hospitals NHS Foundation Trust), Rachael Hunter (University College London Comprehensive Clinical Trials Unit), Tasheeka Jeyapalan (University College London Comprehensive Clinical Trials Unit), Yiannis Kallis (The Blizard Institute, Queen Mary University of London), Stuart McPherson (Liver Unit, The Newcastle upon Tyne Hospitals NHS Foundation Trust; The Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK), Iulia Munteanu (University College London Comprehensive Clinical Trials Unit), Jim Portal (University Hospitals Bristol NHS Foundation Trust), Paul Richardson (Liverpool University Hospitals NHS Foundation Trust), Stephen D Ryder (NIHR Nottingham Biomedical Research Centre at Nottingham University Hospitals NHS Trust; University of Nottingham), Amandeep Virk (University College London Comprehensive Clinical Trials Unit), Gavin Wright (Mid & South Essex NHS Foundation Trust), Alastair O’Brien (University College London Comprehensive Clinical Trials Unit; UCL Institute of Liver and Digestive Health; Sheila Sherlock Liver Centre, Royal Free London NHS Foundation Trust; University College London Hospitals NHS Foundation Trust) |
Name and contact information for the trial sponsor {5b} | University College London (UCL) |
Role of sponsor {5c} | University College London (UCL) is the trial sponsor and has delegated the sponsor responsible for the overall management of the ASEPTIC trial to the UCL Comprehensive Clinical Trials Unit (CCTU). Specific functions delegated to the UCL CCTU include a clinical project manager at UCL CCTU overseeing the trial manager, who will be responsible for the day-to-day management of the trial and providing support to the site staff. The CCTU will be involved in approaching sites, initiation visits, case report form development, database construction, and protocol and patient information development in collaboration with the Trial Management Group (TMG), Independent Data Monitoring Committee (IDMC), and Trial Steering Committee (TSC). |
Introduction
Background and rationale {6a}
For patients with advanced liver disease, bacterial infection is a major cause of morbidity and mortality. In patients with cirrhosis and ascites, spontaneous bacterial peritonitis (SBP) is the most common serious infection [2, 3], and secondary prophylaxis with antibiotic therapy is routinely prescribed to prevent further infection in patients who recover from SBP [4,5,6]. Yet, despite 90% of SBP cases presenting in patients with no previous episode [7], there is considerable uncertainty regarding the use of antibiotic therapy to prevent SBP in patients with ascites and no history of SBP, primary prophylaxis. An episode of SBP triggers a long-term reduction in survival and quality of life in cirrhosis, and therefore, primary prophylaxis to prevent this in the first place may actually offer greater gain than secondary.
Studies have shown that patients with ascites who have a low protein content (< 1.5 g/dL) were associated with a higher risk of developing infection [8,9,10], possibly due to reduced host opsonisation activity [8, 11, 12]. However, more recently, 2 large-scale, post hoc analyses showed no association between low protein count and increased SBP risk [13, 14]. Both the National Institute of Health and Care Excellence (NICE) and the European Association for the Study of the Liver (EASL) recommend antibiotic prophylaxis with quinolones for patients with ascitic fluid concentration < 1.5 g/dL, but the evidence to support this is limited and dated [6, 15, 16]. The American Association for the Study of Liver Disease (AASLD) recommends primary prophylaxis (with norfloxacin or co-trimoxazole) only if the ascitic protein is < 1.5 g/dL in the presence of impaired renal function and liver failure (Child–Pugh score > 9 and bilirubin > 3 mg/dL), which accounts for very few patients [17]. Conversely, the British Society of Gastroenterology (BSG) limits its guidance on primary prophylaxis due to a lack of consensus [18].
With support from the BSG trial development group, we conducted a national survey on the use of primary prophylaxis for SBP, and responses were received from 23 NHS Trusts (unpublished data). Nine centres reported routine use of antibiotics for primary prophylaxis against SBP; for 7 other centres, this was not routine practice, and the remaining 7 reported variable practice differing between clinicians. Evidently, there is considerable uncertainty and therefore great need for robust evidence to guide primary prophylaxis, especially for patients with ascites and ascitic protein > 1.5 g/dL and less advanced liver disease, and regarding the optimal duration of therapy. This is important as no therapies exist that prolong life in these patients outside of liver transplantation, which only occurs in a minority [19, 20].
Objectives {7}
The primary objective of this trial is to assess the effect of primary antibiotic prophylaxis with co-trimoxazole on overall survival compared to placebo in adults with cirrhosis and ascites, utilising a treatment policy estimand. Key secondary objectives include assessing the incidence of SBP, hospital admissions, Clostridium difficile (C. difficile)-associated diarrhoea and antimicrobial resistance, cost-effectiveness, and incidence of cirrhosis-related events, liver transplantation and treatment-related serious adverse events.
Trial design {8}
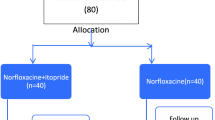
This is a multicentre, randomised, phase 3a, placebo-controlled, double-blind clinical trial. The trial design is displayed in Fig. 1. Patients with cirrhosis and ascites receiving care from at least 30 NHS hospital specialist liver services will be screened using the inclusion and exclusion criteria. We plan to recruit 432 eligible consenting patients that will be allocated in a 1:1 ratio to receive either co-trimoxazole or an identical placebo for 18 months, with a 3-monthly follow-up during this period. Thereafter, post-treatment follow-up will be 6-monthly until the end of the trial, and the maximum possible trial duration for any individual is 48 months.
Methods: participants, interventions, and outcomes
Study setting {9}
The trial will take place in the UK at secondary or tertiary care NHS hospitals that frequently manage patients with advanced liver disease. A list of study sites can be found in the Appendix. Patients with cirrhosis and ascites who have been admitted to an inpatient hospital ward or who are attending hospital for an outpatient clinic appointment (including liver transplant waiting list clinics) or abdominal paracentesis on day-case units, will be identified and approached and asked to participate. The nursing and medical staff on the clinical trial team will be appropriately qualified to manage patients with complications of cirrhosis as for routine clinical care.
Eligibility criteria {10}
The inclusion and exclusion criteria for patient eligibility are listed in Table 1.
Originally, ascitic protein < 2 g/dL was proposed as an inclusion criterion for this trial; however, in the pilot phase, we found a low number of cases of low ascitic protein (38 out of 224 patients), despite only screening patients with refractory ascites and advanced liver disease. Also, there are no large-scale UK data concerning ascitic protein values and the use of ascitic protein in stratifying SBP risk remains contentious [13, 14]. Since it is well recognised that all patients with cirrhotic ascites are at high risk of bacterial infection, it was decided to change the inclusion criteria to persistent ascites without the need for an ascitic protein level. The intention of this amendment was to both increase the number of eligible patients for recruitment and still include patients at the greatest risk of SBP mortality. At the time of this amendment, 15 patients had been recruited. Sub-group analysis will be performed to examine the outcomes in patients with an ascitic protein content ≥ and < 2 g/dL.
The original eligibility requirement regarding the presence of ascites included the condition “despite 3 months of standard treatment”. Feedback from our sites described this time period requisite as a significant limitation to recruitment, and this was formally amended to “patients with cirrhosis of Child–Pugh class B or C and the presence of ascites requiring any diuretic treatment or at least one or more paracentesis within 3 months prior to enrolment”. This enables the recruitment of patients hospitalised with ascites shortly prior to their discharge.
Who will take informed consent? {26a}
A participant information sheet (PIS) will be given to and discussed with potential patient recruits before consent is sought. Written informed consent will be obtained by appropriately qualified nursing or medical staff who are delegated at each site and have received protocol-specific training.
Additional consent provisions for collections and use of participant data and biological specimens {26b}
Eligible patients who give their written informed consent will be investigated with baseline blood tests, ascitic fluid sample for white cell count and/or protein level, and pregnancy test if applicable.
Interventions
Explanation for the choice of comparators {6b}
Co-trimoxazole was chosen as the antibiotic for this trial because it has an existing evidence base in SBP prophylaxis and is well tolerated and inexpensive [21,22,23,24,25]. There is a substantial literature on its use to prevent infection in patients living with human immunodeficiency virus (HIV) in resource-poor settings and little evidence of emergent microbial resistance to it [26,27,28]. Quinolones (e.g. ciprofloxacin or norfloxacin) were also considered as these have been shown to be effective in 2 meta-analyses that included primary prophylaxis trials to reduce the risk of SBP and mortality [4, 5], and they are commonly prescribed for secondary prophylaxis against SBP [15]. However, norfloxacin is not available in the UK, and other systemically absorbed quinolones have been associated with significant rates of antimicrobial resistance and C. difficile diarrhoea [29,30,31,32,33,34]. Rifaximin was not chosen because of its higher cost and widespread use for hepatic encephalopathy in the UK, which would have limited the number of eligible patients and/or risked significantly confounding analyses.
Current EASL guidance (2018) states that norfloxacin prophylaxis should be stopped in patients with long-lasting improvement of their clinical condition and disappearance of ascites but includes no guidance on the duration of therapy in those with persisting ascites [15]. Our trial treatment period of 18 months will test whether there is a beneficial effect on mortality, if there is a waning in efficacy over time, and whether the intervention impacts antimicrobial resistance.
The comparator is placebo, which is an acceptable option as there is no UK consensus regarding the necessity for prescribing primary prophylaxis in SBP and the majority of clinicians who responded to our national survey did not routinely do so.
Intervention description {11a}
The investigational medicinal product (IMP) is either one capsule of co-trimoxazole 960 mg once daily or an identical capsule containing placebo once daily. A bottle containing the IMP will be dispensed to patients via the clinical trials pharmacy department at their respective sites to coincide with randomisation and as close to the patient’s 3-monthly follow-up visits as possible. Patients will be instructed to start taking the IMP either as close to the randomisation date as possible (for those recruited in an outpatient setting) or on discharge from the hospital (for those recruited as inpatients).
Criteria for discontinuing or modifying allocated interventions {11b}
The IMP may be temporarily stopped if the patient experiences any severe adverse reactions or if, in the opinion of the site principal investigator (PI) or delegate, it is necessary. The patient will be encouraged to remain in the trial and continue to attend follow-up visits. The patient may be re-challenged following stopping of the intervention for at least a week if the PI or delegate considers this safe. The IMP will be adjusted in the following specific scenarios:
-
Renal dysfunction: if eGFR falls to < 30 mL/min whilst taking the IMP, dosing will be reduced to one capsule on alternate days and will be increased back to one capsule once daily If eGFR returns to > 30 mL/min. If eGFR reduces to < 15 mL/min whilst taking the IMP, it will be stopped. If the eGFR returns to > 15 mL/min but remains < 30 mL/min, the IMP will be restarted at alternate day dosing but will not be increased to once-daily dosing.
-
Hyperkalaemia: if serum potassium rises to ≥ 6 mmol/L, the IMP will be stopped and re-introduced when the site PI considers it safe. If hyperkalaemia ≥ 6 mmol/L occurs twice or more whilst taking the IMP, it will be reduced to alternate day dosing on re-introduction for the remainder of the study.
-
Thrombocytopaenia: if the platelet count falls to < 50 × 109/L with active bleeding, to < 30 × 109/L without active bleeding, or in a manner that concerns the PI or clinical team regarding increased risk of bleeding, the IMP will be stopped until counts are ≥ 30 × 109/L, any bleeding has resolved, and it is deemed safe to do so.
-
Non-SBP-related hospital admission: the IMP should be stopped upon admission to the hospital and restarted when safe to do so, on or soon after discharge.
-
Recovery from ascites: if ascites resolves for > 6 months without diuretic medications, patients will stop taking the IMP as their health status has improved.
The IMP will be stopped in the following scenarios:
-
Stevens-Johnson syndrome.
-
Hospital admission with SBP. As per routine clinical practice, patients will be commenced on long-term antibiotics as secondary prophylaxis following treatment of SBP.
-
Any significant medical reason deemed by the local PI.
-
Any suspected unexpected serious adverse reaction (SUSAR) event.
Every effort will be made to ensure patients withdrawn from the trial treatment continue to be followed up as per the protocol until the trial end.
Strategies to improve adherence to interventions {11c}
Patients will be educated about the possible risks of non-adherence. Treatment adherence will be assessed at regular intervals using the Medication Adherence Report Scale (MARS) questionnaire [35].
Relevant concomitant care permitted or prohibited during the trial {11d}
Concomitant care with long-term antibiotic prophylaxis is not permitted, except for rifaximin prescribed for hepatic encephalopathy. Patients will be stratified according to their use of rifaximin at randomisation and whether they are presently on the waiting list for a liver transplant. As hyperkalaemia is a known potential side effect of co-trimoxazole, caution will be taken in patients taking other medications that can cause hyperkalaemia: ACE inhibitors, angiotensin receptor blockers, and potassium-sparing diuretics. Patients at increased risk of renal dysfunction and/or hyperkalaemia, namely those with existing kidney dysfunction and those taking spironolactone or amiloride, are not eligible if the PI judges this risk cannot be managed with the adjustment of diuretics.
Provisions for post-trial care {30}
Other medical care during and post-trial will be standard medical care.
Outcomes {12}
The primary outcome is overall survival during follow-up.
The secondary outcomes are as follows:
-
1.
Time to the first incidence of SBP
-
2.
Hospital admission rates at 18 months
-
3.
Incidence of C. difficile-associated diarrhoea at 18 months
-
4.
Incidence of infections other than SBP with hospital admission at 18 months
-
5.
Incidence of other cirrhosis-related events (e.g. variceal haemorrhage) at 18 months
-
6.
Incidence of renal dysfunction with creatinine > 133 µmol/L (1.5 mg/dL) at any point during hospital admission
-
7.
Incidence of anti-microbial resistance at 18 months
-
8.
Incidence of liver transplantation at 18 months
-
9.
We will explore the potential impact of alcohol cessation and recidivism rates in those with alcohol as a cause of cirrhosis and any interaction with treatment
-
10.
Progression of liver disease assessed by an increase in Model for End-Stage Liver Disease (MELD) score between baseline and end of trial follow-up
-
11.
Safety and treatment-related serious adverse events
-
12.
Treatment adherence (assessed by MARS questionnaire)
-
13.
Incidence of resolution of ascites with diuretic treatment not required for 6 months at 18 months
-
14.
Incidence of transjugular intrahepatic portosystemic shunt (TIPS) insertion at 18 months
-
15.
Health-related quality of life assessed using the EQ-5D-5L Questionnaire
-
16.
Health and social care resource use assessed using Hospital Episode Statistics (HES) database
-
17.
Mean incremental cost per quality-adjusted life year (QALY) gained
Participant timeline {13}
The assessments and interventions required at screening, randomisation, and follow-up visits are outlined in Table 2. Eligible patients will be randomised using the secure randomisation website, Sealed Envelope® (www.sealedenvelope.com), which generates anonymised kit codes for trial staff to identify the correct trial medication for the patient, whilst maintaining blinding. Patients should start taking the trial medication as close as possible to the randomisation date (for those enrolled as outpatients) or as soon as they are well enough after discharge from the hospital (for those enrolled as inpatients). Follow-up visits by contact at the outpatient appointment will be on day 10, then at outpatient appointments or by telephone at month 1 and 3-monthly thereafter for 18 months. At month 19, there will be an end-of-study safety telephone call as this marks the end of the treatment period for the trial and denotes the minimum follow-up period. Patients that finish the treatment period prior to the end of the trial will be followed up 6-monthly until the end of the trial.
Sample size {14}
For overall survival as the primary outcome, we anticipate a hazard ratio (HR) of 0.62 and calculated that 432 patients will be required to generate 187 events, incorporating a 10% cumulative probability of loss-to-follow-up by the end of the study. Guided by the Kaplan–Meier 18-month survival estimate amongst patients with cirrhosis and ascites in the control arm of the ANSWER trial [30], and assuming an exponential survival distribution, we anticipate 66% survival in the control arm at 18 months. In the experimental arm, we expect 77% of patients surviving at 18 months. The recruitment will be uniform over a period of 2.5 years, and the follow-up time will be 18 months minimum with a maximum potential follow-up of 48 months. This calculation was based on a two-sided 5% type 1 error rate and 90% power. The primary event rate overall (blinded to allocation) will be monitored by the trial steering committee, and follow-up may be extended beyond the planned period to achieve the required number of events, with any extension agreed with the funder. Disruption to the trial schedule owing to the COVID-19 pandemic may necessitate an extension of the recruitment period.
Recruitment {15}
Both outpatients and inpatients will be recruited from at least 30 secondary or tertiary NHS hospitals that frequently manage patients with advanced liver disease. Outpatients will be screened when attending hepatology or liver transplant waiting list clinic appointments, or day case units for elective ascitic paracentesis. The recruitment period will be 30 months.
Assignment of interventions: allocation
Sequence generation {16a}
The independent, online, computer-generated randomisation service, Sealed Envelope® (www.sealedenvelope.com), will be used to minimise allocation bias. Randomisation will use a minimisation algorithm incorporating a random element, stratifying by active participation on the liver transplant waiting list, rifaximin prescription at enrolment, and centre. To ensure a maximum balance is achieved across the stratification factors, minimisation will be carried out on these factors separately. In mid-2022, we submitted an amendment to include alcohol as a significant cause of cirrhosis and active alcohol consumption at randomisation as stratification variables following review by our oversight committees.
Concealment mechanism {16b}
The Sealed Envelope® software allows concealed allocation. A single labelled bottle with a unique kit code of the IMP will be dispensed following randomisation and at each subsequent 3-monthly follow-up visit.
Implementation {16c}
The responsibility for confirming patient eligibility and prescribing the IMP lies with the site PI or delegated clinicians. Eligibility decisions will be made in line with the approved protocol. Other clinicians employed at the same clinical site (including trial nurses who are registered independent prescribers) may enrol and prescribe trial treatments to patients only if they have received appropriate training on the trial and appear on the trial delegation log, approved by the PI.
Assignment of interventions: blinding
Who will be blinded {17a}
Trial patients and all trial staff including clinicians and dispensing pharmacists will be blinded. The co-trimoxazole and placebo capsules will appear identical.
Procedure for unblinding {17b}
There will be no unblinding unless considered important for the patient’s care as assessed by the attending clinicians. If emergency unblinding is deemed necessary, this can occur at any time through, Sealed Envelope®.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Each patient will be given a unique trial patient identification number (PIN). Data will be collected using paper case report forms (CRFs) and will also be entered directly into an electronic data capture database (InferMed MACRO). Clinical outcomes will be logged by the trial staff. Validated questionnaires (e.g. MARS and EQ-5D-5L) will be used to collect data on certain outcomes.
Plans to promote participant retention and complete follow-up {18b}
The trial follow-up is designed to align with regular clinical follow-up appointments to minimise the additional burden for participants thus promoting retention. Patients that finish the treatment period prior to the end of the trial will be followed up 6-monthly until the end of the trial.
Data management {19}
Identification, screening, and enrolment logs will be kept at each trial site in a locked cabinet in a secured room and/or electronically. Electronic data will be stored on secure servers based at the lead organisation. The database will be password-protected and only accessible to specified trial members. After completion of the trial, these logs will be archived and stored securely by the sites for a minimum of 5 years. All data will be handled in accordance with the Data Protection Act 2018.
Confidentiality {27}
All data will be handled in accordance with the Data Protection Act 2018.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable as no biological specimens for genetic or molecular analysis will be collected as part of this trial.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
A detailed statistical analysis plan will be written prior to the first unblinded analysis and approved in advance by the trial steering committee. The main analyses will be conducted following the intention-to-treat principle. For the primary outcome, Kaplan–Meier survival curves will be compared using the log-rank test, according to the allocated treatment in an intention-to-treat manner. An unadjusted Cox proportional hazards model will be fitted, and the unadjusted hazard ratio and 95% confidence interval will be presented. An adjusted Cox model will then be fitted, adjusting for the stratification factors (active participation on the liver transplant waiting list, rifaximin prescription at enrolment, alcohol as a cause of cirrhosis, and active alcohol consumption at randomisation if the amendment submitted in June 2022 is successful) by including them as covariates in the model. All statistical tests will use a two-sided p-value of 0.05.
If there is evidence of non-proportional hazards, the life expectancy difference and life expectancy ratio between the two arms will be presented [36]. The stratified log-rank test will be reported in this instance as well, and the odds ratio for death at 18 months will be calculated using logistic regression.
Binary secondary outcomes will be analysed using logistic regression, and continuous outcomes using linear regression adjusting for baseline values (i.e. ANCOVA). Time to the first incidence of SBP will be presented with Kaplan–Meier curves and compared between the arms using the log-rank test. Serious adverse event rates will be presented by treatment arm and grade. Quality of life scores will be compared using a hierarchical linear regression model.
Interim analyses {21b}
There are no planned interim analyses. Regular reports regarding patient safety, death, and SBP events will be prepared for IDMC, who will have untrammelled access to study data in order to ensure participants are not placed at avoidable risk. The IDMC processes are described in a separate charter.
Methods for additional analyses (e.g. subgroup analyses) {20b}
The results on the primary efficacy outcome will be presented according to the levels of the stratifying variables used in the randomisation process. Interaction terms between each of the variables below and treatment will be added in turn to the primary analysis model to investigate whether the treatment effect differs according to the levels of these factors. We will report the treatment effect estimates in the subgroups and the p-value of the interaction tests.
The factors for subgroup analysis are as follows:
-
Rifaximin prescription at randomisation
-
Active participation on liver transplant list at randomisation
-
Gender
-
Ascitic fluid protein count (above or below the cut-off of 2 g/dL)
-
Long-term ascites (ascites persistent for more than 3 months at enrolment)
Health economic analysis
We aim to calculate the mean incremental cost per QALY gained from using co-trimoxazole to prevent SBP and improve overall survival in cirrhosis patients. The primary analysis will be a within-trial intention-to-treat analysis. Because an 18-month follow-up is considered enough time to capture all important drivers of the cost-effectiveness of the intervention, no economic modelling will be required. Health-related quality of life will be measured using the EQ-5D-5L, which will be collected at baseline and every 6 months during the 18-month follow-up for each patient. Utility scores will be calculated using UK-specific tariffs [37]. QALYs will be calculated as the area under the curve adjusting for baseline differences and minimisation factors as will be specified in the health economic analysis plan. Cost savings are anticipated to result from the prevention of hospital re-admissions and a reduction in other healthcare resource use. Resource use will be valued from the health and social care services perspective. The data will include hospital admissions (including length of stay and type of ward), cost of co-trimoxazole, day-case visits, any tests undertaken, outpatient attendances, primary care contacts, A&E visits, and concomitant medications. Unit costs will be obtained from publicly available sources such as the British National Formulary [38] and Unit Costs of Health and Social Care [39]. We will report descriptive statistics for all health and social care resource use at 18 months for both arms. 95% confidence intervals for the difference in costs between the two arms will be based on the bootstrapped results adjusting for variables specified in the analysis plan. Bootstrapped results will be also used to report the incremental cost per QALY gained. Cost-effectiveness acceptability curves will be constructed to report the probability that co-trimoxazole is cost-effective compared to placebo for a range of values of the cost-effectiveness threshold recommended by NICE [40].
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
The primary analysis will be conducted with the intention to treat principle, without imputation. We will undertake supportive analyses to consider the potential effects of missing data on the primary objective, using threshold analysis (where intervention group subjects who are missing are considered to have died the day after their last contact, and similarly control group subjects will be censored on the day after their last contact).
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
Enquiries may be addressed to ctu.aseptic@ucl.ac.uk.
Oversight and monitoring
Composition of the coordinating centre and trial steering committee {5d}
Trial oversight is delegated by the trial sponsor, UCL, to the UCL Comprehensive Clinical Trials Unit (CCTU). The trial team assists with the trial design, coordination and day-to-day operational issues in the management of the trial. The Trial Management Group (TMG) assists with the design, coordination, and strategic management of the trial. The TMG is composed of the CI, expert clinicians (hepatologists, microbiologists), PIs, clinical project managers, trial managers, statisticians, data managers, and health economists.
The Independent Trial Steering Committee (TSC) is responsible for the oversight of the trial to safeguard the interests of trial patients. The TSC is composed of independent expert clinicians, independent members and patient representatives, non-independent member clinicians, and observers.
Composition of the data monitoring committee, its role, and reporting structure {21a}
The Independent Data Monitoring Committee (IDMC), comprised of a clinician with expertise in liver disease, a clinical trialist, and a statistician, is responsible for safeguarding the interests of trial participants. They are the only oversight body that can access unblinded accumulating comparative data.
Adverse event reporting and harms {22}
Definitions of harm of the EU Directive 2001/20/EC Article 2, based on the principles of ICH Good Clinical Practice guidelines, apply to this trial. All adverse events (AEs) and serious adverse events (SAEs) occurring during the trial observed by the investigator or reported by the patient will be recorded in the patient’s medical records as per standard practice and, if applicable, on the appropriate case report form. All related SAEs should be notified to the CCTU immediately and within 24 h of the investigator becoming aware of the event. All unrelated SAEs will still be collected but do not require expedited reporting.
Frequency and plans for auditing trial conduct {23}
During the course of the trial, selected sites will be monitored (on-site or remote) to ensure quality assurance in that the site is adhering to the trial protocol, NIHR Good Clinical Practice (GCP) guidelines and regulations, ensure patient safety and that the data collected is accurate. In addition, the sites will be centrally monitored where study data will be regularly checked for any anomalies.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, ethical committees) {25}
We have two liver patient group representatives on our TSC and have the support of the British Liver Trust. In addition, the TSC includes members who have clinical expertise and any proposed changes made to the protocol will be raised through meetings or via email discussions prior to submitting for REC/HRA and MHRA approvals.
Dissemination plans {31a}
The results will be promoted via peer-reviewed publication to maximise the chances of adoption into clinical practice, regardless of the direction of effect on outcomes. Research findings will also be presented at conferences and seminars. Plain language summaries of the research findings will be written and disseminated to trial participants and the wider public. Publicity and engagement with the public and healthcare users will be supported by the British Liver Trust, the Primary Sclerosing Cholangitis Trust, the Liver Patients’ Transplant Consortium, and the UCL Public Engagement Unit.
Discussion
ASEPTIC is a multicentre RCT that aims to evaluate the efficacy, safety, and cost-effectiveness of co-trimoxazole for primary prophylaxis of SBP in patients with cirrhosis and ascites. The current clinical guidelines in this area are inconsistent and those that recommend primary prophylaxis only do so in the context of low ascitic protein levels, despite the evidence underlying this being contested [6, 15, 18, 41]. Our survey of clinicians confirmed the lack of consensus in clinical practice and further highlighted the need for a robust RCT to inform practice (unpublished data). Co-trimoxazole was chosen as it is a widely prescribed and relatively inexpensive antibiotic with a favourable safety profile.
The strengths of the trial design include robust mechanisms of randomisation, blinding, and the use of placebo. The inclusion criteria are deliberately broad to accurately represent the population of patients with advanced liver disease who use secondary and tertiary hepatology services in the UK. This should ensure trial outcomes are as relevant and applicable to this patient group as possible in clinical practice, and thus our primary analysis will present a treatment policy estimand. Pragmatic amendments have also been made to maximise recruitment, such as allowing recruitment of both inpatients and outpatients, and removing the cut-off of ascitic protein level < 2 g/dL as an inclusion criterion. Furthermore, mitigations were added in response to the challenges of the COVID-19 pandemic, for example, the option of delivering the IMP to patients’ homes by courier if required.
One major challenge of running long-term interventional trials in patients with advanced liver disease is the high rate of complications, hospital admissions, and mortality in this population. Recent large-scale clinical trial data from the ANSWER study (n > 400) demonstrated an 18-month mortality rate of > 20% for patients with cirrhosis and persistent ascites [30]. Our trial is recruiting a very similar cohort to ANSWER; therefore, it has been possible to use its results as a guide to power our study using overall survival as a primary outcome. Furthermore, mortality is easier to record accurately compared with infection, and ultimately the clinical trajectory that antibiotic prophylaxis aims to prevent is infection causing organ failure leading to death. If proven effective, primary antibiotic prophylaxis could also provide a bridge to liver transplantation for some patients.
Large-scale clinical trials are rarely performed in patients with cirrhosis and ascites, and there are no treatments that extend life in these patients outside of liver transplant. Therefore, the results of this large, multicentre RCT are anticipated to have a major impact on informing evidence-based practice for the benefit of patients with advanced liver disease.
Trial status
The ASEPTIC trial began recruitment in September 2019 and recruitment is ongoing. Due to the COVID-19 pandemic, recruitment was paused, which affected trial timelines. The new anticipated recruitment end date will be mid-2023.
Availability of data and materials {29}
The main outcomes will be published in a peer-reviewed journal. Unidentifiable participant data will be presented in the trial results. The full study protocol and statistical analysis plans can be requested by email to ctu.aseptic@ucl.ac.uk.
Abbreviations
- AASLD:
-
American Association for the Study of Liver Disease
- AE:
-
Adverse event
- ALT:
-
Alanine aminotransferase
- ANSWER:
-
Human Albumin for the treatmeNt of aScites in patients with hEpatic ciRrhosis
- ASEPTIC:
-
Primary Antibiotic prophylaxis using co-trimoxazole to prevent SpontanEous bacterial PeritoniTIs in Cirrhosis
- BSG:
-
British Society of Gastroenterology
- C. difficile :
-
Clostridium difficile
- CCTU:
-
Comprehensive Clinical Trials Unit
- COVID-19:
-
Coronavirus disease 19
- CRF:
-
Case report form
- EASL:
-
European Society for the Study of the Liver
- GCP:
-
Good Clinical Practice
- HES:
-
Hospital Episode Statistics
- HIV:
-
Human immunodeficiency virus
- IDMC:
-
Independent Data Monitoring Committee
- IMP:
-
Investigational medicinal product
- MARS:
-
Medication Adherence Report Scale
- MELD:
-
Model for End-Stage Liver Disease
- MHRA:
-
Medicines and Healthcare products Regulatory Agency
- NHS:
-
National Health Service
- NICE:
-
National Institute of Health and Care Excellence
- NIHR:
-
National Institute for Health and Care Research
- PI:
-
Principal investigator
- PIN:
-
Patient identification number
- PIS:
-
Patient information sheet
- QALY:
-
Quality of life year
- RCT:
-
Randomised controlled trial
- SAE:
-
Serious adverse event
- SBP:
-
Spontaneous bacterial peritonitis
- SUSAR:
-
Suspected unexpected serious adverse reaction
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
- TMG:
-
Trial Management Group
- TSC:
-
Trail Steering Committee
- UCL:
-
University College London
References
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61(2):297–310.
O’Brien AJ, Welch CA, Singer M, Harrison DA. Prevalence and outcome of cirrhosis patients admitted to UK intensive care: a comparison against dialysis-dependent chronic renal failure patients. Intensive Care Med. 2012;38(6):991–1000.
Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, Soares-Weiser K, Mendez-Sanchez N, Gluud C, et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34(5):509–18.
Cohen MJ, Sahar T, Benenson S, Elinav E, Brezis M, Soares-Weiser K. Antibiotic prophylaxis for spontaneous bacterial peritonitis in cirrhotic patients with ascites, without gastro-intestinal bleeding. Cochrane Database Syst Rev. 2009;(2):CD004791. https://doi.org/10.1002/14651858.CD004791.pub2.
NICE. NICE guideline NG50 | Cirrhosis in over 16s: assessment and management | Guidance | NICE. NICE. Available from: https://www.nice.org.uk/guidance/ng50/resources/endorsed-resource-practical-implications-for-primary-care-nice-guideline-ng50-4540284541. Cited 2022 Jan 29.
Rimola A, Salmerón JM, Clemente G, Rodrigo L, Obrador A, Miranda ML, et al. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology. 1995;21(3):674–9.
Llach J, Rimola A, Navasa M, Ginès P, Salmerón JM, Ginès A, et al. Incidence and predictive factors of first episode of spontaneous bacterial peritonitis in cirrhosis with ascites: relevance of ascitic fluid protein concentration. Hepatology. 1992;16(3):724–7.
Andreu M, Sola R, Sitges-Serra A, Alia C, Gallen M, Vila MC, et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology. 1993;104(4):1133–8.
Guarner C, Solà R, Soriano G, Andreu M, Novella MT, Vila MC, et al. Risk of a first community-acquired spontaneous bacterial peritonitis in cirrhotics with low ascitic fluid protein levels. Gastroenterology. 1999;117(2):414–9.
Runyon BA. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatology. 1988;8(3):632–5.
Guarner C, Runyon BA. Spontaneous bacterial peritonitis: pathogenesis, diagnosis, and management. Gastroenterologist. 1995;3(4):311–28.
Terg R, Casciato P, Garbe C, Cartier M, Stieben T, Mendizabal M, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol. 2015;62(5):1056–60.
Bruns T, Lutz P, Stallmach A, Nischalke HD. Low ascitic fluid protein does not indicate an increased risk for spontaneous bacterial peritonitis in current cohorts. J Hepatol. 2015;63(2):527–8.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–60.
Dever JB, Sheikh MY. Review article: spontaneous bacterial peritonitis–bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther. 2015;41(11):1116–31.
Runyon BA, AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–3. https://doi.org/10.1002/hep.26359.
Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70(1):9–29.
Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372(17):1619–28.
China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808–17.
Alvarez RF, de Mattos AA, Corrêa EBD, Cotrim HP, Nascimento TVSB. Trimethoprim-sulfamethoxazole versus norfloxacin in the prophylaxis of spontaneous bacterial peritonitis in cirrhosis. Arq Gastroenterol. 2005;42(4):256–62.
Singh N, Gayowski T, Yu VL, Wagener MM. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: a randomized trial. Ann Intern Med. 1995;122(8):595–8.
Lontos S, Gow PJ, Vaughan RB, Angus PW. Norfloxacin and trimethoprim-sulfamethoxazole therapy have similar efficacy in prevention of spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2008;23(2):252–5.
Inadomi J, Sonnenberg A. Cost-analysis of prophylactic antibiotics in spontaneous bacterial peritonitis. Gastroenterology. 1997;113(4):1289–94.
Costa T, Linhares I, Ferreira R, Neves J, Almeida A. Frequency and antibiotic resistance of bacteria implicated in community urinary tract infections in North Aveiro between 2011 and 2014. Microb Drug Resist. 2018;24(4):493–504.
Jafferbhoy HM, Miller MH, Gashau W, Chandrashekar C, Henry EB, Lockhart M, et al. Spontaneous bacterial peritonitis prophylaxis in the era of healthcare associated infection. Gut. 2012;61(11):1644–5.
Sibanda EL, Weller IVD, Hakim JG, Cowan FM. Does trimethoprim-sulfamethoxazole prophylaxis for HIV induce bacterial resistance to other antibiotic classes? Results of a systematic review. Clin Infect Dis. 2011;52(9):1184–94.
Everett DB, Mukaka M, Denis B, Gordon SB, Carrol ED, van Oosterhout JJ, et al. Ten years of surveillance for invasive Streptococcus pneumoniae during the era of antiretroviral scale-up and cotrimoxazole prophylaxis in Malawi. PLoS ONE. 2011;6(3):e17765.
Pitiriga V, Vrioni G, Saroglou G, Tsakris A. The impact of antibiotic stewardship programs in combating quinolone resistance: a systematic review and recommendations for more efficient interventions. Adv Ther. 2017;34(4):854–65.
Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417–29.
Owens RC, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;15(46 Suppl 1):S19-31.
Merli M, Lucidi C, Di Gregorio V, Falcone M, Giannelli V, Lattanzi B, et al. The spread of multi drug resistant infections is leading to an increase in the empirical antibiotic treatment failure in cirrhosis: a prospective survey. PLoS ONE. 2015;10(5):e0127448.
Piano S, Fasolato S, Salinas F, Romano A, Tonon M, Morando F, et al. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology. 2016;63(4):1299–309.
O’Brien A, China L, Gant V. The potential danger of empiric antimicrobial therapy for nosocomial SBP. Hepatology. 2016;64(6):2267–8.
Chan AHY, Horne R, Hankins M, Chisari C. The Medication Adherence Report Scale: a measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol. 2020;86(7):1281–8.
Dehbi HM, Royston P, Hackshaw A. Life expectancy difference and life expectancy ratio: two measures of treatment effects in randomised trials with non-proportional hazards. BMJ. 2017;25(357):j2250.
Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–108.
Joint Formulary Committee | About | BNF content published by NICE. Available from: https://bnf.nice.org.uk/about/joint-formulary-committee/. Cited 2022 Jun 16.
Jones KC, Burns A. Unit costs of health and social care 2021. Kent, UK: Personal Social Services Research Unit; 2021. p. 185. Available from: https://www.pssru.ac.uk/research/354/.
National Institute for Health and Care Excellence. Introduction to health technology evaluation | NICE health technology evaluations: the manual | Guidance | NICE. NICE Technology evaluations: the manual (PMG36). NICE; 2022. Available from: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation. Cited 2022 Jun 16.
Runyon BA, AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–3.
Acknowledgements
The ASEPTIC trial team are grateful to all trial participants, without whom this trial would not be possible. We also acknowledge the contributions of the following:
- Independent members of the Trial Steering Committee: Prof Phillip Newsome, Dr. Ahmed Elsharkawy, Martine Walmsley, John Crookenden, and Tim Clayton.
- Independent members of the Independent Data Monitoring Committee: Prof. Shahid Khan, Dr. Nikhil Vergis, and Dr. Zohra Zenasni.
- NIHR local clinical research networks.
- All PIs and trial staff at all recruiting sites.
Funding
This trial is funded by NIHR HTA grant number 17/67/01 and no other external funding. The funding body was not involved in the design of the study, data collection and processing, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
DC is the NIHR associate PI for trial at the Royal Free Hospital; he drafted the manuscript. AO’B is the chief investigator for the trial, contributing to the original idea, study design, proposal, and overall management; he oversaw the drafting, iterative rounds of editing, and final approval of the manuscript. AO’B, JFD, SDR, LCh, LCo, IB, YK, CH, EHF, SM, JP, PR, GW, DPG, MC, NA, NF, and VD are all current or former members of the Trial Management Group (TMG). DPG, LCh, LCo, EHF, YK, SM, and SDR contributed to the original study design and proposal. NF is the lead trial methodologist. TJ and AV are the current trial managers, DPG is a former trial manager, IM is the research nurse, and MC is the clinical project manager: they contributed to the protocol, protocol changes, issues relating to trial management, and data acquisition and analysis. VD is the data manager and contributed to the data management. NA and HMD contributed to the statistical plan for the trial and corresponding sections of the manuscript. IB is the PI leading on the microbiological aspects of the study. EB and RH contributed to the design of the health economic analysis of the trial and the corresponding section of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate {24}
Research ethical approval was granted by Research Ethics Committee (South Central – Oxford B; REC 19/SC/0311) and MHRA. Written, informed consent to participate will be obtained from all participants.
Consent for publication {32}
Not applicable—no identifying images or personal or clinical details of participants are presented here or will be presented in reports of the trial results. Informed consent materials can be made by email to ctu.aseptic@ucl.ac.uk.
Competing interests {28}
NF has acted as a consultant for Gedeon Richter, Abbott Singapore, Galderma, ALK, AstraZeneca, Ipsen, Vertex, Sanofi, Thea, Aimmune, Novatis, Novo Nordisk, Allergan, Alliance, and MSD. No non-financial conflicts. SM has received consultancy/speakers fees from Abbvie, Allergan, BMS, Gilead, Intercept, MSD, Novo Nordisk, Norgine, Novartis, and Sequana outside the submitted work. The other authors declared that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
List of sites recruiting patients at the time of writing (20 June 2022), in alphabetical order.
-
1.
Aintree University Hospital
-
2.
Basildon University Hospital
-
3.
Bedford Hospital
-
4.
Bristol Royal Infirmary
-
5.
Glasgow Royal Infirmary
-
6.
Gloucester Royal Hospital
-
7.
Hull Royal Infirmary
-
8.
James Paget University Hospital (Great Yarmouth)
-
9.
John Radcliffe Hospital (Oxford)
-
10.
Kettering General Hospital
-
11.
King’s College Hospital (London)
-
12.
Luton & Dunstable Hospital
-
13.
Newcastle Freeman Hospital
-
14.
Ninewells Hospital and Medical School (Dundee)
-
15.
Nottingham University Hospital
-
16.
Queen Alexandra Hospital, Portsmouth
-
17.
Queen Elizabeth Hospital, Gateshead
-
18.
Queen Elizabeth University Hospital, Glasgow
-
19.
Royal Berkshire Hospital (Reading)
-
20.
Royal Derby Hospital
-
21.
Royal Free London
-
22.
Royal Infirmary of Edinburgh
-
23.
Royal Liverpool University Hospital
-
24.
Royal London Hospital (Barts Health)
-
25.
Royal Sussex County Hospital
-
26.
St George’s Hospital (London)
-
27.
St James University Hospital, Leeds
-
28.
University College London Hospital
-
29.
University Hospital North Durham
-
30.
University Hospital of North Tees
-
31.
University Hospital of Wales (Cardiff)
-
32.
University Hospital of Coventry and Warwickshire
-
33.
Whittington Hospital
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Crocombe, D., Ahmed, N., Balakrishnan, I. et al. ASEPTIC: primary antibiotic prophylaxis using co-trimoxazole to prevent SpontanEous bacterial PeritoniTIs in Cirrhosis—study protocol for an interventional randomised controlled trial. Trials 23, 812 (2022). https://doi.org/10.1186/s13063-022-06727-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06727-6