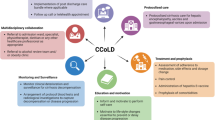
Abstract
Background
Acute-on-chronic liver failure (ACLF) represents a rising global healthcare burden, characterised by increasing prevalence among patients with decompensated cirrhosis who have a 28-day transplantation-free mortality of 33.9%. Due to disease complexity and a high prevalence of socio-economic disadvantage, there are deficits in quality of care and adherence to guideline-based treatment in this cohort. Compared to other chronic conditions such as heart failure, those with liver disease have reduced access to integrated ambulatory care services. The LivR Well programme is a multidisciplinary intervention aimed at improving 28-day mortality and reducing 30-day readmission through a home-based, liver optimisation programme implemented in the first 28 days after an admission with either ACLF or hepatic decompensation. Outcomes from our feasibility study suggest that the intervention is safe and acceptable to patients and carers.
Methods
We will recruit adult patients with chronic liver disease from the emergency departments, in-patient admissions, and an ambulatory liver clinic of a multi-site quaternary health service in Melbourne, Australia. A total of 120 patients meeting EF-Clif criteria will be recruited to the ACLF arm, and 320 patients to the hepatic decompensation arm. Participants in each cohort will be randomised to the intervention arm, a 28-day multidisciplinary programme or to standard ambulatory care in a 1:1 ratio. The intervention arm includes access to nursing, pharmacy, physiotherapy, dietetics, social work, and neuropsychiatry clinicians. For the ACLF cohort, the primary outcome is 28-day mortality. For the hepatic decompensation cohort, the primary outcome is 30-day re-admission. Secondary outcomes assess changes in liver disease severity and quality of life. An interim analysis will be performed at 50% recruitment to consider early cessation of the trial if the intervention is superior to the control, as suggested in our feasibility study. A cost-effectiveness analysis will be performed. Patients will be followed up for 12 weeks from randomisation. Three exploratory subgroup analyses will be conducted by (a) source of referral, (b) unplanned hospitalisation, and (c) concurrent COVID-19. The trial has been registered with the Australian New Zealand Clinical Trials Registry.
Discussion
This study implements a multidisciplinary intervention for ACLF patients with proven benefits in other chronic diseases with the addition of novel digital health tools to enable remote patient monitoring during the COVID-19 pandemic. Our feasibility study demonstrates safety and acceptability and suggests clinical improvement in a small sample size. An RCT is required to generate robust outcomes in this frail, high healthcare resource utilisation cohort with high readmission and mortality risk. Interventions such as LivR Well are urgently required but also need to be evaluated to ensure feasibility, replicability, and scalability across different healthcare systems. The implications of this trial include the generalisability of the programme for implementation across regional and urban centres.
Trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR) ACTRN12621001703897. Registered on 13 December 2021.
WHO Trial Registration Data Set. See Appendix 1
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Main text
Background
Acute-on-chronic liver failure (ACLF) is associated with a rising global healthcare burden, underpinned by increasing prevalence among patients with decompensated cirrhosis [1] and 28-day transplantation-free mortality of 33.9% [2]. A retrospective cost analysis of all ACLF unplanned admissions at our health service demonstrated a 30-day readmission of 34.4% and a 71.0% increase in total hospital costs between 2012 and 2018 [3]. There is evidence of increasing multimorbidity [4] in patients with cirrhosis, with an increasing prevalence of complications from comorbid non-alcoholic fatty liver disease and type 2 diabetes mellitus [5]. ACLF is associated with multi-organ failure, which requires a coordinated, multidisciplinary management approach [6]. Additionally, a high prevalence of socioeconomic disadvantage [7] impedes receipt of guideline-based treatments.
Compared to other conditions such as heart failure, those with ACLF have reduced access to integrated ambulatory care services [8] and care is fragmented through under-resourced outpatient clinics. Alcohol abuse is the most prevalent aetiology and independently contributes to the cirrhotic complications of malnutrition and sarcopenia due to inadequate oral intake of energy and protein, altered gastrointestinal absorption and protein/fat metabolism [9]. The combination of financial disadvantage, low health literacy, and active alcohol use creates a maelstrom of difficult-to-treat malnutrition and sarcopenia. The current model of ambulatory care for chronic liver disease is reactive, fragmented and lacks a robust framework for disease management. For new models of care to be adopted into complex healthcare systems, significant upfront costs need to be justified with high-quality evidence [10], which must address obstacles from patient, staff and healthcare system perspectives [11].
LivR Well is a multidisciplinary intervention aimed at reducing mortality and readmission through a home-based, liver optimisation programme. A prospective, single-arm feasibility study was conducted from March to October 2021 and enrolled 32 patients with ACLF (manuscript in progress). Participants received weekly medical review, nursing support, oral nutritional supplementation and consultation by dietetics, physiotherapy, pharmacy, social work and neuropsychiatry. The primary outcome was safety. Secondary outcomes were attrition, disease severity, healthcare utilisation, and patient-reported outcomes. Participants had a baseline median Charlson Co-Morbidity Index of 4 (IQR 3–5.5) and Model for End-Stage Liver Disease (MELD) score of16.5 (IQR 13–20.5). Handgrip strength in 11/23 (48%) met sex-specific sarcopenia criteria. Three patients died at 16, 148, and 197 days from enrolment. The 28-day mortality was 3% and the 30-day readmission rate was 18.75%. Median MELD score improved to 15 at 28 days (p = 0.16). The Chronic Liver Disease Questionnaire showed improvement in ‘fatigue’ (p = 0.02) and ‘worry’ (p = 0.03), at week 6. Patients, carers, and clinicians reported a positive experience in 100% and generated themes of acceptability, health literacy and insight, and autonomy. LivR Well cost $4947 AUD compared to $16,197 AUD for ACLF hospitalisation [3]. These results indicated that LivR Well was safe and acceptable, and likely to reduce both mortality and costs.
The feasibility study used the Asia-Pacific Association for the Study of Liver (APASL) definition of ACLF as an acute hepatic insult manifesting as jaundice (serum bilirubin ≥85mmol/L) and coagulopathy (International Normalized Ratio (INR) ≥1.5) complicated within 4 weeks by ascites and/or encephalopathy [12]. This study protocol for the RCT uses the European Foundation for the Study of Chronic Liver Failure criteria (page 5, Tables 1 and 2) [13], which grades the severity of ACLF using organ failure scores. Although the APASL definition was used due to our Asia-Pacific location, the decision was made to use EF-CLiF criteria to both identify and stratify the patients with the greatest risk of mortality, and to apply a definition most applicable to the Australian population with an alcohol misuse prevalence of 49.5% in cirrhosis [5] compared to greater chronic hepatitis B prevalence in the Asia-Pacific region at 2.8–7.6% [15] vs. 0.5–0.7% in Europe [16]. Consequentially, the patient population identified using EF-CLiF criteria are anticipated to have greater healthcare requirements and mortality but represent only a proportion of those admitted to hospital with decompensated liver disease. This protocol for a randomised controlled trial (RCT) therefore includes two arms to the trial: (a) those with ACLF and (b) those with hepatic decompensation not meeting EF-CLiF criteria. The RCT aims to provide a robust evaluation of the cost-effectiveness and impact on mortality and re-admission rates in each of these two populations compared to standard care.
Methods/design
Aims
-
1.
To evaluate the impact of the programme on 28-day mortality and 30-day re-admission
-
2.
To model the cost-effectiveness of LivR Well compared to usual ambulatory care
-
3.
To examine the differences in outcomes between those with ACLF and those with hepatic decompensation alone
Outcomes
Primary outcomes
-
ACLF cohort: 28-day mortality
-
Hepatic decompensation cohort: 30-day re-admission
Secondary outcomes
-
Changes in liver disease severity using Model for End-stage Liver Disease Score and Child-Pugh class assessed at day 28 and week 12 compared to baseline
-
Quality of life using Chronic Liver Disease Questionnaire and EQ-5D at week 6 compared to baseline
-
Cost-effectiveness compared to standard care.
Design
The RCT will be a multi-site, non-blinded, parallel-group study with two independent population arms to determine the efficacy and safety of the new model of care (LivR Well) compared to standard care and to inform a cost-effectiveness analysis.
This study protocol was developed in line with Recommendations for Interventional Trials (SPIRIT) guidelines including the SPIRIT checklist [17], which has been adapted in accordance with World Health Organisation registration (Appendix 1) and the CONSORT extension to pilot and feasibility trials [18] (Table 3, Appendix 2).
Participants and setting
The study context is a multicentre tertiary health service in Melbourne, Australia with recruitment from three tertiary hospital campuses. Monash Health is the second largest healthcare service in Australia managing a catchment population greater than 1.2 million people [19], which includes the largest Culturally and Linguistically Diverse (CALD) community, the significant burden of socioeconomic disadvantage and the highest unemployment rates in the state of Victoria [20]. The 28-day intervention will be delivered through ‘Hospital in the Home’ (HITH), a medically-led ambulatory care programme supported by physical clinics and home visits by the nursing team. HITH is able to deliver many aspects of acute hospital care in the community to otherwise stable patients including diagnosis and management of acute conditions that would typically require bed-based care
Patients will have access to up to three home visits per week by nursing staff and physiotherapists. The patients will attend a hospital-based ambulatory care liver clinic weekly for a medical and nursing review with a dietitian, social work, addiction medicine, and pharmacist consultations as required. Patients will be referred to LivR Well from an outpatient context, the emergency department or the inpatient ward. Patients with any severity of liver disease are eligible unless receiving end-of-life care. There are no cut-offs for the MELD score or Child Turcotte Pugh score.
Inclusion criteria
Adult patients
-
with previous or current hepatic decompensation defined as presence of ascites, hepatic encephalopathy, and/or variceal haemorrhage [21].
-
with or without a diagnosis of acute-on-chronic liver failure (ACLF) using the European Foundation for the Study of Chronic Liver Failure criteria (EF-CLIF) including age and white cell count. Severity is graded according to the number of organ failures (Tables 1 and 2) [14].
-
Requiring consultation from ≥3 allied health clinicians (Table 4)
Defining ACLF
-
The diagnosis and grading of ACLF will be performed using the publically-available online calculator incorporating this formula: 10×[0.33×CLIF-OFs + 0.04×Age + 0.63×Ln (White cell count) − 2] [13]. (Tables 1 and 2)
-
SI units of measurement (μmol/L) will be converted to mg/dL for serum bilirubin and serum creatinine for use in the calculator.
Exclusion criteria
-
Greater than grade 2 hepatic encephalopathy
-
Severe chronic extrahepatic disease
-
Active malignancy including hepatocellular carcinoma
-
Inability to provide informed consent
-
Residing outside the local hospital service catchment or deemed ineligible for home visits due to staff safety or occupational hazard concerns
-
Residing in a residential aged care facility
N.B. Concurrent COVID-19 infection is not an exclusion criterion.
Prohibited concomitant care
-
Admission for scheduled procedure or treatment
-
Moribund or receiving end-of-life care
-
Receiving regular albumin infusions for the treatment of diuretic refractory ascites or chronic hepatorenal syndrome (excluding those for periprocedural circulatory support following large volume paracentesis)
-
Refractory ascites managed with an intra-peritoneal catheter in-situ
Processes, interventions and comparisons
LivR Well is a 28-day intensive liver optimisation programme delivered at home and through the outpatient clinic. It is an innovative multidisciplinary model of care co-developed with patients and carers including delivery system re-design (home-based nursing, weekly medical reviews, regular dietitian and pharmacy consults) and adjunct interventions including enteral nutrition for sarcopenia, physiotherapy, large volume paracentesis, addiction medicine consultation and neuropsychiatric assessment for cognitive impairment/hepatic encephalopathy. Referrals to allied health clinicians will be made based on indications outlined in Table 4.
Patients will be enrolled on discharge from the acute admission, directly from the emergency department or the outpatient clinic as demonstrated in the Patient Journey Map (Fig. 1). A standardised checklist template (Appendix 3) will be completed on admission. Patients will have an in-home clinical review by a nurse up to three times per week with observations and blood tests taken weekly, or as requested by the clinic gastroenterologist. A face-to-face appointment at the ambulatory complex care liver clinic will occur weekly and will provide medical and nursing reviews with the opportunity for a dietitian, pharmacist and social worker consultations as required. Key time points and tasks for completion are outlined in Table 5.
Patients on the LivR Well programme will be remotely monitored in between clinic appointments through a custom-built app paired to a Bluetooth scale. Patient-reported outcome measures (PROMS) including quality of life, will be collected through a mobile phone text message link to the online surveys. Patients will also be able to access Lucy LiverBot, a custom-built artificial intelligence ChatBot for all disease-related questions. Quality of life assessments will be performed at day 1 and longitudinally at 6 weeks and 12 weeks from admission in both arms of the RCT. Two validated instruments will be used [EuroQol 5 Dimensions (EQ-5D) [25] and Chronic Liver Disease Questionnaire (CLDQ)] [26]. Outcomes from these assessments will guide the economic evaluation for a cost-effectiveness analysis. Adherence will be monitored through recording attendance at appointments, home visits and regular urinary ethyl glucuronide tests, a biomarker of alcohol consumption [27].
The comparator will be outpatient management in a ‘complex care liver clinic’, an ambulatory care clinic for patients with decompensated cirrhosis, promoting continuity of care by a gastroenterologist, hepatology nurse and pharmacist through regular face-to-face appointments. The control cohort will have no access to the additional allied health clinicians, technology aids or home visits through the LivR Well programme.
Recruitment
Patients will be recruited from the following settings: (a) following an acute hospital admission, (b) directly from the emergency department if able to be discharged home or (c) from the complex liver care clinic if ACLF or decompensation develops. All patients will be assessed by a gastroenterologist and allied health clinicians prior to recruitment. A research assistant or Pharmacist Informatician will provide eligible patients with a Participant Information and Consent Form (PICF) (Appendix 4) and consent will be obtained by a sub-investigator. Patients will be reviewed and admitted to the programme on the day of enrolment. All patients receiving LivR Well will also be onboarded to a mobile application and provided with the requisite hardware and software. Participants will be discontinued from the trial if consent is withdrawn, or if a serious adverse event (as defined on page 9) or death occurs. All participants will be included in the intention-to-treat analysis. If patients are admitted to the hospital after enrolment but before completion, the 28-day programme can be re-started on discharge if the patient still meets eligibility criteria and at the discretion of the treating team.
Screening and randomisation
Patients will be screened against inclusion and exclusion criteria at the time of diagnosis of ACLF or hepatic decompensation. Those who meet the criteria will be enrolled into the study and provided informed consent. These recruited patients will be randomised in a 1:1 ratio to either the LivR Well programme or to complex care liver clinic in the control arm. Participants will be computer randomised and informed of their allocation by the CLD nurse specialist prior to discharge or within 24 hours of obtaining informed consent. Patients from both study arms will be registered on the Virtual Hospital masterlog through the Microsoft Power Apps program. Due to the nature of the intervention, blinding of study participants or clinical staff administering the LivR Well programme will not be possible. However, outcome ascertainment for the primary outcome, as well as the health services utilisation secondary outcomes will be blinded. Furthermore, all data analyses will be undertaken in a blinded manner. The statistician will be blinded to the feasibility study data results and the treatment allocation of the RCT.
Trial status
We anticipate recruitment at a rate of 1-2 participants per week based on the progress of the feasibility study conducted at the same health service. A participant flow chart describing patients from screening, to enrolment, to randomisation, and allocation will be provided and will demonstrate how many patients are eligible for the intervention out of the entire study population.
Data collection
All data will be de-identified at the collection and stored securely on a Power BI database. Recruitment, retention and questionnaire completion numbers will be recorded throughout the trial. Biological specimens will be collected for the study and for patient care and analysed and stored through standard hospital processes. Consent will not be obtained for specimens to be used for genetic, molecular analysis, or in future/ancillary studies.
Data collected will include
-
Demographic information including age, sex, primary language
-
Clinical data at baseline, 28 days and 6-week follow-up
-
Patient-reported outcome measures using EQ-5D and CLDQ quality of life instruments
-
Hospital admissions, referral for liver transplantation and death
Adverse events
Adverse events are defined as “any untoward medical occurrence in a patient or clinical trial participant”. Serious adverse events (SAEs) will be defined as …” an untoward occurrence that results in death; is life-threatening; requires hospitalisation or prolongation of existing hospitalisation; results in persistent or significant disability or incapacity; consists of a congenital anomaly or birth defect” [28]; and will be reported to the PI and local ethics committee immediately. Any patients suffering harm from the trial will receive post-trial care in the liver clinic. Adverse event and SAS data will be collected in the same Power BI database at the time of occurrence by the hepatology nurse consultant.
Interim analysis
A data monitoring committee will consist of 3 senior independent clinician researchers without competing interests. An interim analysis will be conducted and we will adopt a Bayesian approach for an adaptive trial design where efficacy and futility criteria will be based on the probability of treatment effects, given the observed data in our feasibility study. One interim analysis will be performed in the ACLF and decompensated cohort when 50% of patients have been randomised and have completed 12 weeks of follow-up. We will stop the RCT for efficacy if the posterior probability (Pr) that the hazard ratio (HR) is < 1 is > 90% (Pr (HR <1|data) > 0.9. Similarly, we would stop for futility if Pr (HR <1|data) < 0.1. The results are reviewed by the independent data monitoring committee who will ultimately guide the decision to continue or cease the study.
Sample size
Sample size calculations were performed for each of the ACLF and hepatic decompensation arms using a 2-sided alpha of 5%, a power of 80%, and an anticipated 20% dropout.
For the ACLF cohort, we used the primary endpoint 28-day mortality, based on published rates of 22–74%, with a rate of 33.9% demonstrated in a study of 1343 hospitalised patients at 29 liver units in 8 European countries [2]. A 75% reduction in mortality in the intervention group generates a mortality rate of 8.5%, a conservative estimate considering our feasibility study of 59 participants resulted in a 3% 28-day mortality rate. We calculate a total sample size of 120 participants or 60 in each arm for the ACLF cohort. For the hepatic decompensation cohort, the sample size calculation is based on the primary endpoint of 30-day re-admission, which is estimated 17–38% [3, 29, 30]. Re-admission rates in our feasibility were 50% lower than anticipated at 18% by 30-days therefore we used an anticipated 17% 30-day re-admission rate in the intervention arm. We calculate a total sample size of 320 or 160 in each arm for the hepatic decompensation cohort. . Sample size calculation was performed using Stata statistical software, version 16.1 for Mac (StataCorp, College Station, TX, USA).
Statistical analyses
The data analysis for the primary composite endpoint of death, referral for liver transplantation or 30-day readmission from the date of hospital discharge will be undertaken on an intention-to-treat basis. A 28-day mortality rate will be calculated from the date of randomisation. For the primary and other binary outcomes, chi-squared tests will be used to compare the intervention and control groups. Survival analyses will also be undertaken, with the generation of Kaplan-Meier curves and Cox regression analyses to derive (adjusted) hazard ratios. For continuous outcomes, t-tests or non-parametric equivalents (e.g., Mann-Whitney tests) will be applied to compare the intervention and comparator groups. A p value of 0.05 is considered significant and 95% confidence intervals will be reported for all effect estimates Three exploratory subgroup analyses are planned: (a) comparing groups by the source of referral—conducted to address bias created following randomisation whereby in-patients in the intervention arm may be discharged home earlier due to perceived safety with regular home visits compared to those in the control arm. This subgroup analysis will account for differences between those admitted from in-patient admission compared to the emergency department or the ambulatory liver clinic; (b) comparing groups by re-enrolment to LivR Well after drop out due to unplanned re-admission to hospital. Data from the first enrolment to LivR Well will be included in the primary outcomes on an intention-to-treat basis and (c) patients with COVID-19 at the time of referral. Analyses will be performed using Stata statistical software, version 16.1 for Mac (StataCorp, College Station, TX, USA).
Economic analysis
The economic analysis will examine the cost-effectiveness of the LivR Well programme compared to the less resource-intensive control arm and will include both health system and societal cost perspectives. The main output of interest is the incremental cost-effectiveness ratio (ICER) in terms of net costs per unit of health gain. Health gains will be measured in clinical outcomes, estimation of years of life gained, quality-adjusted life years (QALYs) gained and by collection of quality-of-life data. Net costs will comprise the differential costs of the LivR Well programme and usual care minus costs saved from reduction in downstream health service utilisation. All health economic analyses will be undertaken in accordance with recommended approaches such as 5% discounting of estimated future costs and health gains. To account for any uncertainty in the data inputs for health economic modelling, sensitivity and uncertainty analyses will be undertaken via Monte Carlo simulation [31].
Dissemination of trial results
Trial results will be disseminated to healthcare professionals and the public through publication in a peer-reviewed journal and presentation at national and/or international conferences. Results will be distributed to participants during post-trial follow-up in the liver clinic. There are no specific publication restrictions; however, a high-impact hepatology journal will be approached.
Discussion
We will evaluate the feasibility, acceptability, safety, efficacy and cost-effectiveness of LivR Well, a 28-day multidisciplinary liver optimisation programme following hospital admission with ACLF or hepatic decompensation. The programme consists of regular home visits, weekly medical reviews and access to multi-disciplinary team (MDT) care including medical, nursing, pharmacy, dietetics, social work and physiotherapy. Our feasibility study of 32 patients found that the programme was safe, feasible and acceptable and demonstrated a mortality rate of 3% and a re-admission rate of 18.5%.
The single-centre RCT aims to evaluate the impact on mortality and re-admission rate as well as disease severity, quality of life and cost-effectiveness. We anticipate that these results will guide the application of the programme in our quaternary health service, in other similar services and potential adaptation for use in regional centres.
One of the key strengths of this intervention is the use of a MDT approach enabled by technology. The MDT approach has previously been demonstrated to reduce mortality and outcomes in other chronic diseases including heart failure and chronic obstructive pulmonary disease [32]. The additional contributing factors including psychosocial issues associated with alcohol and other substance use disorders that contribute to poor health outcomes in this population must be acknowledged and addressed. The holistic approach of existing chronic disease management strategies can be applied to this similarly complex cohort. A nurse-led chronic disease management programme was assessed by Wigg et al. [8] in 60 patients randomised to the intervention (n=40) or usual care (n=20), which did not demonstrate an improvement in hospital admission rates, disease severity or patient quality of life. However, that study was limited by the sample size and did not incorporate a MDT. Our study aims to further explore these outcomes using a MDT approach, the inclusion of patients during an acute deterioration and with a larger population.
We acknowledge limitations in this study design and the ways we have attempted to address these. The trial is unblinded by nature, which may influence decisions such as referral for liver transplantation, re-admission to and discharge from hospital, clinical decisions especially for the control group, and referral for other procedures eg elective surgery. The statewide liver transplantation unit is external to Monash Health therefore referrals for consideration of transplantation are made using the centre’s own criteria. The other decisions will be challenging to address the influence of knowledge of the allocation group, and as such we will include this in the discussion. Patients in the intervention group may be re-admitted to the hospital faster due to close follow up or conversely, patients in the control group may be re-admitted at a lower threshold due to the absence of home-based support.
The economic evaluation will be a cost-effectiveness analysis and is essential to guide health services to strategically invest in sustainable ambulatory care to reduce emergency presentations and improve healthcare-related quality of life. This is of particular importance in this population with a high healthcare system burden [33], re-admission [34], and mortality rates.
The subgroup analyses are considered ‘exploratory’ as we acknowledge the potential for bias and challenging interpretation of results. It is unlikely with our total sample sizes that the subgroups will be adequately powered to draw conclusions and therefore another consideration could be using these data in a sensitivity analysis.
Trial status
Study protocol V15
The trial is open for recruitment from January 10, 2022. The approximate date for completion of recruitment is March 2023
Availability of data and materials
On completion of the trial, the final datasets generated and/or analysed will be available from the corresponding author on request. Access to the final datasets will be in accordance with governance policies, Good Clinical Practice guidelines and ethics committee policies. The research team will be accountable to an independent data and safety monitoring board throughout the study. Any protocol amendments will be communicated to all relevant parties.
Abbreviations
- ACLF:
-
Acute-on-chronic liver failure
- CLD:
-
Chronic liver disease
- CLDQ:
-
Chronic Liver Disease Questionnaire
- EQ-5D:
-
Euro Quality of Life 5 Dimension
- FiO2 :
-
Fraction of inspired oxygen
- FRAT:
-
Falls Risk Assessment Tool
- HGS:
-
Handgrip strength
- HITH:
-
Hospital in the Home
- INR:
-
International Normalized Ratio
- MAP:
-
Mean arterial pressure
- MUST:
-
Malnutrition Universal Screening Tool
- MDT:
-
Multidisciplinary team
- MELD:
-
Model for End-Stage Liver Disease
- PaO2 :
-
Partial pressure of arterial oxygen
- PI:
-
Primary investigator
- PICF:
-
Patient informed consent form
- PROM:
-
Patient-reported outcome measures
- RCT:
-
Randomised controlled trial
- SAE:
-
Serious adverse event
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
- SpO2 :
-
Pulse oximetric saturation
- WHO:
-
World Health Organization
References
Hernaez R, Kramer JR, Liu Y, Tansel A, Natarajan Y, Hussain KB, et al. Prevalence and short-term mortality of acute-on-chronic liver failure: a national cohort study from the USA. J Hepatol. 2019;70(4):639–47.
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–37 37.e1-9.
Lovett G, Ha P, Spanidis S, Le S. Health care utilization and cost analysis for decompensated chronic liver disease hospitalizations at a Victorian tertiary health care network: 2009 to 2018. J Gastroenterol Hepatol. 2019;34(S2):52–111.
Vaz J, Eriksson B, Strömberg U, Buchebner D, Midlöv P. Incidence, aetiology and related comorbidities of cirrhosis: a Swedish population-based cohort study. BMC Gastroenterol. 2020;20(1):84.
Valery PC, McPhail S, Stuart KA, Hartel G, Clark PJ, O'Beirne J, et al. Changing prevalence of aetiological factors and comorbidities among Australians hospitalised for cirrhosis. Intern Med J. 2020;51(5):691-8.
Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382(22):2137–45.
Powell EE, Skoien R, Rahman T, Clark PJ, O'Beirne J, Hartel G, et al. Increasing hospitalization rates for cirrhosis: overrepresentation of disadvantaged Australians. EClinicalMedicine. 2019;11:44–53.
Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013;11(7):850–8.e1-4.
O'Brien A, Williams R. Nutrition in end-stage liver disease: principles and practice. Gastroenterology. 2008;134(6):1729–40.
Baxter S, Johnson M, Chambers D, Sutton A, Goyder E, Booth A. Understanding new models of integrated care in developed countries: a systematic review; 2018.
Dyb K, Berntsen GR, Kvam L. Adopt, adapt, or abandon technology-supported person-centred care initiatives: healthcare providers' beliefs matter. BMC Health Serv Res. 2021;21(1):240.
Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL). Hepatol Int. 2009;3(1):269–82.
CLIF-C ACLF Calculator. European Foundation for the Study of chronic liver failure; 2019. Available from: https://www.efclif.com/scientific-activity/score-calculators/clif-c-aclf
Arroyo V, Moreau R, Jalan R, Ginès P. Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol. 2015;62(1):S131–S43.
Chen C-J, Wang L-Y, Yu M-W. Epidemiology of hepatitis B virus infection in the Asia–Pacific region. J Gastroenterol Hepatol. 2000;15(s2):E3–6.
Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58(3):593–608.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239.
Welcome and overview of Monash Health Melbourne, Victoria, Australia: Monash Health; 2020 [Available from: https://monashhealth.org/employees/student-orientation/welcome-and-overview-of-monash-health/.
Quality Account 2018-19. Melbourne, Victoria, Australia: Monash Health; 2019.
easloffice@easloffice.eu EAftSotLEa, Liver EAftSot. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–60.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601.
Stapleton C, Hough P, Oldmeadow L, Bull K, Hill K, Greenwood K. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28(3):139–43.
Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Group AHEW. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–36.
Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med. 2001;33(5):337–43.
Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45(2):295–300.
Walsham NE, Sherwood RA. Ethyl glucuronide. Ann Clin Biochem. 2012;49(Pt 2):110–7.
Safety monitoring and reporting in clinical trials involving therapeutic foods. In: Administration TG, editor.: Australian Government; 2016. p. 5.
Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247–52.
Chirapongsathorn S, Poovorawan K, Soonthornworasiri N, Pan-Ngum W, Phaosawasdi K, Treeprasertsuk S. Thirty-day readmission and cost analysis in patients with cirrhosis: a Nationwide population-based data. Hepatol Commun. 2020;4(3):453–60.
Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500.
McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–9.
Wigg AJ, Chin JK, Muller KR, Ramachandran J, Woodman RJ, Kaambwa B. Cost-effectiveness of a chronic disease management model for cirrhosis: analysis of a randomized controlled trial. J Gastroenterol Hepatol. 2018.
Ramachandran J, Hossain M, Hrycek C, Tse E, Muller KR, Woodman RJ, et al. Coordinated care for patients with cirrhosis: fewer liver-related emergency admissions and improved survival. Med J Aust. 2018;209(7):301–5.
Acknowledgements
Nil.
Funding
No funding has been received for this study.
Author information
Authors and Affiliations
Contributions
All authors meet International Committee of Medical Journal Editors (ICJME) guidelines for authorship. NN, ES, PA, LS, JH, BR, DL, WS, SB and SL were involved in the conception and design of the study. NN, ES, TW, PA, LS, AF, JH, AE, PH, IH, AR, DS and GT were involved in data acquisition. NN, DL, BR and SL were involved in planning data analyses. All authors reviewed and approved the submitted manuscript and are accountable for their contributions, and any questions related to the accuracy or integrity of any part of the work. No writing assistance from professional writers is planned.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval has been obtained through the Monash Health Human Research Ethics Committee under an existing approval for The Virtual Hospital.
Registration: QA/76264/MonH-2021-265874(v1)
Consent for publication
Written consent for participation and future publication using a Participant Information and Consent Form (PICF) as a sub-study of The Virtual Hospital, a “digital health ecosystem supporting mobile person-centred care for chronic conditions” (Appendix 4). Written, informed consent will be obtained at the first liver clinic appointment, scheduled within 7 days of admission. The consent process will be performed by a sub-investigator. Language interpreters will be used when required. Consent can be withdrawn at any time. Intention-to-treat analysis will be utilised to account for dropout.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
WHO Trial Registration.
Additional file 2.
SPIRIT Checklist.
Additional file 3.
LivR Well standardised admission checklist.
Additional file 4.
PICF.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ngu, N.L., Saxby, E., Worland, T. et al. A home-based, multidisciplinary liver optimisation programme for the first 28 days after an admission for acute-on-chronic liver failure (LivR well): a study protocol for a randomised controlled trial. Trials 23, 744 (2022). https://doi.org/10.1186/s13063-022-06679-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06679-x