Abstract
Background
Dysmenorrhea is one of the most common disorders among young women. Medicinal herbs are one of the alternative methods for the treatment of dysmenorrhea. This study will investigate the effect of Rosa foetida extract, along with self-care behavior education on primary dysmenorrhea among female students of Babol University of medical sciences.
Methods/design
A randomized clinical trial will be performed on single students, aged 18 to 24 years. The research samples will be divided into three groups. The students will receive self-care behavior education on dysmenorrhea. Following the education, two of the groups will receive Rosa foetida extract capsules and placebo capsules in two consecutive cycles every 8 h for two successive days, respectively. The capsules will have similar physical appearance. The third group will not receive any medication. Data will be collected through demographic characteristic questionnaire, visual analog scale, dysmenorrhea self-care behaviors scale questionnaire, pictorial chart, and menstrual distress scale questionnaire. In order to determine and compare the effect of pharmacological and educational interventions on the severity of dysmenorrhea in groups, an ANOVA analysis of variance test with repeated measures will be used by SPSS software version 22.
Discussion
The results will show the effects of Rosa foetida extract along with self-care behavior education on primary dysmenorrhea, and beneficial effects that may be found in the trial of this plant may be of use for women with the same problem.
Ethics and dissemination
The study is approved by the Ethics Committee of Babol University of Medical Sciences (IR.MUBABOL.REC.1397.059).
Trial registration
IRCT 20190318043086N1. Registered on 14 June 2019.
Similar content being viewed by others
Background
Dysmenorrhea or painful menstruation is referred to as the painful periods which occur before and during menstruation. Dysmenorrhea is divided into two types: primary and secondary [1]. It is reported that 25 to 85% of women in reproductive age suffer from dysmenorrhea [2]. The most recursive symptom of dysmenorrhea is menstrual cramps which happens due to the release of prostaglandins during menstruation. Other symptoms include nausea, vomiting, fatigue, headache, and dizziness, which may lead to 1 to 3 days of unproductivity [3, 4]. Therefore, dysmenorrhea has always been in the spotlight, in social and economical terms, being the most frequent reason behind women’s absence from school or work [5]. Dysmenorrhea causes loss of 600 million work hours and 2 billion dollars, annually in the USA [6]. The same can be said about Japan where dysmenorrhea causes a loss of over 4.2 billion dollars, every year [7]. Women play an important role in every society, be it in employment or their role in families. This causes dysmenorrhea to be one of the most important problems, being researched worldwide [8, 9].
The methods for the management of dysmenorrhea include medicinal treatments and non-medicinal treatments through supplementary and alternative treatment. The most frequently used medications for countering dysmenorrhea include anti-prostaglandin synthesis medicines and non-steroidal anti-inflammatory medicines such as Gelofen, Novafen, naproxen, mefenamic acid, celecoxib, etc. [10]. Many complementary methods have also been proposed, including transcutaneous electrical nerve stimulation (TENS) [11]; exercise [12]; yoga [13]; massage [14, 15]; acupressure [16]; aromatherapy [17, 18]; herbs such as dill, cumin, and ginger [19]; and placebos [20]. These treatments also proved to be effective in soothing dysmenorrhea.
Persian yellow rose, also known as Rosa foetida, is an edible rose. It belongs to the Rosacea family. The chemicals in Rosa foetida are similar to the ones found in a typical rose. According to clinical studies and evidences presented by the Australian Gene Technology Regulator, roses and their products were reported to be non-poisonous in all forms [21, 22]. Traditional treatments also utilize red roses to treat chest pain, stomachache, blood flow, and digestive disorders [23, 24].
Modern researches on Rosa damascena report the anti-microbial, anti-bacterial, anti-inflammation, pain reduction, antioxidant, cancer preventive, anti-viral, anti-epilepsy, anti-depressant, relaxing, and tranquilizing effects of Rosa foetida [24].
Iran is one of the countries where rose and specially Rosa foetida are cultivated. Western and Central regions of Iran, as well as some parts in the South, are reported to offer rose cultivation [25]. Moreover, there has been no clinical research on the effects of Rosa foetida on dysmenorrhea. Hence, we have decided to do this study. The main objective is to investigate the effect of Rosa foetida extract along with self-care behavior education on primary dysmenorrhea, and secondary objectives are to compare the effect of Rosa foetida along with self-care behavior with placebo and self-care behavior education on menstrual bleeding as well as menstrual distress.
Research aims
The study aims are to investigate the effect of Rosa Foetida extract, along with self-care behavior education on menstrual pain intensity, menstrual distress, and blood flow during menstruation.
Methods
Objectives
Our objectives are (1) to compare the severity of menstrual pain before and after intervention among three groups (Rosa foetida, placebo, and self-care); (2) to compare menstrual distress before and after intervention among three groups; and (3) to compare menstrual bleeding severity before and after intervention among three groups.
Design and setting
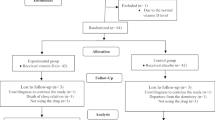
This study is a randomized clinical trial that will be performed on students residing at the dormitory of Babol University of Medical Sciences, Babol, Iran. The study design is in accordance with the Consolidated Standards of Reporting Trials (CONSORT) (Fig. 1). The study was approved by Ethics Committee of Babol University of Medical Sciences (IR.MUBABOL.REC.1397.O59), and the study protocol was recorded in the Iranian Registry of Clinical Trial (IRCT 20190318043086N1).
Participants: eligibility
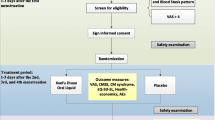
The interested students will be invited to participate in this study and will be given visual analog scale (VAS) for assessing dysmenorrhea, and those with pain scale equal or higher than 5 will be considered as participants for the study. In addition to that, consent form also will be filled out by the participants.
Inclusion criteria
Inclusion criteria are single (unmarried) girls with clinical symptoms of primary dysmenorrhea with pain score (5 or more) based on the visual analogue cale (VAS), who experienced dysmenorrhea in at least 3 of their recent 6 menstruations, aged 18 to 24 with body mass index ranging from 19 to 30 and regular periods, wherein the duration between two consecutive menstruations was ranged from 21 to 35 days. The duration of each bleeding would last 3 to 7 days.
Exclusion criteria
Exclusion criteria are any irregularities in the past 3 months, use of non-steroidal inflammatory medicines and anti-prostaglandins in the week before period, and also use of hormonal medication and contraceptive pills in the past 3 months and history of abdominal or pelvic surgery, known allergy to herbs, taking supplements and vitamins, and history of a prominent physical and psychological problems.
The drop-out criteria:
-
The diagnosis of gynecological disease during the study by a gynecologist
-
Severe stressful events during the study
-
Unwilling to continue being as a participant in clinical trials
Assignment of interventions: allocation
Sequence generation
Eligible subjects will be randomly assigned to three groups by block-randomized allocation method with 6 blocks. A randomization will be made by using a website program (http:// random.org). The table will be managed by an individual who is not included in the research team of the trial.
Concealment mechanism
After completing a baseline evaluation and determination of eligible participants, all the participants who agree to participate and meet the inclusion criteria will be randomized. The numbers, as generated by the mentioned website, will be given to the researcher who will be in the dark regarding what each intervention envelope included. An independent investigator will provide the random sequence within opaque, sealed envelopes for allocation concealment. The group numbers and the participants will be tracked by another researcher.
Implementation
After obtaining permission from the human ethics committee of Babol University of Medical Sciences then registering at the Iranian Clinical Trial Registration Center (IRCT) and obtaining required permissions from university authorities, the researcher will attend at the female dormitory of Babol University of Medical Sciences to invite interested participants for this study. The researcher will provide the information regarding the purpose of the study, kind of intervention, and confidentiality of the information and data. Then participants will be requested to sign the written consent form before enrolling in the study. Moreover, the participants will be ensured that they can leave the study at any stage and they also are allowed to use palliative treatments whenever they are not willing to continue to participate or when they feel need.
A colleague will generate the allocation sequence by using the randomization program. Then, the researcher will get informed consent from the subjects and the colleague will assign the participant to the determined group. The investigator and other related investigators will be blinded to perform proper management.
Measures
In the research, five instruments will be used for data collection:
-
1)
The demographic characteristic questionnaire will be used to collect demographic and reproductive characteristics, including age, menarche age, menstrual cycle length, menstrual bleeding period, and pain killer use history. The questionnaire has been developed based on objectives of the study and the validity will be examined by the content validity method.
-
2)
Visual analogue scale (VAS). Validity and reliability of the VAS is proven to possess an alpha Cronbach of 0.85 in studies by Bani et al. and Williamson et al. [26, 27].
-
3)
Menstrual Distress Questionnaire (MDQ). The reliability of the Persian version of the MDQ is proven to be 0.93 [28].
-
4)
Dysmenorrhea Self-Care Scale (DSCS) is an edited version of a research instrument proposed by Ching et al. and was validated through content method, in a study by Kabirian et al. In addition to that, the reliability of this method was proven and the alpha Cronbach was reported to be 0.78 [29, 30].
-
5)
Pictorial chart is a self-administered pictorial chart which, in addition to record the number of sanitary pads and tampons used, also takes into account the degree to which individual items are soiled with blood, passage of blood clots, and episodes of flooding. Pictorial chart is also proven to be reliable and valid with an alpha Cronbach 0.84 [31, 32].
Primary outcome
The primary outcome is menstrual pain intensity.
Secondary outcome
Secondary outcomes are menstrual distress, menstrual blood flow, and the need for pain killer during menstruation.
Side effects
The number of side effects experienced by each subject and total number of all the side effects will be summarized for each group.
Study procedures
Extract preparation
In the first step, dried Rosa foetida petals will be ground to a fine powder. Then, the powder will be added to a decanter along with 70% ethanol solution for 3–4 h. The solution will be distilled after 24 h. Distillation will be repeated three times. The resultant extract will be added to a rotary device to maximize the concentration of the extract. Finally, the extract will be prepared in the form of a powder which will be encapsulated.
Dysmenorrhea self-care education
The training program will be held in four sessions for all the eligible participants in the study. The first and second sessions of the educational content will include anatomy and physiology of menstruation, and the third and fourth sessions of the educational content will include exercise and nutrition.
Interventions
Group A will receive capsules with 200 mg of Rosa foetida extract to be taken three times a day during menstruation days, in addition to self-care instructions. Group B will received capsules with 200 mg of starch (as the placebo group), in addition to self-care instructions. Group C will receive only self-care instructions.
An assistant will code the capsules and seal them in an envelope; then, the envelopes will be given to the researcher to pass to the participants. Each participant will fill out the Pain VAS, Menstrual Distress Questionnaire (MDQ), and Dysmenorrhea Self-Care Scale (DSCS), as well as the pictorial chart in a menstrual cycle before intervention.
Intervention will be done on the first 2 days of two consecutive cycles, and the participants will be requested to take a capsule and then record their pain on the VAS, 1, 2, 4, 8, 12, 24, and 48 h after the start of intervention. In addition to that, the participants will be requested to fill out the MDQ and DSCS at the end of the second day of interventions.
Sample size
In order to calculate a sample size, the results from similar researches will be used. Considering pain reduction of 1.5 units, variance of 1.8 with CI 95% and 80% power, and 10% dropouts, 135 participants were estimated to be suitable for this study.
Statistical analysis
The comparison of the mean pain intensity of the three groups will be done using repeated measurement, and ANOVA in three different time intervals of before intervention, after the first intervention, and after the second intervention with repeated measures will be used to compare the mean scores of menstrual pain intensity between the groups at different time points. P value of less than 0.05 will be considered significant. The statistical analysis will be performed using SPSS version 22.
Data management and monitoring
In order to optimize the completeness of data collection, researchers will enhance communication with subjects. Therefore, we will strengthen our connection with subjects through social media and phone to remind and monitor their medications. One of the researchers (FS) will be responsible for collecting data and will assign another individual for the task of data entry into the SPSS software. A data manager will check all the data without knowing the treatment allocation. This will be performed under supervision of SO and SK.
Discussion
Persian yellow rose, with the scientific name Rosa foetida, is of the rose family and it is an edible flower as well. It is also a self-seeding, native flower in certain regions of Iran. The most important chemicals found in the rose family are vitamins, fennels, and flavonoids. Clinical studies on animals as well as human showed that fennels and flavonoids exert multiple biologic effects such as anti-microbial, anti-tumor, anti-pain, and anti-inflammatory effects [33, 34]. Moreover, studies reported that contextual factors related to primary dysmenorrhea self-care behaviors, along with self-management, can prove to be effective in pain reduction [30, 35,36,37]. This study will be the first clinical study on Rosa foetida along with self-care on dysmenorrhea. There are limited clinical researches on the effects of rose on dysmenorrhea and there are still questions regarding the effective complementary methods for reducing this bothering problem. Moreover, there is still no clinical study on the effectiveness of Rosa foetida on dysmenorrhea. Hence, if this study find a positive effect of Rosa foetida on dysmenorrhea, it can be suggested as an alternative therapy in dysmenorrhea.
Trial status
Participant enrolment is progressing and data collection is going on. Participant recruitment started on October 2019 and is expected to end in March 2021.
Availability of data and materials
At the end of the study, a completely de-identified data set is scheduled to be delivered to an appropriate data archive.
Abbreviations
- CONSORT:
-
Consolidated Standards of Reporting Trials
- VAS:
-
Visual analog scale
- TENS:
-
Transcutaneous electrical nerve stimulation
- MDQ:
-
Menstrual Distress Questionnaire
- DSCS:
-
Dysmenorrhea Self-Care Scale
- IRCT:
-
Iranian Clinical Trial Registration Center
References
Dawood MY. Primary dysmenorrhea-pathophysiology and management. J Islam Med Assoc North Am. 1981;13(4):125-31.
Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J Altern Complement Med. 2009;15(2):129–32.
Saei Gharenaz M, Ozgoli G. Effect of medicinal plants in the treatment of primary dysmenorrhea in Iran: a review article. Iran J Obstet Gynecol Infertil. 2015;18(160):14–31.
Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144(6):655–60.
Gail B, Slap. Menstrual disorders in adolescence. Best Pract Res Clin Obstet Gynaecol. 2003;17(1):75–92.
Habibi N, Huang MSL, Gan WY, Zulida R, Safavi SM. Prevalence of primary dysmenorrhea and factors associated with its intensity among undergraduate students: a cross-sectional study. Pain Manag Nurs. 2015;16(6):855–61.
Hsu CS, Yang JK, Yang LL. Effect of a dysmenorrhea Chinese medicinal prescription on uterus contractility in vitro. Phytother Res. 2003;17(7):778–83.
Zafari M, Behmanesh F, Mohammadi AA. Comparison of the effect of fish oil and ibuprofen on treatment of severe pain in primary dysmenorrhea. Caspian J Intern Med. 2011;2(3):279.
Obeidat BA, Alchalabi HA, Abdul-Razzak KK, Al-Farras MI. Premenstrual symptoms in dysmenorrheic college students: prevalence and relation to vitamin D and parathyroid hormone levels. Int J Environ Res Public Health. 2012;9(11):4210–22.
Latthe PM, Champaneria R. Dysmenorrhoea. BMJ clinical evidence. 2011;2011.
Igwea SE, Tabansi-Ochuogu CS, Abaraogu UO. TENS and heat therapy for pain relief and quality of life improvement in individuals with primary dysmenorrhea: a systematic review. Complement Ther Clin Pract. 2016;24:86–91.
Siahpour T, Nikbakht M, Rahimi E. The effect of 8 weeks aerobic exercise and yoga on primary dismenorrhea. Yasuj Univ Med Sci J (YUMSJ). 2013;6(18):475–83.
Marzouk TM, El-Nemer AM, Baraka HN. The effect of aromatherapy abdominal massage on alleviating menstrual pain in nursing students: a prospective randomized cross-over study. Evid Based Complement Alternat Med. 2013;2013:742421.
Sajjadi M, Bahri N, Abavisani M. Aromatherapy massage with geranium essence for pain reduction of primary dysmenorrhea: a double blind clinical trial. Iran J Obstet Gynecol Infertil. 2018;20(12):50–7.
Najaf Najafi M, Hadavi F, Vazirinasab Kermani S, Vafisani F, Ghazanfarpour M. Aromatherapy with Iranian herbal medicines for premenstrual syndrome and primary dysmenorrhea: a systematic review and meta-analysis. Int J Pediatr. 2019;7(9):10155–66.
Pour EM, Ousati AF. Attitudes of female adolescents about dysmenorrhea and menstrual hygiene in Tehran suburbs; 2002.
Salarilak S, Mohadesi H, Nabizadeh M, Motaraggeb F. A survey on the rate of knowledge, attitude and practice of hight school girl to menstruation health in Urmia (1999-2000); 2001.
Dabiri F, Abedini S, Shahi A, Kamjoo A. The effect of different methods of health education on knowledge, attitudes and practice of female students regarding menstrual hygiene in Bandar Abbas (2006). Hormozgan Med J. 2009;12(4):271–9.
Omidvar S, Nasiri-Amiri F, Bakhtiari A, Begum K. Clinical trial for the management dysmenorrhea using selected spices. Complement Ther Clin Pract. 2019;36:34–8.
Berek JS. Berek & Novak's Gynecology. Lippincott Williams & Wilkins; 2019.
Government A. the biology and Ecology of Rose Xhybrida (Rose). In: Ageing DoHa, editor. Australian: Government; 2005.
Nagatomo A, Oguri M, Nishida N, Ogawa M, Ichikawa A, Tanaka-Azuma Y. Evaluation of genotoxicity and subchronic toxicity of standardized rose hip extract. Hum Exp Toxicol. 2018;37(7):725–41.
Rezaei A, Mousavi G, Ahmadi C, Garanaz S. Compare palliative effects, pre-anesthetic and anti-anxiety rose extract with diazepam in rats. TUMJ. 2011;69(3):3.
Boskabady MH, Shafei MN, Saberi ZSS. Pharmacological effects of Rosa damascena. Iran J Basic Med Sci. 2011;14:295–307.
Amin G. Popular medicinal plants of Iran. Tehran: University of Medical Sciences; 2005.
Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804.
Bani S, Hasanpour S, Mousavi Z, Mostafa Garehbaghi P, Gojazadeh M. The effect of Rosa damascena extract on primary dysmenorrhea: a double-blind cross-over clinical trial. Iran Red Crescent Med J. 2014;16(1):e14643.
Qorbanalipour K, Ghaderi F, Jafarabadi A, Mohammadalizade M, Charandabi S. Validity and reliability of the Persian version of modified Moos Menstrual Distress Questionnaire. Iran J Obstet Gynecol Infertil. 2016;19(29):11–8.
Ching-Hsing H, Meei-Ling G, Hsin-Chun M, Chung-Yi L. The development and psychometric testing of a self-care scale for dysmenorrhic adolescents. J Nurs Res. 2004;12(2):119–30.
Kabiriyan M, Abedian Z, Mazlom S. Effective conditioning factors on self-care behaviors for primary dysmenorrhea in both peer and health provider-led education. IJOGI. 2014;17:8–15.
Higham JM, O'brien P, Shaw R. Assessment of menstrual blood loss using a pictorial chart. BJOG. 1990;97(8):734–9.
Janssen CA, Scholten PC, Heintz APM. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol. 1995;85(6):977–82.
Hajhashemi V, Ghannadi A, Hajiloo M. Analgesic and anti-inflammatory effects of Rosa damascena hydroalcoholic extract and its essential oil in animal models. Iran J Pharmaceut Res. 2010;9(2):163.
Mostafa-Gharabaghi P, Delazar A, Gharabaghi MM, Shobeiri MJ, Khaki A. The view of cesarean pain after preemptive use of Rosa damascena extract in women with elective cesarean section. World Sci J. 2013;4:226–35.
Kabirian M, Abedian Z, Mazlom SR, Mahram B. Self-management in primary dysmenorrhea: toward evidence-based education. Life Sci J. 2011;8(2):13–8.
Tang J, Xu H. Analysis of self-management education on improving the symptoms of female college students with primary dysmenorrhea in Shaoyang. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41(4):434–9.
Wong CL, Ip WY, Lam LW. Self-care strategies among Chinese adolescent girls with dysmenorrhea: a qualitative study. Pain Manag Nurs. 2016;17(4):262–71.
Acknowledgements
We appreciate the Research and Technology Deputy of Babol University of Medical Sciences for the approval and funding.
Protocol amendments
Any modification or changes to the current protocol that may affect to the potential benefit to the subjects or the conduct of the study requires a formal modification to the protocol. Such change will be approved by the sponsor and the Ethical Committee of Babol University of Medical Sciences before implementation.
Dissemination policy
The project findings will be published in an international journal.
Funding
The research deputy of Babol University of Medical Sciences will provide financial support. Study funders have no role in the study design; the collection, management, analysis, and interpretation of the data; the writing of the report; and the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
FS and SO contributed to the concept and design of this study protocol; HP, PS, and HN contributed to the design of this study; SK contributed to the planning of data acquiring and analysis; SK and SO will contribute to the interpretation of the data; SO drafted the manuscript, and MF, FN, and ZB prepared the final version of manuscript. All authors revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the study, and read and approved the final version of the manuscript to be published. All the authors met the criteria for authorship and listed as co-authors on the title page.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research protocol is approved by the research review board of Babol University of Medical Sciences (IR.MUBABOL.REC.1397.O59 human ethics committee of Babol University of Medical Sciences), and the study protocol is recorded in the Iranian Registry of Clinical Trial (IRCT 20190318043086N1). The permissions were obtained from university authorities for attending at the female dormitory of Babol University of Medical Sciences to invite interested participants for this study. During recruitment, the researcher will introduce herself to the participants. After the research objectives, the confidentiality of data maintenance, and the freedom to withdraw from the study are introduced, the written informed consent form will be signed by the participants who are willing to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shabani, F., Omidvar, S., Sajadi Kaboudi, P. et al. The effects of Rosa foetida extract along with self-care education on primary dysmenorrhea: study protocol for a randomized clinical trial. Trials 23, 637 (2022). https://doi.org/10.1186/s13063-022-06583-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06583-4