Abstract
Background
Chronic low back pain is a public health problem, and there is strong evidence that it is associated with a complex interaction of biopsychosocial factors. Cognitive functional therapy (CFT) is a promising new intervention that deals with potentially modifiable multidimensional aspects of pain (e.g., provocative cognitive, movement, and lifestyle behaviors).
Methods
To investigate the efficacy of CFT compared with a sham intervention for pain intensity and disability post-intervention (immediately after the last session) in patients with non-specific chronic low back pain (CLBP). This study is a randomized controlled trial in which 152 (18–60 years old) patients with CLBP will be enrolled. The patients will be randomly allocated to receive (1) CFT intervention or (2) sham intervention. The experimental group will receive individualized CFT in a pragmatic manner (5 to 7 sessions) based on the clinical progression of the participants. The sham group will attend six sessions: consisting of 30 min of photobiomodulation using a detuned device and more than 15 min of talking about neutral topics. Patients from both groups also will receive an educational booklet (for ethical reasons). Participants will be assessed pre and post-intervention, 3 months, and 6 months after randomization. The primary outcomes will be pain intensity and disability post-intervention. The secondary outcomes will be: pain intensity and disability at 3- and 6-month follow-up, as well as self-efficacy, global perceived effect of improvement, and functioning post-intervention, 3-, and 6-month follow-up. The patients and the assessor will be blinded to the treatment administered (active vs. sham).
Statistical analysis
The between-group differences (effects of treatment), as well as the treatment effect for the primary and secondary outcomes, and their respective 95% confidence intervals will be calculated by constructing linear mixed models.
Discussion
To the best of our knowledge, the current study will be the first to compare CFT vs. sham intervention. Sham-controlled RCTs may help to understand the influence of non-specific factors on treatment outcomes. Considering complex interventions as CFT, it is imperative to understand the impact of contextual factors on outcomes.
Trial registration
ClinicalTrials.gov NCT04518891. First Posted: August 19, 2020.
Similar content being viewed by others
Background
Low back pain is a challenge in terms of public health worldwide [1], due to its high prevalence [2] and socioeconomic impact [3]. It is considered the number one cause of disability worldwide [4]. For the management of non-specific chronic low back pain (CLBP), assessment and interventions focused on the biopsychosocial model are recommended by guidelines [5,6,7]. However, a large number of interventions for CLBP are not personalized to encompass specific individualized patient needs [8].
Cognitive functional therapy (CFT) is a flexible and integrated behavioral approach for individualizing the management of people with disabling CLBP [9]. Previous randomized clinical trials (RCTs) showed reductions in disability for the group submitted to CFT compared with manual therapy and exercise [10, 11] and with pain education and group-based exercise [12]. However, methodological shortcomings such as a high loss of follow-up of participants precluded confirmatory results. There are additional studies in progress [13, 14]; however, to the best of our knowledge, there is no previous study comparing CFT against a placebo treatment.
Previous findings have demonstrated that several therapies for CLBP are actually not superior or have only marginal efficacy compared with placebo for the management of CLBP in RCTs [15,16,17,18,19,20] or systematic reviews with meta-analysis [21]. In this way, it is important to conduct clinical trials comparing interventions commonly used in clinical practice against placebo ones.
The placebo could be defined as any therapy or component of therapy used for its nonspecific, psychological, or psychophysiological effect, or that is used for its presumed specific effect, but is without specific activity for the condition being treated [22]. In randomized placebo-controlled clinical trials, the placebo (term properly used in the context of pharmacological interventions) or sham (when the placebo mimics a procedure/surgery intervention) treatment arm is designed to capture and control for the nonspecific variables that can influence clinical outcomes [23]. These possible confounding could be regression to the mean, spontaneous remission, changes due to the natural course of the disease, Hawthorne effect, and placebo effects. In addition, the placebo control in a randomized trial enables the blinding of the patients and/or the investigators which facilitates outcome blinding [21, 24].
One can argue that for complex interventions such as CFT, the comparison with a sham procedure is not suitable. Consequently, the challenge is to deliver the same amount of fake therapeutic ingredients and patient-therapist interactions, which could be better called as a sham intervention. In such a scenario, it is compelling the importance of controlling for the intervention fidelity - the extent to which a behavioral intervention (active or sham) was designed, implemented, and received as intended [25, 26].
In this way, the aim of this study is to investigate the efficacy of CFT compared with a sham intervention (photobiomodulation using a detuned device + talking about neutral topics), for pain intensity and disability post-intervention in patients with CLBP. The secondary aims will be to investigate the effect of CFT for pain intensity and disability at 3- and 6-month follow-up, as well as the effect of the active treatment on self-efficacy, global perceived effect of improvement and functioning post-intervention and at 3- and 6-month follow-up.
Methods
Ethical considerations and protocol registration
The study was submitted to and approved by the ethics committee for research involving human subjects of the Ribeirão Preto Medical School (Ethics Committee Board from Centro Saúde Escola Cuaibá) of the University of São Paulo (CAAE: 30367320.4.0000.5414.). The study was registered prospectively on Clinical Trials (NCT04518891). The blinded assessor will clarify all details of the research and will obtain informed consent from all volunteers prior to allocation. No significant adverse reactions are anticipated in the study, but these will be monitored. The intervention is planned to be discontinued if there is withdrawal of participant consent.
Setting and participants
Study participants and eligibility criteria
This study will be a superiority RCT, sham-controlled, prospectively registered, two-arm with a blinded assessor. The sample will be comprised of 152 participants (both genders) with non-specific CLBP who will be referred to the physiotherapy outpatient clinic from Ribeirão Preto Medical School – University of São Paulo (Brazil), as well as community volunteers recruited through invitations announced by social media (Instagram and Facebook). Participants who met the following criteria will be considered eligible for the study: (1) aged between 18 and 60 years; (2) current episode of non-specific CLBP for at least 3 months of duration diagnosed by a general practitioner (different qualified physicians will be in charge of the diagnosis), that is located between T12 and the gluteal folds; (3) pain intensity equal to or greater than three on a numerical pain rating scale (NPRS); (4) score greater than 14% on the Oswestry Lumbar Disability Index [27]; and (5) fluent in Brazilian Portuguese.
Participants will be excluded due to the following conditions: (1) red flags (neoplastic diseases or tumors in the spine, inflammatory diseases, infections, and fractures); (2) serious neurological (or central and peripheral neurological) symptoms and psychiatric, rheumatologic, and cardiac diseases; (3) radiculopathy with symptoms (evidence of nerve root compromise tested by clinical neurological examination—identifying motor, reflex, or sensory abnormalities); (4) lumbar stenosis; (5) spondylolisthesis; (6) history of spinal surgeries; (7) pregnancy; (8) underwent other physical therapy treatments for low back pain or chronic pain in the last 6 months; and (9) illiterate people. Patients will be oriented to not use pain relief medications during the application of the interventions and during the one-month follow-up, they will be encouraged to register in a pain log the use of pain killers or rescue medications [28].
Procedures, randomization, and allocation
The study will follow the recommendations described on Consolidated Standards of Reporting Trials (CONSORT) statement [29], the current report followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) document [30], and the Template for Intervention Description and Replication (TIDieR) checklist for placebo and sham controls [31]. The description of interventions as recommended by TIDieR can be found in supplementary material 1.
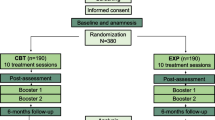
Patients will be informed that this study will involve a sham intervention arm (fake intervention in which an inert treatment will be provided but it still can result in positive outcomes due to its psychological effects) vs. an active treatment arm, but the nature of the sham will not be elucidated (equipment detuned). After this initial assessment, participants will be randomly assigned using block randomization by simple computerized procedures to one of the two treatment groups through the use of cards previously placed in opaque sealed envelopes: (i) CFT group or (ii) sham group. The allocation sequence will be generated by a researcher not involved in the assessment and interventions (TCC), and another research assistant will assign participants to interventions (Fig. 1). Each participant will be treated by a single physiotherapist, who will not be involved in the assessment of the patients. Participants will not have knowledge about treatment allocation. One blinded researcher regarding treatment group allocation will run the assessments pre-treatment, immediately after, and at the follow-ups.
Outcome measures
The primary outcomes in the current study will be pain intensity [32] and low back pain-related disability [27] collected post-intervention. The secondary outcomes will be pain intensity and low back pain-related disability at 3 and 6 months after randomization, as well as self-efficacy [33], global perceived effect of improvement [32], and functioning [32] post-intervention, 3, and 6 months after randomization. In the baseline, we will administer scales to assess moderating effect: expectation [34], depression [35], anxiety [36], kinesiofobia [37], and pain catastrophizing [38]. Mini-Mental State Examination (MMSE) [39] will be administered to assess the cognitive function of the volunteers. Patients with a score lower than 24 will be excluded [40]. The questionnaires will be administered in an assisted interview mode.
Primary outcomes
Pain intensity
Numeric pain rating scale (NPRS) will be used to assess pain intensity. The NPRS used in this trial will consist of numbers from 0 to 10, in which 0 represents “no pain” and 10 represents “worst pain imaginable” [32]. NPRS showed responsiveness to change, with a minimum clinically important difference (MCID) of 2 among patients with CLBP [41].
Low back pain-related disability
The Brazilian Portuguese version of the Oswestry Disability Index (ODI) [27] consists of 10 items, each of which has six response options. The total score will be calculated by summing up all the points, with the largest possible sum being 50. This sum will be transformed into a percentage (0 to 100). ODI showed responsiveness to change for patients with CLBP, with MCID of 10–12 points [41].
Secondary outcomes
Pain Self-Efficacy Questionnaire (PSEQ)
The PSEQ has 10 items related individual’s confidence to perform a certain task which are rated on a 7-point ordinal scale (ranging from 0: “not at all confident” to 6: “completely confident”). It was adapted and validated to Brazilian Portuguese [33].
Global Perceived Effect of improvement with treatment (GPE)
GPE used for this trial is an 11-point scale that ranges from −5 (“vastly worse”) through 0 (“no change”) to +5 (“completely recovered”) and participants are asked: “Compared to when this episode first started, how would you describe your back these days?” [32].
Patient-Specific Functional Scale (PSFS)
The PSFS using the average of three items scored from 0 (unable to perform) to 10 (able to perform at preinjury level) [32]. In the PSFS patients are asked to identify up to three important activities that they are having difficulties with or are unable to perform due to their condition. In addition, the patients are asked to rate, on an 11-point scale (ranging from 0 to 10) their current level of ability associated with each activity.
Baseline assessment
Standford ExpectationTreatment Scale (SETS)
The SETS is a scale with six items, three covering positive treatment-related expectations and three regarding negative treatment-related expectations [34]. The SETS is comprised of a seven-point Likert-type response scale that was chosen, varying from (1) “strongly disagree” to (7) “strongly agree.” An average of items 1, 3, and 5 yields the positive expectancy score, while an average of 2, 4, and 6 yields the negative expectancy score. This scale is under cross-cultural adaptation into Brazilian Portuguese.
Patient Health Questionnaire 9-item (PHQ-9)
The PHQ-9 is a nine-item questionnaire designed to screen for depression in primary care and other medical settings. They incorporate DSM-IV depression criteria with other leading major depressive symptoms into a brief self-report instrument. The standard cut-off score for screening to identify possible major depression is 10 or above. It was adapted and validated to Brazilian Portuguese [35].
The Generalized Anxiety Disorder 7-item scale (GAD7)
The GAD-7 [36] is a 7-item self-report measure of generalized anxiety symptoms grouped into one factor of generalized anxiety. Respondents score each item in a 4-point scale based on how often they have been bothered by the described symptoms over the last two weeks (not at all = 0; several days = 1; more than half the days = 2; nearly every day = 3). Total scores range from 0 to 21, with higher scores reflecting higher severity levels of anxiety. It was adapted and validated to Brazilian Portuguese [42].
The Tampa Scale for Kinesiophobia (TSK)
The Brazilian Portuguese version of TSK has 17 statements scored on 4-point scales from “strongly disagree” to “strongly agree,” yielding a total range from 17 to 68. Higher scores indicate more severe fear-avoidance beliefs. The Tampa Scale for Kinesiophobia was cross-culturally translated and validated into Brazilian Portuguese [37].
Pain Catastrophizing Scale (PCS)
The PCS translated and validated to Brazilian Portuguese [38] is composed of 13 items staggered on a Likert scale, ranging from 0 to 5 points. The total score is the sum of the items divided by the number of items answered. Higher scores indicated a greater presence of catastrophic thoughts. The total score of the scale could vary between 0 and 52 points [38].
Blinding
Despite it being a sham-controlled RCT, considering the differences in the interventions administered in both study arms, it will not be possible to blind the therapist and the patients. The assessor, as well as the participants, will not be aware of the arm of the study that he/she will be allocated. However, considering the different nature of the interventions, we cannot guarantee that the patients’ blinding will work. The blinding codes will be kept at the monitoring office of research and research ethics till the end of the trial unless an emergency developed which requires unblinding. At the 6-month follow-up participants will be asked if they received the sham or active intervention, as a mean to confirm the blinding.
Interventions
All the patients will receive a booklet with information regarding low back pain and advice on strategies of self-management [43]. The patients in the current study will be randomly allocated to receive one of two possible interventions: (1) CFT or (2) sham intervention. The same physiotherapist will deliver the interventions.
Patients in the sham intervention group will receive 6 sessions, lasting 45 min, once a week. Similarly, CFT treatment duration will vary from approximately 5 to 7 sessions [10, 12], once a week [44]. Patients will receive weekly reminders of the next sessions, as a strategy to improve adherence to intervention protocols. As a strategy to control for the treatment fidelity on both arms of the study, we will adopt the framework developed by the NIH Behavior Change Consortium (BCC) [45]. To control for the “Intervention Delivery,” the time of therapist-patient interaction, number of sessions, and the different components of the intervention administered, as well as video recordings along the trial, will be registered. Also, regular meetings to discuss the clinical cases will be performed. Furthermore, the “Intervention receipt” will be assessed in two different manners: (i) immediately post-intervention, each participant will be submitted to a manipulation check and they will be asked about the group they believe they were allocated, and (ii) patients in both groups will be invited to summarize at the beginning of each session how the treatment impacts their lives.
Sham intervention
Patients allocated to this group will receive two interventions: sham photobiomodulation + neutral talking. Patients will be treated with detuned photobiomodulation device (904Nm Ibramed Infrared – no-visible beam), without any emission of therapeutic dose (0J). The points for applying fake stimulation are described in the Fig. 2. Three minutes of fake stimulation will be administered, summing up to 27 min [19].
Sham photobiomodulation application sites: nine sites will be applicated on the patient’s lumbar region: three central sites on top of the spinous processes (between T11 and T12, L2 and L3, L5 and S1); in the same direction, but laterally, three sites on the left and three on the right (on the paravertebral muscles), without any emission of therapeutic dose
The photobiomodulation will be adopted in the current study because of the absence of sensory perceptions during the application. The device will be used with the internal cables disconnected, however, it will be possible to handle it and adjust doses and alarms as if they were simulating a real clinical situation as well as to increase the credibility. Sham intervention has been used in previous trials with patients with CLBP [15, 16]. A physiotherapist with 5 years of clinical experience will administer this intervention.
In addition, a neutral talking control therapy of at least 15 min will be provided to patients in each session [46]. Maladaptive beliefs will not be challenged; however, the therapists will be trained to show interest and warmth, empathy, and encouraging participants to discuss neutral topics such as hobbies, sports, and current affairs. No advice or problem solving will be given, and any attempt to talk about emotional issues will be kindly discouraged and the talking will be redirected to neutral tropics. For example, if the patient says, “I guess I’ll never play soccer again because of my back pain”, the therapist will be trained to ask, “Do you like soccer? Did you watch the game on TV last week?”
After finishing the 6-month follow-up, all the participants will be invited to receive the CFT intervention.
Cognitive Functional Therapy (CFT) Group
The specific training for the CFT intervention involved the following: (1) submit the treating physiotherapist with a training of at least 106 h of CFT by an experienced CFT tutor (NAMMF) and (2) videos of the physiotherapist (MRL) administering the CFT approach will be analyzed by a certified physiotherapist. The physiotherapist responsible to deliver CFT has a clinical experience of 5 years.
CFT is underpinned by a strong therapeutic alliance, active listening, motivational interviewing style (open, non-judgmental, reflective), providing validation, and using strategies to establish rapport. A customized progressive self-management program will be provided tailored to the individual’s valued goals, directed at changing cognitive, movement, and lifestyle behaviors considered provocative or aggravating of their condition.
The CFT intervention has three broad components [44]:
-
1.
Making sense of pain: a reflective process that combines the person’s own narrative (interview) and experience to develop a customized relevant, multidimensional understanding of pain for the patient. In this process, maladaptive beliefs related to a vicious cycle of persistent pain and disability are discouraged.
-
2.
Exposure with “control”: The exposure with control will be directed to activities reported by patients as painful, feared or avoided like: bend the trunk forward to catch an object on the floor or to keep in a sitting position. The patient will be invited to confront such activities.
-
3.
Lifestyle change: physical activity and lifestyle advice will include an invitation to gradually increase physical activity levels based on their preference and presentation, advice on sleep hygiene, stress management strategies, and social re-engagement behavioral modification.
Statistical analysis
All randomized patients will be analyzed on an intention-to-treat basis. Baseline characteristics will be presented by the treatment group. Binary and categorical variables will be summarized by frequencies and percentages. The between-group differences (effects of treatment) and their respective 95% CIs will be calculated by constructing linear mixed models. Treatment effect for the primary and continuous secondary outcomes will be estimated using mixed linear models, considering the correlation within the individual. The mixed linear model will include random intercept adjusted with the baseline score, time as categorical, and the interaction between treatment and time. We will use strategies to reduce the attrition rate during follow-ups as telephone calls and an active search of information.
Two primary outcome variables were considered for the calculation of sample size. For pain intensity, an improvement of at least 1-point on the NPRS (SD=1) and a mean difference of at least 5 points for the ODI score (SD=10). The following specifications were considered: α=5%, 80% statistical power, effect size of 0.27 for F-test, and a follow-up attrition rate of 15%. The final sample size will be comprised of 152 patients (76 per group) (G*Power©, University of Dusseldorf, Germany). A moderator effect of baseline variables including the expectation to treatment will be calculated using regression analysis and structural equation modeling (path analysis) (AMOS (SPSS, v.22). Data will be coded and entered into the Statistical Package for the Social Sciences (SPSS), version 22 (Chicago, IL, IBM corp., USA) program for analysis and the significance level will be established at 0.05. The Bonferroni correction will be used to adjust the p-values for multiple primary outcomes [47]. The data collected will be stored and coded to protect patient confidentiality. In order to handle missing data, we will replace the value with the last observation carried forward. The researchers involved in the study will have access to the final trial dataset and, upon request, it can be shared for the purposes of results conference and publication. The results of the study will be disseminated to interested parties through publications in an appropriate journal.
Discussion
The pain and disability related to CLBP may compromise many aspects of individual daily life. Non-specific CLBP is best seen as neurobiological and behavioral responses to individuals’ actual and/or perceived threat to their body, lifestyle, or social circumstances and/ or disruption to their homeostasis. In such a scenario, CFT was developed to consider these interacting processes involved in CLBP which demands a flexible multidimensional clinical reasoning framework [9].
Despite its promising approach centered on the biopsychosocial model, there are few RCTs available in the literature [10,11,12], and the majority are conducted always by the same researcher group, noteworthy are the limitations of the first trials [48, 49]. The RCTs previously published [10,11,12] showed high attrition rates during follow-up (greater than 24%), failed to follow the intention-to-treat principles, and to blind assessors involved in the follow-up assessments.
To the best of our knowledge, the current study will be the first to compare CFT vs. sham intervention. Sham-controlled RCTs may help to understand the influence of non-specific factors on treatment outcomes. Considering complex interventions as CFT, it is imperative to understand the impact of contextual factors on outcomes.
Trial status
Protocol: The study was registered prospectively on ClinicalTrials.gov (Protocol Number: NCT04518891). Last Update Posted: June 18, 2021.
Date recruitment began: May 17, 2021
Approximate date when recruitment will be completed: July 17, 2023
Availability of data and materials
The datasets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Abbreviations
- CFT:
-
Cognitive functional therapy
- CLBP:
-
Chronic low back pain
- RCTs:
-
Randomized clinical trials
- NPRS:
-
Numerical pain rating scale
- CONSORT:
-
Consolidated Standards of Reporting Trials
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
- TIDieR:
-
Template for Intervention Description and Replication
- MCDID:
-
Minimum clinically important difference
- ODI:
-
Oswestry Disability Index
- PSEQ:
-
Pain Self-Efficacy Questionnaire
- GPE:
-
Global Perceived Effect of improvement with treatment
- STES:
-
Standford ExpectationTreatment Scale
- PHQ-9:
-
Patient Health Questionnaire 9-item
- TKS:
-
The Tampa Scale for Kinesiophobia
- PCS:
-
Pain catastrophizing scale
- SPSS:
-
Statistical Package for the Social Sciences
- SD:
-
Standard deviation
References
Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–67. https://doi.org/10.1016/S0140-6736(18)30480-X Epub 2018 Mar 21.
Hoy D, Bain C, Williams L, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–37. https://doi.org/10.1002/art.34347 Epub 2012 Jan 9.
Hoy D, March L, Brooks P, et al. Measuring the global burden of low back pain. Best Pract Res Clin Rheumatol. 2010;24(2):155–65. https://doi.org/10.1016/j.berh.2009.11.002.
Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. https://doi.org/10.1016/S0140-6736(16)31678-6.
Michaleff ZA, Kamper SJ, Maher CG. Low back pain in children and adolescents: a systematic review and meta-analysis evaluating the effectiveness of conservative interventions. Eur Spine J. 2014;23(10):2046–58. https://doi.org/10.1007/s00586-014-3461-1 Epub 2014 Jul 29.
Qaseem A, Wilt TJ, McLean RM. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–30. https://doi.org/10.7326/M16-2367 Epub 2017 Feb 14.
Stochkendahl MJ, Kjaer P, Hartvigsen J. National Clinical Guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J. 2018;27(1):60–75. https://doi.org/10.1007/s00586-017-5099-2 Epub 2017 Apr 20.
O'Sullivan P. It's time for change with the management of non-specific chronic low back pain. Br J Sports Med. 2012;46(4):224–7. https://doi.org/10.1136/bjsm.2010.081638 Epub 2011 Aug 4.
O'Sullivan PB, Caneiro JP, O'Keeffe M. Cognitive Functional Therapy: An Integrated Behavioral Approach for the Targeted Management of Disabling Low Back Pain. Phys Ther. 2018;98(5):408–23. https://doi.org/10.1093/ptj/pzy022.
Fersum VK, O'Sullivan P, Skouen JS, Smith A, Kvåle A. Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: a randomized controlled trial. Eur J Pain. 2013;17(6):916–28. https://doi.org/10.1002/j.1532-2149.2012.00252.x Epub 2012 Dec 4.
Fersum VK, Smith A, Kvåle A, Skouen JS, O'Sullivan P. Cognitive functional therapy in patients with non-specific chronic low back pain-a randomized controlled trial 3-year follow-up. Eur J Pain. 2019. https://doi.org/10.1002/ejp.1399.
O'Keeffe M, O'Sullivan P, Purtill H, Bargary, O'Sullivan K. Cognitive functional therapy compared with a group-based exercise and education intervention for chronic low back pain: a multicentre randomised controlled trial (RCT). Br J Sports Med. 2019. https://doi.org/10.1136/bjsports-2019-100780.
Belache FTC, Souza CP, Fernandez J, et al. Trial Protocol: cognitive functional therapy compared with combined manual therapy and motor control exercise for people with non-specific chronic low back pain: protocol for a randomised, controlled trial. J Physiother. 2018;64(3):192. https://doi.org/10.1016/j.jphys.2018.02.018 Epub 2018 Jun 11.
Kent P, O'Sullivan P, Smith A, et al. RESTORE-Cognitive functional therapy with or without movement sensor biofeedback versus usual care for chronic, disabling low back pain: study protocol for a randomised controlled trial. BMJ Open. 2019;9(8):e031133. https://doi.org/10.1136/bmjopen-2019-031133.
Garcia AN, Costa LDCM, Hancock MJ, et al. McKenzie Method of Mechanical Diagnosis and Therapy was slightly more effective than placebo for pain, but not for disability, in patients with chronic non-specific low back pain: a randomised placebo-controlled trial with short and longer term follow-up. Br J Sports Med. 2018;52(9):594–600. https://doi.org/10.1136/bjsports-2016-097327.
Costa LO, Maher CG, Latimer J, et al. Motor control exercise for chronic low back pain: a randomized placebo-controlled trial. Phys Ther. 2009;89:1275–86. https://doi.org/10.2522/ptj.20090218.
Kumar SP. Efficacy of segmental stabilization exercise for lumbar segmental instability in patients with mechanical low back pain: a randomized placebo-controlled crossover study. N Am J Med Sci. 2011;3:456–61. https://doi.org/10.4297/najms.2011.3456.
Balthazard de GP, Goumoens P, Rivier G, Demeulenaere P, Ballabeni P, Dériaz O. Manual therapy followed by specific active exercises versus a placebo followed by specific active exercises on the improvement of functional disability in patients with chronic non-specific low back pain: a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:162. https://doi.org/10.1186/1471-2474-13-162.
Tomazoni SS, Costa LDCM, Guimarães LS, et al. Effects of photobiomodulation therapy in patients with chronic non-specific low back pain: protocol for a randomised placebo-controlled trial. BMJ Open. 2017;7(10):e017202. https://doi.org/10.1097/MD.0000000000015177.
Alzayed KA, Alsaadi SM. Efficacy of pulsed low-frequency magnetic field therapy on patients with chronic low back pain: a randomized double-blind placebo-controlled trial. Asian Spine J. 2019. https://doi.org/10.31616/asj.2019.0043.
Machado LA, Kamper SJ, Herbert RD, Maher CG, McAuley JH. Analgesic effects of treatments for non-specific low back pain: a meta-analysis of placebo-controlled randomized trials. Rheumatology (Oxford). 2009;48(5):520–7. https://doi.org/10.1093/rheumatology/ken470.
Linde K, Fässler M, Meissner K. Placebo interventions, placebo effects and clinical practice. Philos Trans R Soc Lond Ser B Biol Sci. 2011. https://doi.org/10.1098/rstb.2010.0383.
Hall KT, Loscalzo J. Drug-placebo additivity in randomized clinical trials. Clin Pharmacol Ther. 2019;106(6):1191–7. https://doi.org/10.1002/cpt.1626.
Vickers AJ, de Craen AJ. Why use placebos in clinical trials? A narrative review of the methodological literature. J Clin Epidemiol. 2000;53(2):157–61. https://doi.org/10.1016/s0895-4356(99)00139-0.
Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443. https://doi.org/10.1037/0278-6133.23.5.443.
Lambert JD, Greaves CJ, Farrand P, Cross R, Haase AM, Taylor AH. Assessment of fidelity in individual level behaviour change interventions promoting physical activity among adults: a systematic review. BMC Public Health. 2017;17(1):765. Published 2017 Oct 2. https://doi.org/10.1186/s12889-017-4778-6.
Vigatto R, Alexandre NMC, Correa-Filho HR. Development of a brazilian portuguese version of the oswestry disability index: cross-cultural adaptation, reliability and validity. Spine. 2007;32(4):481–6. https://doi.org/10.1097/01.brs.0000255075.11496.47.
Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. https://doi.org/10.1016/j.pain.2004.09.012.
Moher D, Hopewell S, Schulz KF, CONSORT, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. Int J Surg. 2012;10(1):28–55. https://doi.org/10.1136/bmj.c869.
Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7. https://doi.org/10.7326/0003-4819-158-3-201302050-00583.
Howick J, Webster RK, Rees JL, et al. TIDieR-Placebo: A guide and checklist for reporting placebo and sham controls. PLoS Med. 2020;17(9):e1003294.
Costa LO, Maher CG, Latimer J, et al. Clinimetric testing of three self-report outcome measures for low back pain patients in Brazil: which one is the best? Spine (Phila Pa 1976). 2008;33(22):2459–63. https://doi.org/10.1097/BRS.0b013e3181849dbe.
Sardá J Jr, Nicholas MK, Pimenta CAM, Asghari A. Pain-related self-efficacy beliefs in a Brazilian chronic pain patient sample: psychometric analysis. Stress Health. 2007;23(3):185–90. https://doi.org/10.1002/smi.1135.
Younger J, Gandhi V, Hubbard E, Mackey S. Development of the Stanford Expectations of Treatment Scale (SETS): a tool for measuring patient outcome expectancy in clinical trials. Clin Trials. 2012;9:767–76. https://doi.org/10.1177/1740774512465064.
Osório FL, Mendes AV, Crippa JA, Loureiro SR. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspect Psychiatr Care. 2009;45:216–27. https://doi.org/10.1111/j.1744-6163.2009.00224.x.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. JAMA Intern Med. 2006;166:1092–7. https://doi.org/10.1001/archinte.166.10.1092.
de Souza FS, Marinho Cda S, Siqueira FB, Maher CG, Costa LO. Psychometric testing confirms that the Brazilian-Portuguese adaptations, the original versions of the Fear-Avoidance Beliefs Questionnaire, and the Tampa Scale of Kinesiophobia have similar measurement properties. Spine (Phila Pa 1976). 2008;33(9):1028–33. https://doi.org/10.1097/BRS.0b013e31816c8329.
Sehn F, Chachamovich E, Vidor LP, et al. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the pain catastrophizing scale. Pain Med. 2012;13(11):1425–35. https://doi.org/10.1111/j.1526-4637.2012.01492.x Epub 2012 Oct 4.
de Melo DM, Barbosa AJG, Castro NR, Neri AL. Mini-mental state examination in Brazil: an item response theory analysis. Psychol Eval. 2020;30. https://doi.org/10.1590/1982-4327e3014.
Brucki SMD, Nitrini R, Caramelli P, et al. Sugestões para o uso do Mini-Exame do Estado Mental no Brasil. Arqui Neuropsiquiatr. 2003;61:777–781B. https://doi.org/10.1590/S0004-282X2003000500014.
Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90–4. https://doi.org/10.1097/BRS.0b013e31815e3a10.
Moreno AL, DeSousa D, Souza AMFLP, et al. Factor structure, reliability, and item parameters of the brazilian-portuguese version of the GAD-7 questionnaire. Temas Psicol. 2016;24(1):367–76. https://doi.org/10.9788/TP2016.1-25.
Jenkins HJ, Moloney NA, French SD, et al. Using behaviour change theory and preliminary testing to develop an implementation intervention to reduce imaging for low back pain. BMC Health Serv Res. 2018;18(1):734. https://doi.org/10.1186/s12913-018-3526-7.
O'Keeffe M, Purtill H, Kennedy N, O'Sullivan P, et al. Individualised cognitive functional therapy compared with a combined exercise and pain education class for patients with non-specific chronic low back pain: study protocol for a multicentre randomised controlled trial. BMJ Open. 2015;5(6):e007156. https://doi.org/10.1136/bmjopen-2014-007156.
Borrelli B, Sepinwall D, Ernst D, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol. 2005;73(5):852–60. https://doi.org/10.1037/0022-006X.73.5.852.
Serfaty MA, Haworth D, Blanchard M, Buszewicz M, Murad S, King M. Clinical effectiveness of individual cognitive behavioral therapy for depressed older people in primary care: a randomized controlled trial. Arch Gen Psychiatry. 2009;66(12):1332–40. https://doi.org/10.1001/archgenpsychiatry.2009.165.
Vickerstaff V, Ambler G, King M, Nazareth I, Omar RZ. Are multiple primary outcomes analysed appropriately in randomised controlled trials? A review. Contemp Clin Trials. 2015;45(Pt A):8–12.
George SZ. Commentary on “Cognitive Functional Therapy in Patients with Non-Specific Chronic Low Back Pain”. Eur J Pain. 2019. https://doi.org/10.1002/ejp.1434.
Fernandez J, Ferreira AS, Castro J, Correia LCL, Meziat-Filho N. Comment on the paper “Cognitive functional therapy in patients with non specific chronic low back pain”, by Vibe Fersum et al. Eur J Pain. 2019. https://doi.org/10.1002/ejp.1441.
Acknowledgements
Not applicable.
Data sharing statement
Trying to publish protocol, and protocol available at Clinical Trials.
Funding
No funding to declare.
Author information
Authors and Affiliations
Contributions
TCC and MRL developed the central idea and written the protocol. MRL and GZMS are participating in data collection. NAMMF provided CFT training and reviewed the paper. All authors read and approved the final manuscript. Authorship eligibility guideline will involve the principal investigators, according to the level of contribution. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the local ethics committee of the Centro de Saúde Escola Cuiabá from Ribeirão Preto School of Medicine – University of São Paulo (process number: CAAE: 30367320.4.0000.5414.). A written informed consent will be provided from each patient.
Consent for publication
Not applicable.
Competing interests
Not applicable. Prof Ney Meziat runs private workshops on CFT for chronic back pain. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary material 1.
Description of the active treatment and sham control of the current study according to TIDieR (Template for Intervention Description and Replication)-Placebo.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Lira, M.R., de Mello Meziat-Filho, N.A., Silva, G.Z.M. et al. Efficacy of the cognitive functional therapy (CFT) in patients with chronic nonspecific low back pain: a study protocol for a randomized sham-controlled trial. Trials 23, 544 (2022). https://doi.org/10.1186/s13063-022-06466-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06466-8