Abstract
Background
The trial investigates the efficacy of internet-based cognitive behavioral therapy (iCBT) in improving health-related QoL in patients with endometriosis, which is a chronic gynecological condition affecting up to 15% of people with female-assigned reproductive organs. Endometriosis is stress-related and comes with various physical symptoms such as pelvic pain and infertility. It has a substantial impact on health-related quality of life (QoL), and mind-body interventions seem promising in reducing the psychological burden.
Methods
This is a monocentric randomized-controlled trial recruiting 120 patients with endometriosis. The intervention consists of eight iCBT modules focusing on psychoeducation, cognitive restructuring, pacing, and emotion regulation. Participants will receive written feedback from a trained therapist weekly. The comparator is a waitlist control group. All participants will be followed up 3 months after the intervention, and the intervention group will additionally be followed up 12 months after the intervention. Trial participants will not be blinded to the allocated trial arm. Primary outcome measures are endometriosis-related QoL, pain, and pain-related disability. Secondary outcomes include coping, illness representations, and psychological flexibility. Statistical analyses will be performed following intention-to-treat principles.
Discussion
This randomized-controlled trial is the first trial to test the efficacy of iCBT for improving endometriosis-related QoL. Potential predictor variables and key mechanisms in treatment will be investigated to enable further progression in medical and psychological care for patients with endometriosis.
Trial registration
ClinicalTrials.gov, NCT05098444 Registered on October 28, 2021
Similar content being viewed by others
Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
Title {1} | Internet-based Cognitive Behavioral Therapy for Improving Health-Related Quality of Life in Patients with Endometriosis: Study Protocol for a Randomized Controlled Trial. |
Trial registration {2a and 2b}. | ClinicalTrials.gov, Identifier NCT05098444. Date of registration: 10/28/2021 |
Protocol version {3} | First version (dated 11/11/2021) |
Funding {4} | Philipps-University Marburg, Marburg, Germany. This trial is non-commercial. We received a local grant called UMRvernetzt from Philipps-University Marburg, which is set up to encourage interdisciplinary exchange. The grant went to the construction of the iCBT platform. There is no external funding for this study. |
Author details {5a} | 1, 2, 5 Philipps-University Marburg, Dept. of Psychology, Division of Clinical Psychology and Psychotherapy, Marburg, Germany 3, 4 Clinic for Gynecology and Obstetrics, University Hospital of Giessen and Marburg (UKGM), Marburg, Germany. |
Name and contact information for the trial sponsor {5b} | Trial Sponsor: UMR 2027, Philipps-University Marburg. Address: UMR 2027, Biegenstraße 36, 35032 Marburg, Germany. Email: interaktion@uni-marburg.de |
Role of sponsor {5c} | The funder has no role in study design, analysis, interpretation, or publication of the study protocol and trial results. |
Introduction
Background and rationale {6a}
Background
Endometriosis, defined by the presence of endometrial-like tissue outside the uterine cavity, is an inflammatory, chronic disease often associated with pelvic pain [1], infertility [2], and reduced quality of life (QoL) [3]. Prevalence of 5% up to 15% in women of reproductive age has been reported [4,5,6]. The societal and economic burden of endometriosis is severe, with high costs for medical treatment, reduced productivity at work [7], and high rates of absence from work [8]. The diagnosis of endometriosis is obtained only through laparoscopic surgery and histological confirmation. Due to a wide array of symptoms (both cyclic and acyclic [9]) and lack of awareness [10], practitioners often experience difficulties in diagnosing endometriosis correctly and in a timely manner [11]. This can lead to diagnostic delays of up to 10 years [12, 13] and prevents patients from receiving adequate treatment, adding further distress to the experienced psychological burden. Endometriosis is associated with symptoms of depression, anxiety [14, 15], and heightened feelings of uncertainty [16], leading to substantial reduction [3] in health-related quality of life (HRQoL). Dyspareunia, infertility, or decreased general ability to cope with everyday life [17] might put a strain especially on intimate relationships [18], impairing relationship satisfaction [19], and adding to the social burden of endometriosis [20]. The presentation of physical symptoms seems consistent across populations with different cultural backgrounds and geographical locations [7, 21]. However, HRQoL might be further reduced by culturally specific societal expectations, with, for example, women of Arab ancestry in the Middle Eastern region experiencing greater distress when suffering from infertility [22].
The exact etiology and pathogenesis of endometriosis remain unclear, although some biological and psychosomatic mechanisms and circuits involved in the progression of endometriosis have been identified: endometriosis is stress-related, with symptoms of endometriosis causing an increase in perceived psychological stress [23, 24] as well as high levels of chronic stress promoting overregulation of the hypothalamic–pituitary–adrenal (HPA) axis [25], leading to chronic inflammatory dysregulation and further growth of endometriotic lesions [26]. Chronic inflammation contributes to enhanced sensibility of nociceptive afferents [27], supporting peripheral and central sensitization processes [28] in regard to chronic pelvic pain (CPP) as one of the cardinal symptoms [1]. Overall, the complex pathogenesis might contribute to the existing knowledge gap regarding the precise mental health sequelae of endometriosis and unsubstantiated presumptions about the causality between physical and psychological symptoms [29].
There is no remedial treatment for patients with endometriosis available: it is a chronic disease with high recurrence rates after surgical or pharmacological treatment. Medical treatment of endometriosis is symptom-based, often focused on pain relief and treating infertility [30]. Surgical treatment is directed at ablating or excising endometriotic tissue. Pharmacological treatment is often directed at either symptomatic relief of pain through the use of analgesics (e.g., non-steroidal anti-inflammatory drugs) or at reducing circulating estrogen to a constantly lower level [31], leading to reduced ovarian function, less pain and inhibition in further growth of endometriotic tissue [32].
Given the high impact of endometriosis on HRQoL and the immediate influence of high psychological distress on its pathogenesis, developing complementary, non-medical treatment options for stress reduction and better psychological coping seems sensible. Diverse approaches have been tested over the last few decades. Different mind-body interventions for improving HRQoL in patients with endometriosis have been proven effective so far, including yoga [33], progressive muscle relaxation [33], and Traditional Chinese Medicine–based acupuncture combined with psychological consultations [35]. Kold, Hansen, Vedsted-Hansen, and Forman [36] conducted a prospective pilot single-arm trial, showing that mindfulness-based psychotherapy can lastingly improve HRQoL in patients with endometriosis. Yet overall, few studies have examined the effects of psychological and mind-body-interventions, most of them with limiting conclusions about efficacy due to rather low methodological quality (see [37, 38] for an overview).
Study rationale
There is an ongoing need for an evaluated, standardized, effective intervention for improving QoL in patients with endometriosis [39], although the exact interaction between physical and psychological symptoms is still to be further investigated. CBT teaches restructuring of unhelpful thoughts and core beliefs as well as behavioral activation and relaxation, leading to greater self-efficacy and enabling patients to display improved health behavior [40]. There is strong evidence for the effectiveness of CBT in treatment of chronic pain [41] and for stress reduction [42]. With perceived stress perpetuating experienced mental health sequelae and CPP as a cardinal symptom, we believe that the implementation CBT might be of great value for patients with endometriosis. Its efficacy in easing the burden of other disorders in women’s health (e.g., premenstrual dysphoric disorder [PMDD] [43]) further supports the implementation of CBT in treatment of endometriosis. Additionally, patients with endometriosis supported the implementation of CBT when informed about possible benefits [44]. However, to our knowledge, no study has yet investigated the effect of CBT in improving HRQoL in patients with endometriosis. We want to address this gap by testing the efficacy of CBT in improving HRQoL and examining potential moderators for new insights on key mechanisms in treatment.
An internet-based approach offers better accessibility alongside with reduced stigma associated with seeing a psychologist. Especially in patients with endometriosis, fear of not being taken seriously by a medical provider, fear of stigmatization, or disappointment in former practitioner-patient-relationships might otherwise hinder them from seeking psychological treatment [45]. We therefore decided to develop an internet-based CBT (iCBT) program. Such programs have previously been proven effective in the treatment of psychological [46] as well as psychosomatic [47] and somatopsychic [48] disorders and are considered as effective as face-to-face-therapy [49, 50]. We hypothesize that endometriosis-related QoL will be significantly improved when patients take part in our iCBT training.
Objectives {7}
The trial objectives are:
-
To test the efficacy of iCBT in improving HRQoL in patients with endometriosis
-
To investigate potential predictor and mediator/moderator variables of treatment outcomes
-
To improve medical and psychological care for patients with endometriosis, whose symptoms are often dismissed by medical providers
-
To support further development of research within the field of women’s mental health with a clear focus on little-researched disorders relevant to people with female-assigned reproductive systems
-
To foster the availability of online-interventions as a way of reducing barriers to appropriate care for patients with stigmatized diagnoses
Trial design {8}
This is a randomized-controlled, two-armed, superiority trial testing the efficacy of an 8-week iCBT training in improving HRQoL in patients with endometriosis against a waitlist control group. Randomization will be balanced with a 1:1 ratio for patient allocation to the intervention group and waitlist control group. Identifying potential moderators and mediators of treatment outcome will also be a component of this trial.
Methods: participants, interventions, and outcomes
Study setting {9}
Patients with endometriosis will be recruited in Germany, and participants will access iCBT using their own technical devices (e.g., notebook, PC).
Eligibility criteria {10}
The inclusion criteria are:
-
Endometriosis diagnosed through laparoscopic surgery
-
Age between 18 and 45
-
Reduced HRQoL assessed with EHP-30 + 23 [52]
-
Sufficient German language skills
-
Access to an appropriate technical device (PC, laptop) with a stable internet connection
The exclusion criteria are:
-
Current diagnosis of one of the following mental illnesses: unipolar severe depression, acute suicidal tendencies, bipolar affective disorder, unipolar manic episodes, psychotic disorder, alcohol or substance use disorder (assessed using the Web-Based Screening Questionnaire (WSQ) [53], and Beck Depression Inventory–II (BDI-II) [54]
-
Receiving CBT treatment, current or in the past 2 years
-
Regular intake of benzodiazepines, changes in intake (e.g., start/change in dosage/discontinuation) of antidepressants or hormonal contraceptives within the last 3 months
-
Current or planned IUI, IVF, or ICSI treatment with hormonal stimulation within the next 8 months
-
Current pregnancy or birth of a child within the last 6 months, breastfeeding within the last 6 months
-
Current diagnosis of one of the following: malignant tumor, esp. breast cancer, cervical cancer, or ovarian cancer; ulcerative colitis; Crohn’s disease; or any bacterial or viral infection such as TC, HAV, HBV, or HCV
Who will take informed consent? {26a}
Informed consent will be obtained through local researchers experienced in working with patients with endometriosis. Potential trial participants receive detailed written information about trial scope, time schedule, randomization process, and so forth. During an extensive discussion of general trial benefits and risks, as well as use of trial participation via telephone, potential participants will be given the chance to address individual questions and concerns. Afterwards, participants will be asked to send written informed consent within the next few days to ensure that consent is given voluntarily without situational pressure.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
After trial completion, participants will be asked if they agree to be contacted in the future about further research participation. No collection of biological specimens is planned.
Interventions
Explanation for the choice of comparators {6b}
The comparator is a waitlist control. Patients in the waitlist control group can continue with their usual treatment plan during trial participation (e.g., medication or physiotherapy). Because there is, as yet, no established treatment as usual (TAU) for patients with endometriosis, using a waitlist control seemed reasonable to capture real-life conditions. Patients currently receiving psychotherapy or seeking psychotherapeutic treatment in the past 2 years will be excluded from trial participation.
Intervention description {11a}
The online intervention lasts 8 weeks with one assigned interactive module per week, each of which consists of psychoeducational information, exercises, and homework. Different formats, methods, and media (e.g., infographics, videos, audio files, and text) will be used to convey relevant knowledge and strategies. For greater vividness, four fictional personas are introduced in the beginning; their answers and thoughts on certain exercises are displayed as encouraging examples. The intervention content was developed with helpful support from researchers with different scientific backgrounds and expertise in psychology, medicine, and gender studies. Additionally, the first three modules were tested by five endometriosis patients interested in iCBT. They were interviewed about their experience and provided valuable feedback on usability, psychoeducational comprehensiveness, and overall benefit.
Participants generate an individual biopsychosocial model about the impact of endometriosis on different areas of life and get to learn about cognitive restructuring. Relations between stress and pain are explored, and participants are encouraged to try out different pacing and relaxation methods. Acceptance and regulation of difficult emotions are practiced, as well as communication of healthy boundaries in different social settings. The last module focuses on reflection and long-term maintenance of achieved progression. Table 1 describes the intervention content in detail.
Participants are asked to spend 1 to 2 h on each module. After each week, participants receive written feedback from a trained psychologist based on their input into the treatment platform. The participants can ask questions regarding the module they have been working on via text messages to their psychologist on the online platform. The psychologist is at least a bachelor-level clinical psychologist and receives regular supervision by a licensed CBT therapist.
Criteria for discontinuing or modifying allocated interventions {11b}
There are no criteria for discontinuing or modifying the intervention. Participants may withdraw from voluntary trial participation at any point for any given reason. Withdrawal from participation has no negative consequences or disadvantages for the participant. The investigators might also terminate participation if severe adverse events arise during the intervention.
Strategies to improve adherence to interventions {11c}
Adherence (e.g., taking part in the online intervention weekly) is encouraged through weekly contact with trained psychologists; strategies may include encouragement as well as setting specific goals and a specific date for working on the weekly modules or for certain exercises.
Relevant concomitant care permitted or prohibited during the trial {11d}
Any treatment or change in treatment with direct influence on trial outcomes is permitted during the trial as defined in the exclusion criteria (see {10}).
Provisions for post-trial care {30}
No special provisions are offered.
Outcomes {12}
Primary outcome measures
The primary outcomes are improvements in endometrioses-related QoL and pain during menstruation and across the cycle, as well as pain-related disability in the time between baseline and follow-up. Greater improvements are expected in the intervention group compared to the control group. Endometriosis-related QoL will be assessed using the Endometriosis Health Profile-30 + 23 (EHP-30+23) [55], which measures endometriosis-related burden across different domains of everyday life considered relevant to patients with endometriosis. The core questionnaire of 30 items is complemented by a 23-item modular questionnaire with six subscales (work life, relationship with children, sexual intercourse, medical profession, treatment, and infertility). Items are rated on a Likert scale ranging from 0 to 4. A score ranging from 0 to 100 is calculated for the core questionnaire as well as for each modular subscale, with 0 indicating the best possible quality of life.
Pain will be measured using visual analog scales. Pain-related disability will be assessed using the Pain Disability Index (PDI) [56], a 7-item questionnaire measuring the degree of disruption in different aspects of everyday life caused by chronic pain (e.g., family/home responsibilities, recreation). Each item is rated on an 11-point Likert Scale (0–10). Raw scores for all items are added together, with higher scores indicating higher degrees of disruption caused by chronic pain. Primary outcomes will be assessed at baseline, post-treatment, and at 3 months past treatment, as well as at 12 months past treatment for the intervention group.
Secondary outcome measures
All secondary outcome measures will be assessed at the same points of measurement as the primary outcome measures.
Depressive mood
Endometriosis is often associated with a wide array of depressive symptoms. Depressive mood is assessed using the Patient Health Questionnaire-9 (PHQ-9) [57], a 9-item depression subscale from the Patient Health Questionnaire which has reliable sensitivity to change.
Perceived stress
As described in {6a}, endometriosis and its impact on HRQoL are closely linked to perceived stress. The perception of psychological stress (e.g., the extent to which participants experience themselves not being able to cope with their situation) will be measured using the Perceived Stress Scale-10 (PSS-10) [58].
Coping ability
General abilities in coping with difficulties and challenging situations play an important role in adapting to life with a chronic illness. The improvement of adaptive coping skills can reduce perceived stress [59]. The Brief COPE [60] is a 28-item measurement that captures various facets of coping using the subscales of problem-focused, emotion-focused, and avoidant coping.
Cognitive illness representations
Individuals suffering from chronic illness develop a set of cognitive representations about their disease, its cause and impact, and their ability to control progression. These representations form health-related behavior and are assessed using the Illness Perception Questionnaire Revised (IPQ-R) [61].
Psychological flexibility
The construct of psychological flexibility derives from research about therapeutic processes in Acceptance and Commitment Therapy [62]. Although CBT is tested in this trial, a shift from experiential avoidance of negative inner experience (e.g., unpleasant thoughts and emotions) toward a more accepting approach seems helpful in living with a chronic illness. Therefore, psychological flexibility will be captured with the 7-item Acceptance and Action Questionnaire-II (AAQ-II) [63].
Control variables
The control variables will be assessed at baseline only.
Relationship satisfaction
Living with endometriosis can have a negative impact on relations with others, and especially on committed relationships with romantic partners. Endometriosis leaves couples to deal with many different difficulties such as reduced ability to cope with stressors, infertility, and sexual distress [64]. At the same time, a functional, healthy relationship can be a source of continuous support and may improve resilience in living with the disease [44]. Relationship satisfaction will be assessed using the 7-item Relationship Assessment Scale (RAS) [65].
Personality traits
To determine the role of personality traits as potential moderators of treatment outcome, the Big Five Inventory-10 (BFI-10) [66] will be used to capture extraversion, agreeableness, conscientiousness, emotional stability, and openness.
Process evaluation
To evaluate the planned CBT treatment and for identification of elements to focus on for further improvement, the participants are asked to provide weekly feedback on the module. We will therefore monitor symptoms and impact on HRQoL weekly throughout the training and present the questionnaire alongside a section for further comments on the module and aspects that have been helpful or unnecessary for each participant. Negative effects of treatment will be measured post-treatment and at follow-up.
Endometriosis-related quality of life
For a quick assessment of HRQoL, the Endometriosis Health Profile-5 (EHP-5) [67] will be used weekly after each module. This 5-item short form of the EHP-30 + 23 provides sufficient reliability and validity [67].
Negative effects of treatment
To detect possible negative effects of treatment in this trial (e.g., increased stress, new symptoms, hopelessness, and dependency), the Negative Effect Questionnaire (NEQ) [68] will be implemented.
Participant timeline {13}
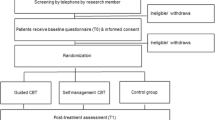
For the participant timeline, see Fig. 1.
Sample size {14}
Because this is the first published study to test the efficacy of iCBT for patients with endometriosis, there is not much data available to guide sample size calculations. We estimated an effect size of f = 0.25, drawing on another trial in women’s health that tested efficacy of iCBT in PMDD [42]. A total number of 120 participants are needed with significance of p < .05 and 95% power.
Recruitment {15}
Participants will be recruited online via social media and mailing lists, as well as through local inpatient clinics and gynecologists. Patients interested in participation will receive a link to the online screening for our trial.
Assignment of interventions: allocation
Sequence generation {16a}
Participants will be randomized to either the treatment or the control group using blockwise randomization. This is to ensure balanced group size even when the target sample size cannot be reached. Block size will not be shared with researchers running the trial.
Concealment mechanism {16b}
A web-based randomization system will be used to implement the planned allocation sequence.
Implementation {16c}
Randomization will be implemented by a research assistant who is not involved in the trial in any form. Participants will be informed about their allocation via mail.
Assignment of interventions: blinding
Who will be blinded {17a}
Due to the nature of the planned intervention and trial design, researchers and participants will not be blinded to the allocated trial arm.
Procedure for unblinding if needed {17b}
There is no need for any kind of unblinding procedure, as no one involved is blinded.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Data will be assessed online via self-report measures. Primary and secondary outcomes will be assessed at baseline, post-treatment, at a 3-month follow-up and, for the intervention group, at a 12-month follow-up. Control variables will be assessed at baseline only; measures for process evaluation will be assessed weekly during treatment and at post treatment as well as follow-up. For more details on outcome measures see {12}.
Plans to promote participant retention and complete follow-up {18b}
Participants will be contacted for follow-up via e-mail. The data are collected online, reducing as many barriers as possible (e.g., having to schedule an in-person appointment or sending the questionnaires back). There is no additional incentive for participants to complete the trial. Based on our experience working with patients who suffer from endometriosis, the sample is usually grateful for any research conducted on endometriosis and tends to be very eager to participate.
Data management {19}
Every participant will get an individual trial subject number used to identify them throughout the trial. Researchers involved in the trial will monitor data entry and check for any missing data or anomalies.
Confidentiality {27}
All data will be stored on local servers of the Philipps-University Marburg according to GDPR and local policy. All data, including collected personal information, will be kept strictly confidential.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
There are no plans for collection and evaluation of biological specimens.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Analysis will be conducted based on the intention-to-treat (ITT) principle, and two-sided p-values < .05 will be considered statistically significant. Results will be reported according to the Consolidated Standards of Reporting Trials (CONSORT, [53]). Baseline data will be summarized by treatment group and total. We will report categorical data as N (%), continuous data as mean (SD). Primary outcome analysis will be performed as a general linear model comparing the difference in mean EHP-30 + 23 score and pain scores between groups with repeated measures (baseline, post-treatment, 3-months follow-up). With this approach, all participants providing data for at least one point of measurement will be included. The Holm-Bonferroni Method [54] will be implemented to account for the testing of multiple hypotheses due to nomination of two primary outcomes. For secondary outcomes, we will use analysis of covariance models to conduct possibly necessary adjustment for baseline characteristics. If any non-foreseeable changes occur during the trial, we will adjust the statistical analysis plan accordingly. Sensitivity analyses will be performed to investigate the possible influence of missing data on the robustness of the results.
Interim analyses {21b}
No interim analyses are planned.
Methods for additional analyses (e.g., subgroup analyses) {20b}
Subgroup analyses
We will test for possible subgroup factors such as being partnered and currently trying to conceive. These are known moderators for HRQoL reductions through endometriosis. As our sample is expected to be quite homogenous in age and gender, other subgroup analyses might not be functional.
Process evaluation
To evaluate the development of iCBT for patients with endometriosis, the weekly measures of endometriosis-related QoL and qualitative feedback are taken into account. We hope to identify helpful aspects and key mechanisms in improving HRQoL to further improve the intervention. No systematic qualitative approach in assessing participant feedback will be implemented.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
Data will be analyzed following the ITT principle. Missing data will be explored. Imputation of missing values is not intended, although multiple imputations might be used within sensitivity analyses assuming data are missing at random.
Plans to give access to the full protocol, participant level-data, and statistical code {31c}
There is no additional protocol to this version. Because gynecological diseases still carry some stigma, we will decide on whether to publish participant-level data anonymously after trial completion. Psychological treatment of endometriosis is not yet common, so participants might easily be identified through some of the collected data. Only if we reach a sample size large enough to rule out identification of participants by some of their data (N ≥ 80) will we proceed to provide access to participant-level data without any kind of demographic information. Any other data, statistical code, or documentation may be provided by the corresponding author at request.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The immediate trial team responsible for trial management, day-to-day support, and administration of the intervention will meet weekly. The wider team—including medical experts—will meet every 3 months. Due to the small size of our trial and the few researchers being involved, other committees such as a Trial Steering Committee or Project Management Group will not be formed.
Composition of the data monitoring committee, its role and reporting structure {21a}
Given the small size of our monocentric trial with few researchers involved and without external funding, there is no need for an additional data monitoring committee.
Adverse event reporting and harms {22}
Adverse events (AEs) will be collected and monitored by the therapists running the intervention. Any disadvantageous event occurring during the trial (e.g., worsening of symptoms, acute suicidal ideation, hospitalization due to mood deterioration) will be classified as an adverse event. Both AEs that are assumed to be a consequence of treatment and those that are not causally related to the treatment will be collected. All AEs will be discussed weekly regarding causality and severity, and possible further actions will be considered.
Frequency and plans for auditing trial conduct {23}
The trial will be monitored by the principal investigator (CW) and the team members KS and JL. There will be no additional independent auditing of trial conduct.
Plans for communicating important protocol amendments to relevant parties (e.g. trial participants, ethical committees) {25}
The local ethic committee will be notified for approval if any protocol modifications are required. Trial registries and the trial protocol would be updated.
Dissemination plans {31a}
Trial results will be communicated via journal publications and conference presentations. If participants state their interest in trial results, they will be informed after trial completion.
Discussion
This trial is designed to test the efficacy of iCBT in improving HRQoL in patients with endometriosis. Endometriosis is a chronic disease linked to symptoms of stress, depression, and anxiety. There is a need for additional, non-medical interventions targeting mental health and reducing the burden of living with endometriosis [55]. The intervention is investigated using a waitlist control as the comparator.
Limitations
The decision to use waitlist control in our study comes with the risk of possible overestimation of treatment effects [56]. There is no TAU established for treatment of endometriosis, so using a TAU control group was not considered. Because it seemed ethical not to withhold a potential effective treatment for the control group, including a simple control group or placebo control group was also ruled out. We will investigate key mechanisms in improvement of HRQoL, allowing for the development of potential active control groups in future trials. The online setting of our invention limits access for patients without a suitable electronic device and/or skills needed to participate in iCBT (e.g., basic computer skills); however, if iCBT is proven effective in reducing the burden of living with endometriosis in this trial, the intervention can easily be adapted to face-to-face therapy for further research.
Strengths
Research about mind-body interventions for improvement of HRQoL in patients with endometriosis is still limited, and the demand for more RCTs has been mentioned [36, 37]. To our knowledge, this is the first RCT testing the efficacy of iCBT for patients with endometriosis in a cost-efficient way. The study offers iCBT as an established mind-body intervention to patients with a diagnosis that comes with an array of symptoms and who often are not taken seriously by both practitioners and in their private lives. Our ultimate goal is to discover effective support for patients with endometriosis and to encourage further research within this domain.
Trial status
The trial is registered (dated October 28, 2021) at ClinicalTrials.gov, Identifier NCT05098444, recruitment will start in January 2022. The current protocol is version 1 (dated November 11, 2021).
Abbreviations
- AAQ-II:
-
Acceptance and Action Questionnaire II
- AE:
-
Adverse event
- BDI-II:
-
Beck’s Depression Inventory II
- BFI-10:
-
Big Five Inventory 10
- CBT:
-
Cognitive behavioral therapy
- CONSORT:
-
Consolidated Standards of Reporting Trials
- CPP:
-
Chronic pelvic pain
- EHP-30 + 23:
-
Endometriosis Health Profile 30 + 23
- EHP-5:
-
Endometriosis Health Profile 5
- GDPR:
-
General Data Protection Regulation
- HAV:
-
Hepatitis A virus
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HRQoL:
-
Health-related quality of life
- iCBT:
-
Internet-based cognitive behavioral Therapy
- ICSI:
-
Intracytoplasmic sperm injection
- IPQ-R:
-
Illness Perceptions Questionnaire Revised
- ITT:
-
Intention-to-treat principle
- IUI:
-
Intrauterine insemination
- IVF:
-
In vitro fertilization
- NEQ:
-
Negative Effects Questionnaire
- PC:
-
Personal computer
- PDI:
-
Pain Disability Index
- PMDD:
-
Premenstrual dysphoric disorder
- PSS-10:
-
Perceived Stress Scale 10
- QoL:
-
Quality of life
- RAS:
-
Relationship Assessment Scale
- SD:
-
Standard derivation
- TAU:
-
Treatment as usual
- TBC:
-
Tuberculosis
- TCM:
-
Traditional Chinese Medicine
- WSQ:
-
Web-Based Screening Questionnaire
References
Chiantera V, Abesadze E, Mechsner S. How to understand the complexity of endometriosis-related pain. J Endometr Pelvic Pain Disord. 2017;9(1):30–8. https://doi.org/10.5301/je.5000271.
Prescott J. Farland L v., Tobias DK, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod. 2016;31(7):1475–82. https://doi.org/10.1093/humrep/dew085.
Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19(6):625–39. https://doi.org/10.1093/humupd/dmt027.
Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Investig. 2017;82(5):453–61. https://doi.org/10.1159/000452660.
Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–96. https://doi.org/10.1093/aje/kwh275.
Carey ET, Martin CE, Siedhoff MT, Bair ED, As-Sanie S. Biopsychosocial correlates of persistent postsurgical pain in women with endometriosis. Int J Gynaecol Obstet. 2014;124(2):169–73. https://doi.org/10.1016/j.ijgo.2013.07.033.
Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–373.e8. https://doi.org/10.1016/J.FERTNSTERT.2011.05.090.
Soliman AM, Yang H, Du EX, et al. The direct and indirect costs associated with endometriosis: a systematic literature review. Hum Reprod. 2016;31(4):712–22. https://doi.org/10.1093/humrep/dev335.
Olliges E, Bobinger A, Weber A, Hoffmann V, Schmitz T, Popovici RM, et al. The physical, psychological, and social day-to-day experience of women living with endometriosis compared to healthy age-matched controls—a mixed-methods study. Front Glob Womens Health. 2021;2:87. https://doi.org/10.3389/fgwh.2021.767114.
Roullier C, Sanguin S, Parent C, Lombart M, Sergent F, Foulon A. General practitioners and endometriosis: level of knowledge and the impact of training. J Gynecol Obstet Hum Reprod. 2021;50(10):102227. https://doi.org/10.1016/j.jogoh.2021.102227.
Staal AHJ, van der Zanden M, Nap AW. Diagnostic delay of endometriosis in the Netherlands. Gynecol Obstet Investig. 2016;81(4):321–4. https://doi.org/10.1159/000441911.
Hudelist G, Fritzer N, Thomas A, Niehues C, Oppelt P, Haas D, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412–6. https://doi.org/10.1093/humrep/des316.
Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756–9. https://doi.org/10.1093/humrep/deg136.
Pope CJ, Sharma V, Sharma S, Mazmanian D. A systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can. 2015;37(11):1006–15. https://doi.org/10.1016/S1701-2163(16)30050-0.
Estes SJ, Huisingh CE, Chiuve SE, Petruski-Ivleva N, Missmer SA. Depression, anxiety, and self-directed violence in women with endometriosis: a retrospective matched-cohort study. Am J Epidemiol. 2021;190(5):843–52. https://doi.org/10.1093/aje/kwaa249.
Roomaney R, Kagee A. Salient aspects of quality of life among women diagnosed with endometriosis: a qualitative study. J Health Psychol. 2018;23(7):905–16. https://doi.org/10.1177/1359105316643069.
Carbone MG, Campo G, Papaleo E, Marazziti D, Maremmani I. The importance of a multi-disciplinary approach to the endometriotic patients: the relationship between endometriosis and psychic vulnerability. J Clin Med. 2021;10(8):1616. https://doi.org/10.3390/jcm10081616.
Namazi M, Behboodi Moghadam Z, Zareiyan A, Jafarabadi M. Impact of endometriosis on reproductive health: an integrative review. J Obstet Gynaecol. 2021;41(8):1183–91. https://doi.org/10.1080/01443615.2020.1862772.
Facchin F, Buggio L, Vercellini P, Frassineti A, Beltrami S, Saita E. Quality of intimate relationships, dyadic coping, and psychological health in women with endometriosis: results from an online survey. J Psychosom Res. 2021;146:110502. https://doi.org/10.1016/j.jpsychores.2021.110502.
da Pereira M. G, Ribeiro I, Ferreira H, et al. Psychological morbidity in endometriosis: a couple’s study. Int J Environ Res Public Health. 2021;18(20):10598. https://doi.org/10.3390/ijerph182010598.
Muhaidat N, Saleh S, Fram K, Nabhan M, Almahallawi N, Alryalat SA, et al. Prevalence of endometriosis in women undergoing laparoscopic surgery for various gynaecological indications at a Jordanian referral centre: gaining insight into the epidemiology of an important women’s health problem. BMC Womens Health. 2021;21(1):1–8. https://doi.org/10.1186/s12905-021-01530-y.
Mousa M, Al-Jefout M, Alsafar H, et al. Impact of endometriosis in women of Arab ancestry on: health-related quality of life, work productivity, and diagnostic delay. Front Glob Womens Health 2021;0:66, 2, https://doi.org/10.3389/fgwh.2021.708410.
Toth B. Stress, inflammation and endometriosis: are patients stuck between a rock and a hard place? J Mol Med. 2010;88(3):223–5. https://doi.org/10.1007/s00109-010-0595-4.
Lazzeri L, Orlandini C, Vannuccini S, Pinzauti S, Tosti C, Zupi E, et al. Endometriosis and perceived stress: impact of surgical and medical treatment. Gynecol Obstet Investig. 2015;79(4):229–33. https://doi.org/10.1159/000368776.
Reis FM, Coutinho LM, Vannuccini S, Luisi S, Petraglia F. Is stress a cause or a consequence of endometriosis? Reprod Sci. 2020;27(1):39–45. https://doi.org/10.1007/s43032-019-00053-0.
Tariverdian N, Theoharides TC, Siedentopf F, Gutiérrez G, Jeschke U, Rabinovich GA, et al. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007;29(2):193–210. https://doi.org/10.1007/s00281-007-0077-0.
Maddern J, Grundy L, Castro J, Brierley SM. Pain in endometriosis. Front Cell Neurosci. 2020;14:335. https://doi.org/10.3389/fncel.2020.590823.
Zheng P, Zhang W, Leng J, Lang J. Research on central sensitization of endometriosis-associated pain: a systematic review of the literature. J Pain Res. 2019;12:1447–56. https://doi.org/10.2147/JPR.S197667.
Delanerolle G, Ramakrishnan R, Hapangama D, et al. A systematic review and meta-analysis of the endometriosis and mental-health sequelae; the ELEMI Project. Womens Health (Lond). 2021;17:17455065211019717. https://doi.org/10.1177/17455065211019717.
Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, de Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–12. https://doi.org/10.1093/humrep/det457.
Vannuccini S, Clemenza S, Rossi M, Petraglia F. Hormonal treatments for endometriosis: the endocrine background. Rev Endocr Metab Disord. 2021;1:1–23. https://doi.org/10.1007/s11154-021-09666-w.
Donnez J, Dolmans M-M. Endometriosis and medical therapy: from progestogens to progesterone resistance to GnRH antagonists: a review. J Clin Med. 2021;10(5):1085. https://doi.org/10.3390/jcm10051085.
Gonçalves AV, Barros NF, Bahamondes L. The practice of hatha yoga for the treatment of pain associated with endometriosis. J Altern Complement Med. 2017;23(1):45–52. https://doi.org/10.1089/acm.2015.0343.
Zhao L, Wu H, Zhou X, Wang Q, Zhu W, Chen J. Effects of progressive muscular relaxation training on anxiety, depression and quality of life of endometriosis patients under gonadotrophin-releasing hormone agonist therapy. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):211–5. https://doi.org/10.1016/j.ejogrb.2012.02.029.
Meissner K, Schweizer-Arau A, Limmer A, Preibisch C, Popovici RM, Lange I, et al. Psychotherapy with somatosensory stimulation for endometriosis-associated pain. Obstet Gynecol. 2016;128(5):1134–42. https://doi.org/10.1097/AOG.0000000000001691.
Kold M, Hansen T, Vedsted-Hansen H, Forman A. Mindfulness-based psychological intervention for coping with pain in endometriosis. Nordic Psychol. 2012;64(1):2–16. https://doi.org/10.1080/19012276.2012.693727.
Evans S, Fernandez S, Olive L, Payne LA, Mikocka-Walus A. Psychological and mind-body interventions for endometriosis: a systematic review. J Psychosom Res. 2019;124:109–756. https://doi.org/10.1016/j.jpsychores.2019.109756.
van Niekerk L, Weaver-Pirie B, Matthewson M. Psychological interventions for endometriosis-related symptoms: a systematic review with narrative data synthesis. Arch Womens Ment Health. 2019;22(6):723–35. https://doi.org/10.1007/s00737-019-00972-6.
Sullivan-Myers C, Sherman KA, Beath AP, Duckworth TJ, Cooper MJW. Delineating sociodemographic, medical and quality of life factors associated with psychological distress in individuals with endometriosis. Hum Reprod. 2021;36(8):2170–80. https://doi.org/10.1093/humrep/deab138.
Ciharova M, Furukawa TA, Efthimiou O, Karyotaki E, Miguel C, Noma H, et al. Cognitive restructuring, behavioral activation and cognitive-behavioral therapy in the treatment of adult depression: A network meta-analysis. J Consult Clin Psychol. 2021;89(6):563–74. https://doi.org/10.1037/ccp0000654.
Williams AC De C, Fisher E, Hearn L, et al. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2020. Epub ahead of print August 14, 2020. https://doi.org/10.1002/14651858.CD007407.pub4.
Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res. 2012;36(5):427–40. https://doi.org/10.1007/s10608-012-9476-1.
Weise C, Kaiser G, Janda C, Kues JN, Andersson G, Strahler J, et al. Internet-based cognitive-behavioural intervention for women with premenstrual dysphoric disorder: a randomized controlled trial. Psychother Psychosom. 2019;88(1):16–29. https://doi.org/10.1159/000496237.
Boersen Z, de Kok L, van der Zanden M, Braat D, Oosterman J, Nap A. Patients’ perspective on cognitive behavioural therapy after surgical treatment of endometriosis: a qualitative study. Reprod BioMed Online. 2021;42(4):819–25. https://doi.org/10.1016/j.rbmo.2021.01.010.
Butt FS, Chesla C. Relational patterns of couples living with chronic pelvic pain from endometriosis. Qual Health Res. 2007;17(5):571–85. https://doi.org/10.1177/1049732307299907.
Sims OT, Gupta J, Missmer SA, Aninye IO. Stigma and endometriosis: a brief overview and recommendations to improve psychosocial well-being and diagnostic delay. Int J Environ Res Public Health. 2021;18(15):8210. https://doi.org/10.3390/ijerph18158210.
Loughnan SA, Joubert AE, Grierson A, Andrews G, Newby JM. Internet-delivered psychological interventions for clinical anxiety and depression in perinatal women: a systematic review and meta-analysis. Arch Womens Ment Health. 2019;22(6):737–50. https://doi.org/10.1007/s00737-019-00961-9.
Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E. Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry. 2014;13(3):288–95. https://doi.org/10.1002/wps.20151.
Bernardy K, Füber N, Köllner V, et al. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37(10):1991–2005. https://doi.org/10.3899/jrheum.100104.
Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cognit Behav Ther. 2018;47(1):1–18. https://doi.org/10.1080/16506073.2017.1401115.
Andersson G, Carlbring P, Rozental A. Response and remission rates in internet-based cognitive behavior therapy: an individual patient data meta-analysis. Front Psychol. 10. Epub ahead of print October 25, 2019. https://doi.org/10.3389/fpsyt.2019.00749.
Jones G, Kennedy S, Barnard A, Wong J, Jenkinson C. Development of an endometriosis quality-of-life instrument: the Endometriosis Health Profile-30. Obstet Gynecol. 2001;98(2):258–64. https://doi.org/10.1097/00006250-200108000-00014.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:1–8.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70.
Facchin F, Barbara G, Saita E, Mosconi P, Roberto A, Fedele L, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynecol. 2015;36(4):135–41. https://doi.org/10.3109/0167482X.2015.1074173.
Tait RC, Pollard CA, Margolis RB. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil. 1987;68(7):438–41.
Cunningham JA, Kypri K, McCambridge J. Exploratory randomized controlled trial evaluating the impact of a waiting list control design. BMC Med Res Methodol. 2013;13(1):150. https://doi.org/10.1186/1471-2288-13-150.
Donker T, van Straten A, Marks I, et al. A brief web-based screening questionnaire for common mental disorders: development and validation. J Med Internet Res. 2009;11(3):e1134. https://doi.org/10.2196/jmir.1134.
Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1996.
Zipfel S, Herzog W, Löwe B. Screening psychischer Störungen mit dem “Gesundheitsfragebogen für Patienten (PHQ-D)”. Diagnostica. 2004;50(4):171–81. https://doi.org/10.1026/0012-1924.50.4.171.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. https://doi.org/10.2307/2136404.
Zarbo C, Brugnera A, Frigerio L, Malandrino C, Rabboni M, Bondi E, et al. Behavioral, cognitive, and emotional coping strategies of women with endometriosis: a critical narrative review. Arch Womens Ment Health. 2018;21(1):1–13. https://doi.org/10.1007/s00737-017-0779-9.
Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4(1):92–100. https://doi.org/10.1207/s15327558ijbm0401_6.
Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, Buick D. The revised Illness Perception Questionnaire (IPQ-R). Psychol Health. 2002;17(1):1–16. https://doi.org/10.1080/08870440290001494.
Gloster AT, Klotsche J, Chaker S, Hummel KV, Hoyer J. Assessing psychological flexibility: what does it add above and beyond existing constructs? Psychol Assess. 2011;23(4):970–82. https://doi.org/10.1037/a0024135.
Bond FW, Hayes SC, Baer RA, Carpenter KM, Guenole N, Orcutt HK, et al. Preliminary psychometric properties of the Acceptance and Action Questionnaire–II: a revised measure of psychological inflexibility and experiential avoidance. Behav Ther. 2011;42(4):676–88. https://doi.org/10.1016/j.beth.2011.03.007.
Pluchino N, Wenger JM, Petignat P, Tal R, Bolmont M, Taylor HS, et al. Sexual function in endometriosis patients and their partners: effect of the disease and consequences of treatment. Hum Reprod Update. 2016;22(6):762–74. https://doi.org/10.1093/humupd/dmw031.
Hendrick SS. A generic measure of relationship satisfaction. J Marriage Fam. 1988;50(1):93. https://doi.org/10.2307/352430.
Rammstedt B, John OP. Measuring personality in one minute or less: a 10-item short version of the Big Five Inventory in English and German. J Res Pers. 2007;41(1):203–12. https://doi.org/10.1016/j.jrp.2006.02.001.
Jones G, Jenkinson C, Kennedy S. Development of the short form endometriosis health profile questionnaire: the EHP-5. Qual Life Res. 2004;13(3):695–704. https://doi.org/10.1023/B:QURE.0000021321.48041.0e.
Rozental A, Kottorp A, Boettcher J, Andersson G, Carlbring P. Negative effects of psychological treatments: an exploratory factor analysis of the negative effects questionnaire for monitoring and reporting adverse and unwanted events. PLoS One. 2016;11(6):e0157503. Epub ahead of print. https://doi.org/10.1371/journal.pone.0157503.
Acknowledgements
Not applicable.
Authors’ contributions {31b}
KS and CW conceived the study. KS, JL, and CW designed the intervention content and will conduct the study. MK and VZ provided medical advice and critical evaluation. KS drafted the manuscript. CW is the lead investigator. All authors contributed substantially to the study and read and approved the final manuscript.
Funding {4}
Open Access funding enabled and organized by Projekt DEAL. The researchers working on this trial are employed at Philipps-University Marburg through budgetary funds. No external funding was received for this trial. We received the local grant UMRvernetzt from Philipps-University Marburg for interdisciplinary exchange of medical and psychological expertise. The grant went to the construction of our iCBT platform.
Availability of data and materials {29}
Data will be available for all researchers involved in the trial. For public access to participant-level datasets, see {31c}.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate {24}
Ethics approval was granted by the Philipps University Department of Psychology Ethics Committee (2021-45v). Written informed consent to participate in the trial will be obtained from all participants. For more information, see {26a}.
Consent for publication {32}
Not applicable, as we will not include any data relating to an individual person in our protocol. A model consent form will be provided by the corresponding author on request.
Competing interests {28}
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schubert, K., Lohse, J., Kalder, M. et al. Internet-based cognitive behavioral therapy for improving health-related quality of life in patients with endometriosis: study protocol for a randomized controlled trial. Trials 23, 300 (2022). https://doi.org/10.1186/s13063-022-06204-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06204-0