Abstract
Background
Self-stigma is highly prevalent in serious mental illness (SMI) and is associated with poorer clinical and functional outcomes. Narrative enhancement and cognitive therapy (NECT) is a group-based intervention combining psychoeducation, cognitive restructuring and story-telling exercises to reduce self-stigma and its impact on recovery-related outcomes. Despite evidence of its effectiveness on self-stigma in schizophrenia-related disorders, it is unclear whether NECT can impact social functioning.
Methods
This is a 12-centre stepped-wedge cluster randomized controlled trial of NECT effectiveness on social functioning in SMI, compared to treatment as usual. One hundred and twenty participants diagnosed with schizophrenia, bipolar disorder or borderline personality disorder will be recruited across the 12 sites. The 12 centres participating to the study will be randomized into two groups: one group (group 1) receiving the intervention at the beginning of the study (T0) and one group (group 2) being a control group for the first 6 months and receiving the intervention after (T1). Outcomes will be compared in both groups at T0 and T1, and 6-month and 12-month outcomes for groups 1 and 2 will be measured without a control group at T2 (to evaluate the stability of the effects over time). Evaluations will be conducted by assessors blind to treatment allocation. The primary outcome is personal and social performance compared across randomization groups. Secondary outcomes include self-stigma, self-esteem, wellbeing, quality of life, illness severity, depressive symptoms and personal recovery.
Discussion
NECT is a promising intervention for reducing self-stigma and improving recovery-related outcomes in SMI. If shown to be effective in this trial, it is likely that NECT will be implemented in psychiatric rehabilitation services with subsequent implications for routine clinical practice.
Trial registration
ClinicalTrials.gov NCT03972735. Trial registration date 31 May 2019.
Similar content being viewed by others
Introduction
Mental illness stigma refers to the negative beliefs, emotional reactions and attitudes towards people with serious mental illness (SMI) endorsed by the general population (also referred to as public stigma [1]). People with SMI are generally acutely aware of public stigma and expect to be rejected by others (69.4% of the 1229 participants with schizophrenia (SZ) and 71.6% of the 1182 participants with mood disorders in the GAMIAN-Europe study had high perceived stigma [2, 3]). Self-stigma occurs when someone moves beyond awareness of stigma to accepting the negative stereotypes about SMI as true to describe him/herself [1]. Self-stigma refers to the process whereby a person’s previously held social identity (defined by social roles such as son, brother, sister, friend, employee or potential partner) is progressively replaced by a devalued and stigmatized view of oneself. Self-stigma is highly frequent in Europe (41.7% in SZ and 21.7% in mood disorders [2, 3]) and the USA (36.1% out of 144 people with SMI [4]). The ‘illness identity model’ [5] proposed that self-stigma can have pervasive effects on outcomes related to recovery from SMI, including self-esteem, hopefulness, social interaction, employment and symptom severity. The evidence consistently supports many of the model’s predictions, including that self-stigma is negatively associated with self-esteem, motivation to achieve personal life goals, shared-decision making, adherence into treatment, well-being, quality of life, personal recovery and social function [6,7,8,9,10,11,12,13,14]. People with elevated self-stigma report more dysfunctional attitudes, social withdrawal, depressive symptoms and increased suicidal ideation [5, 15,16,17,18,19,20,21]. High insight into illness directly predicts and compounds the effects of self-stigma on depression [15, 17, 22,23,24]. Impairments in cognitive functioning, metacognition and social cognition predict increased self-stigma [12, 24,25,26,27,28,29].
Several psychosocial interventions have been designed to reduce self-stigma and its impact on patient’s outcomes, with preliminary results on self-stigma, insight and self-efficacy [30]. Narrative enhancement and cognitive therapy (NECT) is a structured, 20 session group-based approach which combines psychoeducation to counteract SMI stereotypes, cognitive restructuring to challenge dysfunctional attitudes about the self and story-telling exercises to enhance meaning-making and the person’s ability to move beyond their illness-identity to a more integrated sense of self [31]. NECT is manualized and can be implemented with fidelity in routine treatment settings [31]. Four controlled studies, including three randomized controlled trials (RCT) in three different countries (USA, Israel, Sweden) have been published to date in psychotic-related disorders. NECT was effective in improving self-stigma, hope and self-esteem [32,33,34] with persisting effects after 6 months’ follow-up [33, 34]. The results on subjective quality of life were mixed, one study found improvements [32] but another found no effectiveness [33]. NECT was effective in reducing social withdrawal and avoidant coping strategies in participants with schizophrenia (SZ) in comparison with an active control condition [34]. However, NECT was not effective on social functioning, measured with the Quality of Life Scale [35], a global measure of psychosocial function [34]. In almost all studies of NECT, the samples were mixed, with some participants showing low levels of self-stigma (Internalized Stigma of Mental Illness (ISMI) total score < 2.5) and others moderate to high self-stigma (ISMI > 2.5). Changes in coping strategies were higher in participants from centres providing the intervention within the context of psychiatric rehabilitation [34].
In summary, NECT has demonstrated effectiveness on self-stigma and subjective aspects of recovery (hope, self-esteem and quality of life) in participants with psychotic-related disorders and SZ. Its effectiveness on social function and in other stigmatized SMI conditions (bipolar disorder (BD), borderline personality disorder (BPD)) are, however, still unclear. Similarly, NECT’s effectiveness in improving depression, treatment adherence, wellbeing and personal recovery is still unknown.
The objectives of the present study are to investigate (i) NECT effectiveness on social functioning in SMI participants, (ii) NECT effectiveness on self-stigma, psychiatric symptoms, depression, well-being, subjective quality of life and personal recovery, (iii) whether insight into illness and cognitive functioning at baseline predict improvements at follow-up and (iv) whether the effects on primary and secondary outcomes persist after 6 and 12 months’ follow-up.
The primary hypothesis to be tested is whether NECT significantly improves social functioning in comparison with the control group. The secondary hypotheses are to determine if, when compared to the control condition, NECT results in significant improvements in self-stigma, psychiatric symptoms, depression, wellbeing, self-esteem, subjective quality of life and personal recovery.
Methods and design
Design
This stepped wedge cluster randomized controlled study is multi-centric, prospective, interventional and exploratory. For ethical purposes, we have chosen a stepped wedge cluster randomized trial design, with a waiting list type control condition. Previous research from the French national REHABase network showed that (i) most patients were dissatisfied with their interpersonal relationships at the time of admission and asked for interventions improving social function [36] and (ii) elevated self-stigma was frequent (31.2% of the 738 participants [37]) and was negatively associated with recovery-related outcomes. Self-stigma has been negatively associated with patient’s longitudinal outcomes and to lower benefits from psychiatric rehabilitation [38]. Providing NECT to all participants aims at not excluding participants from interventions improving social function (e.g. social skills training or social-cognitive remediation) during the follow-up period without proposing them a credible therapeutic alternative (i.e. self-stigma reduction). This also aims at reducing potential deception bias and dropouts in participants allocated to the control group. Each centre will represent a cluster. In stepped-wedge RCTs, all clusters are evaluated before and after providing the intervention and each cluster switches from control to become exposed, but not at the same point in time [39, 40]. The 12 centres participating to the study will be randomized into two groups: one group (group 1) receiving the intervention at the beginning of the study (T0) and one group (group 2) being a control group for the first 6 months and receiving the intervention after (T1). The stepped-wedge design of this 2-group trial can be seen on Fig. 1.
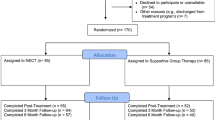
The total duration of the study will be 18 months to allow for recruitment of the target number of participants, delivery of the intervention, follow-up assessments and data analysis using intention-to-treat methods. A schematic overview of the study design is presented in Fig. 2.
Participants
Inclusion and exclusion criteria
Eligible participants will be adults (age > 18) diagnosed with schizophrenia, schizoaffective disorder, schizotypal disorder, schizophreniform disorder, bipolar I or II disorder or borderline personality disorder (DSM-5 criteria [41]). For the purpose of the study only stabilized outpatients will be recruited (defined as 3 months of clinical stability and no changes in pharmacological treatment in the past 3 months). Participants unable to give informed consent or with a comorbid intellectual disability will be excluded. Participation in other programs having an impact on social function or self-stigma (social cognitive remediation, social skills training) will be prohibited during the follow-up period.
Recruitment and randomization
Patients diagnosed with schizophrenia, bipolar disorder or borderline personality disorder (DSM-5 criteria [41]) will be recruited through six Rehabase psychiatric rehabilitation network centres [36], three FondaMental Academic Centres of Expertise (FACE) for SZ or BD [42, 43], one unit specialized in mood disorders at the Geneva university hospital, one psychiatric day-care clinic specialized in mood and personality disorders in Grenoble and one psychiatric rehabilitation centre located in the psychiatric hospital in Caen. The selected sites are already actively involved in treating patients with SZ, BD or BPD and have previous research experience in the field of psychiatric rehabilitation. Regular group meetings will be organized to monitor quality control and ensure good inter-rater reliability. A list of eligible participants will be generated under the supervision of the coordinating centre clinical research officer. Each eligible patient will be approached by a trained member of the research team and invited to participate in the study, thereby eliminating selection biases. Eligible patients will be given a leaflet describing the program and the research. Informed consent will be taken during a medical interview with one of the investigators. The clinical research officer of the coordinating centre (Grenoble) will be in charge of the randomization process for participants from all centres. Clusters will be randomized to receive the intervention at either T0 or T1. Stratified randomization will be used to ensure that cluster types are well balanced across groups. Outcomes will be compared in all groups at T0 and T1 (duration of intervention= 6 months). Six-month and 12-month outcomes for groups 1 and 2 will be measured at T2 (18 months after T0) to evaluate the stability of the effects over time. A 7-point improvement in social functioning measured using Personal and Social Performance Scale [44] (PSP) is generally considered as clinically significant. We plan to include 5 patients per centre and per group, meaning that there will be 10 participants included per centre for a total of 120 patients included (60 in group 1, 60 in group 2). This sample size was calculated using R package ‘pwr’ [45] based on Cohen [46] (d = 0.50, alpha level of 0.05) without allowing for intra-cluster correlations and the cluster and time effects found in stepped-wedge designs [47, 48]. This could be a considerable limitation, as it might have underpowered the trial [47]. Most of the outcome measures are self-rated questionnaires; the other evaluations will be conducted by an evaluator who has not taken part in the intervention. The schedule of enrolment, interventions and assessments is shown in Fig. 3 (SPIRIT diagram).
Schedule of enrolment, interventions and assessments (SPIRIT diagram). BIS, Birchwood Insight Scale; ISMI, Internalized Stigma of Mental Illness; MARS, Medication Adherence Rating Scale; NECT, narrative enhancement and cognitive therapy; PSP, Personal and Social Performance Scale; S-QoL, Subjective-Quality of Life; TAU, treatment as usual; TMT, Trail Making Test; V-SIR, Versailles-Situational Intention Reading; WAIS IV, Wechsler Adult Intelligence Scale-4th edition; WEWMBS, Warwick-Edinburgh Mental Wellbeing Scale; YMRS, Young Mania Rating Scale
NECT
Translation procedure
A consistent translation and cross-cultural adaptation procedure was realized by two independent research teams (JD, MF and MA in Grenoble; HRL, SF and FJ in Geneva). All materials were translated from English into French, the first language of all translators. Each team reviewed the material translated by the other team so all translations were crosschecked. Regular group meetings between the two teams were conducted to discuss any problematic translations and resolve any disputed items, in line with established translation methods.
Training and fidelity monitoring
All the clinicians involved will attend a 1-day NECT training session, delivered by the creators of the program (Yanos PT, Roe D). Training will include discussion and role-playing exercises, with corrective feedback from the trainers. Regular group supervisions will be organized to ensure treatment fidelity. NECT fidelity will be monitored using the NECT Fidelity Scale, developed by Yanos et al. [34].
The experimental intervention
The French adaptation of NECT is a manualized, structured, 12-session, group-based intervention conducted by two facilitators. The number of sessions was reduced in the French adaptation and their duration extended to match the typical duration of psychosocial interventions in French-speaking countries (up to 15 sessions). Figure 4 provides an overview of the NECT topics. The 2-h sessions are divided into three main parts: (1) psychoeducation about stigma, self-stigma and myths about mental disorders; (2) cognitive restructuring targeting negative thoughts about the self and dysfunctional attitudes and (3) story-telling exercises in which participants tell stories about themselves, receive feedback from other group members and facilitators and are invited to apply the cognitive restructuring skills learnt in the previous sessions to their personal life narratives. At-home practice exercises are carried out between the sessions.
The control condition
The control condition refers to the group receiving current standard care during the first 6 months of the study before attending NECT after T1. There is no definition of standard care. The association of pharmacological treatment with psychiatric rehabilitation is however recommended in all the international guidelines on SMI [49,50,51]. All participants will attend day-care recovery-oriented services offering several forms of psychiatric rehabilitation (group CBT on positive symptoms, general psychoeducation, cognitive remediation, vocational rehabilitation, supported employment). Information about the interventions received during the follow-up period (with their duration and the number of sessions) will be systematically collected by the clinical research officer and taken into account when analysing the results.
Assessments
At baseline and at follow-up, socio-demographic and illness-related information (education level, employment and marital status, psychiatric diagnosis, age of onset, illness duration, current pharmacological treatment) will be collected. All outcome measures listed below are part of routine clinical evaluations in the national REHABase and FACE networks for SZ and BD [36, 42, 43] approved by the relevant ethical committees.
Primary outcome
Social function will be assessed with the Personal and Social Performance Scale [44] (PSP), a clinician-reported measure of social dysfunction using six-point severity scale in four life domains (socially useful activities, personal and social relationships, self-care and disturbing or aggressive behaviours). PSP provides an overall rating score ranging from 1 to 100, higher scores representing better personal and social functioning. PSP showed acceptable internal consistency [52], excellent inter-rater reliability [53] and a satisfactory ability to detect changes [44]. A seven-point improvement during clinical trials is considered to be clinically significant [44].
Secondary outcomes
Self-stigma will be assessed using the Internalized Stigma of Mental Illness scale (ISMI [2, 54]), a 29-item self-report measure designed to assess a person’s personal experience of stigma related to mental disorders and is rated on a four-point Likert scale. Items are summed to provide a mean total score and five subscale scores (alienation or feeling of being a devalued member of the society; stereotype endorsement or agreement with the negative attitudes about SMI; discrimination experience; social withdrawal as a coping strategy and stigma resistance). Stigma resistance is generally excluded because of poor correlations with other subscales [32, 34]. Higher scores reflect higher levels of self-stigma. Scores above 2.5 indicate moderate to high levels of self-stigma [2, 3]. Illness severity will be assessed in patients with SZ using the Positive and Negative Syndrome [55] (PANSS) scales. Current depressive symptoms were evaluated using the Montgomery-Asberg Depression Rating Scale [56] (MADRS) and current manic symptoms with the Young Mania Rating Scale [57] (YMRS). Insight and treatment adherence will be assessed with self-reported measures (Birchwood Insight Scale (BIS) [58]; Medication Adherence Rating Scale (MARS) [59]). Quality of life will be evaluated with the self-reported Subjective Quality of Life scale [60] (S-QoL) and wellbeing using the Warwick-Edinburgh Mental Well-being Scale [61] (WEMBS). Self-esteem will be measured with the Self-Esteem Rating Scale [62] (SERS) and personal recovery using the self-reported Stage of Recovery Instrument [63] (STORI). Baseline neuropsychological cognitive assessments include Wechsler Adult Intelligence Scale-4th edition [64] (WAIS-IV) subscales assessing working memory, verbal abstraction and hypothetico-deductive reasoning, BEM-144 story subtest for auditive-verbal memory [65], Trail Making Test A and B (TMT-A or B) [66] respectively for speed of processing and reactive mental flexibility, D2-R for selective attention, concentration and speed of processing [67], and shopping test for planning abilities [68]. Theory of mind will be assessed at baseline using the Versailles-Situational Intention Reading Test [69] (V-SIR).
Statistical analysis
Intention-to-treat analyses will be conducted in the first instance. The analyses will be conducted using parametric tests for normal distributed data (Student’s t and covariance analysis with repeat measures ANCOVA) and with non-parametric tests (Mann-Whitney U and Kruskal-Wallis test) in case of significant deviations from the normal distribution. The primary analysis was done by intention to treat.
We will analyse all outcomes using multilevel regression models with time point and intervention status as fixed effects and clusters as a random effect. Timepoint will be included as a categorical variable [39]. The statistical analysis will be done using the R software, version 3.2.3 [70]. The psych package version 1.5.8 will be used [71]. Effect size (Cohen’s d) will be calculated using the effsize package [71]. Size effects inferior to 0.20 will be considered as negligible, from 0.20 to 0.40 as small, from 0.40 to 0.60 as moderate and superior to 0.60 as strong [71]. The leaps package version 2.9 will be used for variable selection [72]. The level of confidence intervals will be set at 0.95 and the significance level of tests at 0.05.
Trial status
The present trial has not yet started. The recruitment will begin on September 1, 2019, with an estimated study completion date of April, 3, 2023. Protocol version 1.5 was written on April 2, 2019, and approved by the relevant ethical committee (CPP Nord-Ouest I) on April 26, 2019.
Discussion and conclusion
NECT has shown effectiveness in reducing self-stigma in patients with psychotic-related disorders in a range of contexts in previous research. Considering the prevalence of moderate to high self-stigma in SMI and its impact on functional outcomes, NECT appears to be a promising intervention to complete the basket of services proposed by psychiatric rehabilitation centres (cognitive and social cognitive remediation, psychoeducation, social skills training, group cognitive behaviour therapy, vocational rehabilitation, peer-support [36]). If shown to be effective on social function and for different SMI, it is likely that NECT will have widespread implications for clinical practice.
Availability of data and materials
The study protocol is available online on Clinical Trials: https://clinicaltrials.gov/ct2/show/NCT03972735?term=nect&rank=1
References
Corrigan PW, Watson AC. Understanding the impact of stigma on people with mental illness. World Psychiatry. 2002;1(1):16–20.
Brohan E, Elgie R, Sartorius N, Thornicroft G, GAMIAN-Europe Study Group. Self-stigma, empowerment and perceived discrimination among people with schizophrenia in 14 European countries: the GAMIAN-Europe study. Schizophr Res. 2010;122(1–3):232–8.
Brohan E, Gauci D, Sartorius N, Thornicroft G, GAMIAN-Europe Study Group. Self-stigma, empowerment and perceived discrimination among people with bipolar disorder or depression in 13 European countries: the GAMIAN-Europe study. J Affect Disord. 2011;129(1–3):56–63.
West ML, Yanos PT, Smith SM, Roe D, Lysaker PH. Prevalence of internalized stigma among persons with severe mental illness. Stigma Res Action. 2011;1(1):3–10.
Yanos PT, Roe D, Markus K, Lysaker PH. Pathways between internalized stigma and outcomes related to recovery in schizophrenia spectrum disorders. Psychiatr Serv. 2008;59(12):1437–42.
Gerlinger G, Hauser M, De Hert M, Lacluyse K, Wampers M, Correll CU. Personal stigma in schizophrenia spectrum disorders: a systematic review of prevalence rates, correlates, impact and interventions. Word Psychiatry. 2013;12:155–64.
Corrigan PW, Larson JE, Rüsch N. Self-stigma and the “why try” effect: impact on life goals and evidence-based practices. World Psychiatry. 2009;8:75–81.
Hamann J, Bühner M, Rüsch N. Self-stigma and consumer participation in shared decision making in mental health services. Psychiatr Serv. 2017;68(8):783–8.
Hill K, Startup M. The relationship between internalized stigma, negative symptoms and social functioning in schizophrenia: the mediating role of self-efficacy. Psychiatry Res. 2013;206(2–3):151–7.
Cerit C, Filizer A, Tural Ü, Tufan AE. Stigma: a core factor on predicting functionality in bipolar disorder. Compr Psychiatry. 2012;53(5):484–9.
Mersh L, Jones F, Oliver J. Mindfulness, self-stigma and social functioning in first episode psychosis: a brief report. Psychosis. 2015;7(3):1–4.
Firmin RL, Lysaker PH, Luther L, Yanos PT, Leonhardt B, Breier A, et al. Internalized stigma in adults with early phase versus prolonged psychosis. Early Interv Psychiatry. 2019;13(4):745–51.
Xu Z, Lay B, Oexle N, Drack T, Bleiker M, Lengler S, et al. Involuntary psychiatric hospitalisation, stigma stress and recovery: a 2-year study. Epidemiol Psychiatr Sci. 2018;31:1–8.
Oexle N, Müller M, Kawohl W, Xu Z, Viering S, Wyss C, et al. Self-stigma as a barrier to recovery: a longitudinal study. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):209–12.
Schrank B, Amering M, Hay AG, Weber M, Sibitz I. Insight, positive and negative symptoms, hope, depression and self-stigma: a comprehensive model of mutual influences in schizophrenia spectrum disorders. Epidemiol Psychiatr Sci. 2014;23(3):271–9.
Park SG, Bennett ME, Couture SM, Blanchard JJ. Internalized stigma in schizophrenia: relations with dysfunctional attitudes, symptoms, and quality of life. Psychiatry Res. 2013;205(1–2):43–7.
Valiente C, Provencio M, Espinosa R, Duque A, Everts F. Insight in paranoia: the role of experiential avoidance and internalized stigma. Schizophr Res. 2015;164(1–3):214–20.
Oexle N, Rüsch N, Viering S, Wyss C, Seifritz E, Xu Z, et al. Self-stigma and suicidality: a longitudinal study. Eur Arch Psychiatry Clin Neurosci. 2017;267(4):359–61.
Oexle N, Waldmann T, Staiger T, Xu Z, Rüsch N. Mental illness stigma and suicidality: the role of public and individual stigma. Epidemiol Psychiatr Sci. 2018;27:169–75.
Espinosa R, Valiente C, Rigabert A, Song H. Recovery style and stigma in psychosis: the healing power of integrating. Cogn Neuropsychiatry. 2016;21(2):146–55.
Au CH, Wong CS, Law CW, Wong MC, Chung KF. Self-stigma, stigma coping and functioning in remitted bipolar disorder. Gen Hosp Psychiatry. 2019;57:7–12.
Cavelti M, Kvrgic S, Beck EM, Rüsch N, Vauth R. Self-stigma and its relationship with insight, demoralization, and clinical outcome among people with schizophrenia spectrum disorders. Compr Psychiatry. 2012;53(5):468–79.
Lysaker PH, Roe D, Yanos PT. Toward understanding the insight paradox: internalized stigma moderates the association between insight and social functioning, hope, and self-esteem among people with schizophrenia spectrum disorders. Schizophr Bull. 2007;33(1):192–9.
Lysaker PH, Vohs J, Hasson-Ohayon I, Kukla M, Wierwille J, Dimaggio G. Depression and insight in schizophrenia: comparisons of levels of deficits in social cognition and metacognition and internalized stigma across three profiles. Schizophr Res. 2013;148(1–3):18–23.
Lysaker PH, Buck KD, Taylor AC, Roe D. Associations of metacognition and internalized stigma with quantitative assessments of self-experience in narratives of schizophrenia. Psychiatry Res. 2008;157(1–3):31–8.
Lysaker PH, Vohs JL, Tsai J. Negative symptoms and concordant impairments in attention in schizophrenia: associations with social functioning, hope, self-esteem and internalized stigma. Schizophr Res. 2009;110(1–3):165–72.
Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. 2014;13(3):275–87.
Hasson-Ohayon I, Mashiach-Eizenberg M, Elhasid N, Yanos PT, Lysaker PH, Roe D. Between self-clarity and recovery in schizophrenia: reducing the self-stigma and finding meaning. Compr Psychiatry. 2014;55(3):675–80.
Hasson-Ohayon I, Mashiach-Eizenberg M, Lysaker PH, Roe D. Self-clarity and different clusters of insight and self-stigma in mental illness. Psychiatry Res. 2016;240:308–13.
Wood L, Byrne R, Burke E, Enache G, Morrison AP. The impact of stigma on emotional distress and recovery from psychosis: the mediatory role of internalised shame and self-esteem. Psychiatry Res. 2017;255:94–100 (Wood, 2015???).
Yanos PT, Lysaker PH, Roe D. Internalized stigma as a barrier to improvement in vocational functioning among people with schizophrenia-spectrum disorders. Psychiatry Res. 2010;178(1):211–3 (Yanos, 2011???).
Roe D, Hasson-Ohayon I, Mashiach-Eizenberg M, Derhy O, Lysaker PH, Yanos PT. Narrative enhancement and cognitive therapy (NECT) effectiveness: a quasi-experimental study. J Clin Psychol. 2014;70(4):303–12.
Hansson L, Lexén A, Holmén J. The effectiveness of narrative enhancement and cognitive therapy: a randomized controlled study of a self-stigma intervention. Soc Psychiatry Psychiatr Epidemiol. 2017;52(11):1415–23.
Yanos PT, Lysaker PH, Silverstein SM, Vayshenker B, Gonzales L, West ML, et al. A randomized-controlled trial of treatment for self-stigma among persons diagnosed with schizophrenia-spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. 2019. https://doi.org/10.1007/s00127-019-01702-0.
Heinrichs DW, Hanlon TE, Carpenter WT. The quality of life scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–96.
Franck N, Bon L, Dekerle M, Plasse J, Massoubre C, Pommier R, et al. Satisfaction and needs in serious mental illness and autism spectrum disorder: the REHABase psychosocial rehabilitation project. Psychiatr Serv. 2019;70(4):316–23.
Dubreucq J, Plasse J, Gabayet F, Faraldo M (…) REHABase network and Franck N. Self-stigma in serious mental illness and autism spectrum disorder: results from the REHABase national psychiatric rehabilitation cohort. Eur Psychiatry. Accepted for publication.
Dubreucq J, Plasse J, Franck N. Self-stigma in Serious Mental Illness: A Systematic Review of Frequency, Correlates, and Consequences, Schizophrenia Bulletin. 2021;sbaa181. https://doi.org/10.1093/schbul/sbaa181.
Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350 Available from: https://www.bmj.com/content/350/bmj.h391.long. [cited 2019 Jul 17].
Hemming K, Taljaard M, McKenzie JE, Hooper R, Copas A, Thompson JA, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. https://www.bmj.com/content/363/bmj.k1614. Accessed 18 July 2019.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). 5th ed. Washington: DC Author; 2013.
Schürhoff F, Fond G, Berna F, Bulzacka E, Vilain J, Capde-vielle D, et al. A national network of schizophrenia expert centres: an innovative tool to bridge the research-practice gap. Eur Psychiatry. 2015;30(6):728–35.
Henry C, Etain B, Mathieu F, Raust A, Vibert JF, Scott J, et al. A French network of bipolar expert centres: a model to close the gap between evidence-based medicine and routine practice. J Affect Disord. 2011;131(1–3):358–63.
Nasrallah H, Morosini PL, Gagnon DD. Reliability, validity and ability to detect change of the Personal and Social Performance Scale in patients with stable schizophrenia. Psychiatry Res. 2008;161:213–24.
Champely S. pwr: Basic functions for power analysis [Computer software] (version 1.2-2); 2018. Retrieved from https://CRAN.R-project.org/package=pwr.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988.
Hemming K, Taljaard M. Sample size calculations for stepped wedge and cluster randomised trials: a unified approach. J Clin Epidemiol. 2016;69:137–46. https://doi.org/10.1016/j.jclinepi.2015.08.015 Epub 2015 Sep 5. PMID: 26344808; PMCID: PMC4687983.
Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the Shiny CRT Calculator. Int J Epidemiol. 2020;49(3):979–95. https://doi.org/10.1093/ije/dyz237 PMID: 32087011; PMCID: PMC7394950.
Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, et al. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):48–70.
Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Primers. 2018;4:18008.
Gunderson JG, Herpertz SC, Skodol AE, Torgersen S, Zanarini MC. Borderline personality disorder. Nat Rev Dis Primers. 2018;4:18029.
Kawata AK, Revicki DA. Psychometric properties of the Personal and Social Performance scale (PSP) among individuals with schizophrenia living in the -community. Qual Life Res. 2008;17(10):1247–56.
Patrick DC, Burns T, Morosini P, Rothman M, Gagnon DD, Wild D, et al. Reliability, validity and ability to detect change of the clinician-rated Personal and Social Performance scale in patients with acute symptoms of schizophrenia. Curr Med Res Opin. 2009;25(2):325–35.
Boyd Ritsher J, Otilingam PG, Grajales M. Internalized stigma of mental illness: psychometric properties of a new measure. Psychiatry Res. 2003;121:31–49.
Kay S, Fiszbein A, Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Brit J Psychiatry. 1979;134:382–9.
Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35.
Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self-report insight scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89(1):62–7.
Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res. 2000;42(3):241–7.
Auquier P, Simeoni MC, Sapin C, Reine G, Aghababian V, Cramer J, et al. Development and validation of a patient-based health-related quality of life questionnaire in schizophrenia: the S-QoL. Schizophr Res. 2003;63(1–2):137–49.
Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-being Scale 19 (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5:63.
Lecomte T, Corbière M, Laisné F. Investigating self-esteem in individuals with schizophrenia: relevance of the Self-Esteem Rating Scale-Short Form. Psychiatry Res. 2006;143(1):99–108.
Andresen R, Caputi P, Oades LG. Stages of recovery instrument: development of a measure of recovery from serious mental illness. Aust NZ J Psychiatry. 2006;40:972–80.
Wechsler D. WAIS IV Nouvelle version de l’échelle d’intelligence de Weschler pour adultes. 4th ed. Paris: Edition de Centre de Psychologie Appliquée; 2011.
Signoret JL. Batterie d’efficience mnésique BEM 144. Paris: Elsevier; 1991.
Godefroy O, GREFEX (Groupe de Réflexion pour l’Evaluation des Fonctions Exécutives). Fonctions exécutives et pathologies neurologiques et 675 psychiatriques. Evaluation en pratique clinique. Marseille: DeBoeck supérieur; 2012:84.
Brickenkamp R, Schmidt-Atzert L, Liepmann D. D2-R : Test d’attention concentrée révisé. Paris: Editions Hogrefe; 2015.
Pelletier Y, Lemoine L, Gros C, Fournet N. Test des commissions révisé. GREMOIRE II Tests et échelles des maladies neurologiques avec symptomatologie cognitive. Paris: De Boeck-Solal; 2009.
Bazin N, Brunet-Gouet E, Bourdet C, Kayser N, Falissard B, Hardy-Baylé MC, et al. Quantitative assessment of attribution of intentions to others in schizophrenia using an ecological video-based task: a comparison with manic and depressed patients. Psychiatry Res. 2009;167:28–35.
R Core Team. R: a language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, 2015. https://www.R-project.org/. Accessed 18 July 2019.
Revelle W. Psych: procedures for psychological, psychometric, and personality research, Northwestern University. Evanston, Illinois: R package; 2015.
Lumley T. Leaps: regression subset selection. 2009. https://CRAN.R-project.org/package=leaps. Accessed 18 July 2019.
Funding
This research did not receive any specific grants from funding bodies in the public, private or not-for-profit sectors. This trial will be conducted in day-care community services funded by the Auvergne-Rhône-Alpes, Nouvelle-Aquitaine and Normandie regional health agencies.
Author information
Authors and Affiliations
Contributions
The authors had full access to the data in the study and take the responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Julien Dubreucq drafted the article and made the literature review. Mrs. Megane Faraldo, Mrs. Marie Abbes, Mr. Thomas Cohen, Pr Paul Lysaker, Pr Philip Yanos and Pr Nicolas Franck contributed to design the study protocol and critically revised the article. All the authors will be involved in the collection and analysis of the data. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study obtained all the authorizations required under French regulations and was approved by the relevant ethical committee (CPP Nord-Ouest I) on April 26, 2019. Informed written consent will be systematically sought for all participants.
Consent for publication
Not applicable
Competing interests
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dubreucq, J., Faraldo, M., Abbes, M. et al. Narrative enhancement and cognitive therapy (NECT) to improve social functioning in people with serious mental illness: study protocol for a stepped-wedge cluster randomized controlled trial. Trials 22, 124 (2021). https://doi.org/10.1186/s13063-021-05067-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-021-05067-1