Abstract
Background
Different non-pharmacological techniques, including hypnosis and virtual reality (VR) are currently used as complementary tools in the treatment of anxiety, acute and chronic pain. A new technique called virtual reality hypnosis (VRH), which encompasses a combination of both tools, is regularly used although its benefits and underlying mechanisms remain unknown to date. With the goal to improve our understanding of VRH combination effects, it is necessary to conduct randomised and controlled research trials in order to understand their clinical interest and potential benefits.
Methods
Patients (n = 100) undergoing cardiac surgery at the Liège University Hospital will be randomly assigned to one of four conditions (control, hypnosis, VR or VRH). Each patient will receive two sessions of one of the techniques: one the day before the surgery and one the day after. Physiological assessments will be made on the monitor and patients will rate their levels of anxiety, fatigue, pain, absorption and dissociation.
Discussion
This study will help to expand knowledge on the application of virtual reality, hypnosis and VRH in the specific context of cardiac and intensive care procedures, and the influence of these non-pharmacological techniques on patient’s anxiety, fatigue, pain and phenomenological experience.
Trial registration
ClinicalTrials.gov: NCT03820700. Date registered on 29 January 2019.
Study recruitment date: October 6, 2018. Study anticipated completion date: December 28, 2020.
Similar content being viewed by others
Background
The aim of this study is to better understand the use of non-pharmacological approaches to reduce anxiety among patients in intensive care units (ICUs), and their potential clinical benefits in one specific population of patients, i.e. patients undergoing cardiac surgery.
Anxiety, pain and fatigue are important factors influencing the recovery of patients after surgery. The definition of preoperative anxiety is “an unpleasant state of uneasiness or tension that is secondary to a patient being concerned about a disease, hospitalization, anaesthesia and surgery, or the unknown” [1]. Among patients admitted to hospital for surgery, 20–28% reported high preoperative anxiety and 60% reported minimal anxiety in two studies investigating this issue [2, 3]. Patients’ anxiety before surgery is a risk for postoperative recovery problems and its consequences are of paramount importance in delaying wound healing [4]. In addition, preoperative anxiety is reported to significantly influence the intensity of postoperative pain [5, 6]. Studies have shown that among patients in the ICU, pain is associated with an unpleasant stay, sleep deprivation, increased agitation, high rates of post-traumatic stress disorder and feeling unsafe in the ICU environment [7,8,9,10,11,12,13].
Currently, pharmacological treatments for anxiety and pain are well-developed in the ICU environment, where pain and fatigue are most commonly managed by opioid analgesics, propofol and benzodiazepines [14]. Pharmacological treatment could be considered efficient when the patient feels comfortable, with no adverse effects [15]. Yet, opioid analgesics (e.g. morphine) often lead to respiratory sedation, hyperalgesia, depression, nausea, opioid-induced tolerance and dependence [15]. Benzodiazepines can be used in the short term but can lead to strong dependence, with important adverse effects: ataraxia, irritability, nervousness, depression and risk of suicide [16]. Further, some pharmacological interventions (e.g. lorazepam and pregabalin), intended to treat anxiety, fail to decrease preoperative anxiety and postoperative pain [17, 18]. In the ICU environment, deep levels of sedation potentially lead to increased mortality and lengths of stay [19]. In the light of this, non-pharmacological approaches are of interest as complementary techniques to reduce anxiety and pain. Techniques such as hypnosis and virtual reality (VR) have been investigated in numerous studies in the medical field (e.g. algology, oncology, anaesthesia) to reduce pain and anxiety and increase patients’ comfort [20,21,22]. Hypnosis is defined as a “state of modified consciousness involving focused attention and reduced peripheral awareness, characterized by an enhanced capacity for response to suggestions” [23]. Hypnosis has three main components: absorption, dissociation and suggestibility. Absorption is the tendency to become fully involved in a perceptual, imaginative or ideational experience; dissociation is the mental separation from the environment; and suggestibility is the responsiveness to social cues, leading to an enhanced tendency to comply with instructions and a relative suspension of critical judgment [24]. This technique is considered safe, and one that allows the patient to be focused on his or her inner world, by including cognitive and behavioural components that enable the mind to influence body sensations and perceptions [25,26,27]. Hypnotic suggestions can be used to modify perception of symptoms such as pain, anxiety and fatigue, in different health-related disorders (e.g. oncology, chronic pain, surgery). In some cases, hypnosis can be a complement to other medication therapy to reduce anxiety before surgery (e.g. presurgical anxiety in coronary artery bypass and cataract surgery) [28,29,30] and also after surgery (e.g. during weaning from mechanical ventilation) [31]. A recent meta-analysis showed that hypnosis is a highly effective intervention for anxiety and is more effective when combined with other psychological interventions and various clinical applications [28]. Hypnosis is known to reduce acute and chronic pain [32,33,34,35,36] and improve sleep quality [37, 38]. A variety of relaxation techniques have been investigated to improve the quality of sleep in ICU patients (e.g. aromatherapy, earplugs and masks, noise bundle) but results are not convincing in all studies [39, 40]. One review of the literature showed that hypnosis seems to be a promising technique for management of sleep problems; however, more randomised studies are required to support these results [34]. Hypnosis is an efficient treatment in health care, and one that can save time and costs to healthcare providers in some instances [35, 41].
There has been growing interest in the use of virtual reality (VR) in medicine [20]. VR involves computer-generated, immersive and three-dimensional technologies. VR subjective experience is characterized by senses of immersion and presence. Presence refers to the degree to which the subject experiences being in the virtual environment [42, 43], while immersion is the amount of sensory input the VR system creates [44]. Feedback systems with trackers - and often helmet and gloves - allow individuals to be distracted by interacting with a virtual world and make it as “real” as possible [45]. According to Patterson et al. (2006), immersion in VR can isolate the patient from the outside environment and it is effective in distracting the subject’s attention from a painful stimulus [46, 47]. VR has been shown to divert attention from painful stimulation in both highly hypnotizable and less hypnotizable individuals in experimental and clinical settings [48, 49]. VR can also be considered as an efficient non-pharmacological tool to decrease anxiety (e.g. during dental treatment or phobia therapy) [50, 51]. In our experiment, VR is not used during a painful stimulus but before and after a painful and stressful intervention, i.e. surgery. VR distraction is an adjunctive tool to clinical interventions for such issues as acute and chronic pain management, clinical education, cognitive and motor rehabilitation, anxiety management and communication skills training; however, less is known about its efficacy in decreasing fatigue [20, 52].
Virtual reality hypnosis (VRH) is a technique that combines VR hardware/software and hypnotic induction followed by analgesic suggestions [53]. According to Patterson et al. (2004; 2010), “VRH uses a high-resolution, head-mounted display that delivers absorbing visual images and high-fidelity audio that provide an induction […] followed by suggestions for comfort and pain relief” [53, 54]. Studies have demonstrated positive effects of VRH on pain and anxiety [48], but the actual mechanisms of this treatment are not well-known. Hence, it seems that even if immersion is present in VRH and hypnosis, it would not necessarily bring about the same effects as it does with VR distraction. For example, in hypnosis, absorption and dissociation come from the subject who constructs his own world with the hypnotherapist’s suggestions. In VR, that world is imposed on the subject with existing technology. To our knowledge, two randomised studies have previously been conducted to compare hypnosis, VR and VRH in experimental setups [47, 48]. However, there is a need to compare the efficiency of these techniques in clinical practice.
One of the main goals of caregivers is to create the best environment for reducing the patient’s anxiety in surgery and in the ICU by using the patient’s own resources. In this study, we wish to compare three non-pharmacological methods in patients undergoing cardiac surgery and hospitalized in the ICU: hypnosis, VR and VRH. By comparing these non-pharmacological tools, we would be able to better understand their relative efficacy and mechanisms in making the patient more comfortable. Randomised, controlled research trials are necessary to evaluate how the patient’s cognition and perception of these tools can impact the outcomes [20, 26].
Methods/design
Aim
The aim of the project is to better understand the impact of VR, hypnosis and VRH on individual perception and sensation in patients hospitalized in cardiac surgery and ICU departments. The primary outcome will be patients’ anxiety levels preoperatively and in postoperative recovery. Secondary assessments will include assessment of pain, fatigue, relaxation, physiological parameters, absorption, dissociation and presence concepts.
Study registration
This study has been approved by the Ethic Committee of the Faculty of Medicine and the Ethic Committee of the Faculty of Psychology, Speech Therapy and Educational Sciences of the University of Liège. This trial was registered on clinicaltrials.gov with the trial identification number NCT03820700 in January 2019. The trial is currently ongoing and recruiting.
Eligibility criteria
This study will have a prospective randomised design, and will be a single-centre trial with four arms, including three experimental and one control group. The study sample will comprise adult patients undergoing cardiac surgery (coronary artery bypass graft; mitral heart valve replacement; aortic valve replacement; others). All the study procedures and surgery will be conducted in the University Hospital of Liège (Belgium). Informed consent will be obtained before inclusion of patients.
There will be 100 patients included in the study (25 patients per condition). The participants will be adults undergoing cardiac surgery who have provided informed consent for their participation in the study. This choice is due to the high prevalence of patients undergoing cardiac surgery and accessibility to these patients at the university treatment centre, and the possibility of easily collecting physiological data and patients’ reports. The age of the patients will range from 18 to 90 years. Patients have to be conscious, awake and able to understand and answer in fluent French. Exclusion criteria are psychiatric diseases like dementia, claustrophobia, acrophobia, severe hearing problems, visual impairment or a state of confusion (Table 1).
Design
Participants will be randomly included in the following conditions:
- 1.
Control group: daily care only.
- 2.
Hypnosis: taped hypnosis called “Soothing white clouds”.
The hypnosis session will consist of a 20-min hypnosis recording created by M-E Faymonville and A-S Nyssen, both experts in clinical and experimental hypnosis. The recording, named “Soothing white clouds”, includes suggestions about relaxation, positive body sensations and invitation to observe a sunrise and a beautiful landscape, while relaxing in a white cloud chair. Suggestions are focused on variables we wish to improve with the patient (i.e. relaxation) and not on symptom relief. An example of the text (the original text is in French) is as follows: “This experience invites you to discover your resources to find more comfort, calm and healing… I suggest you to find a comfortable position to take full advantage of this moment […] You can discover new perspectives in this soothing white clouds, note others details, and appreciate to be present in this moment […] appreciate the air around you, breathing oxygen, this energy source, which give energy to your body, everywhere it’s needed…”
- 3.
Virtual reality (VR): mountain landscape 3D animation and sounds of nature
For the VR session we will use a head-mounted 3D graphical display with goggles. This VR environment consists of visualization of a landscape accompanied by sounds of nature. The 3D immersive landscape features a shed near a lake at sunrise followed by a relaxing moment in the clouds. This device was constructed by Oncomfort© according to what we wish the patient to visualize in the hypnosis condition (i.e. landscape, sunrise, clouds). The session lasts 20 min and ends on the lake’s edge. There is no verbal suggestion in this VR device. Sounds consist of water sound, birds and the cicada’s song. Participants will not interact with the environment; they are invited to simply watch the 3D animation and relax during the session.
- 4.
Virtual reality hypnosis combination (VRH): mountain landscape 3D animation plus taped hypnosis “Soothing white clouds”.
In VRH, we replace the sounds of nature used for the VR device by the hypnotic tape in order to have the ideal experimental conditions to compare the techniques. The Soothing white clouds hypnosis session is combined with a 3D visual movie (immersive landscape and relaxing moment in the clouds (Oncomfort©)), with a duration of 20 min. The text for hypnosis is the same as in the hypnosis group and includes suggestions about relaxation, positive body sensations, invitation to observe a sunrise and a beautiful landscape, while relaxing in a white cloud chair. The hypnosis script was previously recorded (see hypnosis group description) and then integrated into the VR device. In that way, participants can listen to the hypnosis record throughout the VR session.
Initial contact between the investigator and the patient will take place in the patient’s room one day before cardiac surgery. The investigator will request each patient to consent for participation in the study and subsequently record their demographic data (age, gender, surgery type, alcohol and tobacco use). Hypnosis, VR or VRH will be applied in 20 min session: the day before surgery (day − 1 at 5.00 p.m.) and the day after surgery (day + 1 at 2.00 p.m.). Before and after each session, physiological measurements will be recorded (heartbeat, arterial pressure), and a visual analogical scale (VAS) will be used to assess anxiety, fatigue, pain and relaxation. The VAS is a continuous scale subjectively assessed by the patient and ranges from 0 (no pain/anxiety/fatigue/relaxation) to 10 (maximum pain/anxiety/fatigue/relaxation). We ask the patients to assess their “current pain intensity/anxiety/fatigue/relaxation at the moment”. This score determines the intensity of these variables at a given time [57]. After the session, patients will complete questions about absorption and dissociation (Table 2).
Recruitment and randomisation
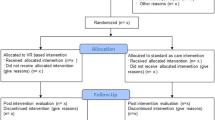
Each patient scheduled for cardiac surgery who meets our exclusion and inclusion criteria will be asked to participate. The number of patients who refuse to participate will be recorded, and demographic data will be collected to allow a comparison with those who participate. Written informed consent will be obtained before inclusion. The selected patients will be randomly assigned to one of the four groups, using block randomisation with a block size of 5 to obtain a good balance of participants between the four groups during the recruitment period. The recruitment started in October 2018 and is ongoing. Sample size has been determined by a power analysis calculated to detect a difference in the evolution of data between the four groups. The sample size calculation was based on repeated measures analysis of variance (ANOVA). Alpha was set at 0.05, power at 95% and the standardized effect size at 0.5. In other studies designed to assess the effect of hypnosis on patients’ anxiety pre-surgery, an effect size of 0.2 has been considered small, 0.5 moderate and 0.8 large [29]. According to this analysis, 12 patients are required in each group giving a total of 48 patients. We decided to enrol 100 patients (25 per group) at day 1 to compensate for dropouts on the day after surgery (Fig. 1).
Assessments
Qualitative data
We will record dropouts and the reasons for dropouts. We will record patients’ subjective opinions on hypnosis, VR and VRH, collected through an interview. We also record the nurses’ opinions on hypnosis, VR and VRH collected through an interview about the applicability and the usability of the tools.
Demographic factors
We will collect data on age and gender, duration of surgery and type of cardiac surgery (aortic valve replacement, mitral valve replacement or coronary artery bypass surgery). Patients in the ICU are not currently consuming alcohol, but their treatment can be influenced by their previous daily alcohol consumption. The investigator will therefore ask patients about their habitual alcohol and social drug consumption per day and per week. Tobacco withdrawal symptoms (e.g. nervous behaviour) could also influence the participant’s behaviour on the postoperative day. The investigator will therefore ask patients whether they are smokers (yes/no).
Psychological outcomes
Anxiety, pain, relaxation and fatigue will be evaluated using a VAS before and after the 20-min sessions. The VAS score helps to determine the intensity of these psychological variables, as subjectively assessed by the patient, on a scale ranging from 0 to 10. The daily dissociative profile will be assessed using the Dissociative Experience Scale (DES) 28-items [58].
Absorption is the “tendency to become fully involved in a perceptual, imaginative, or ideational experience” [59, 60]. We asked subjects to answer this question: “Could you estimate on a 0- (not at all) to 10- (fully) scale how deeply you felt absorbed and felt your attention as focalized and focused by the experience you have just lived?” [60].
Dissociation: is “a mental separation of components of experience that would ordinarily be processed together” [24, 60]. We asked subjects to answer this question: “Could you estimate on a 0-to-10 scale if you felt a dissociation between your bodily sensation and the actual environment? Zero means you were in the reality, in this room; 10 means that you completely escaped in your subjective experience, totally disconnected from the here-and-now reality” [60].
Immersion and presence will be assessed using a VAS. The questions will be “From 0 to 10, how much did you feel present in the environment?” and “From 0 to 10, how much did you really feel the sensations suggested by the therapist?”
Time perception: we will ask subjects to estimate the time elapsed in minutes since they started the session. Time perception will be calculated as the absolute value of the real duration of the experience (hypnosis, VR, VRH) minus the subjects’ estimated duration [60]. The experimenter will record the start and end times of the session.
Physiological outcomes
We will assess heart rate: normal heart rate is from 60 to 110 heart beats per minute (depending on whether or not the patient regularly pratices sport) [61]; arterial pressure: normal arterial pressure is from 9.5 to 14.9 [62]; respiratory rate: normal respiratory rate is from 12 to 20 cycles per minute [61]; oxygen saturation: normal values of oxygen saturation are from 95% to 100% [61]; and pupil size: pupil diameter varies from 1 to 10 mm and normal range is from 2 to 8 mm [63].
Data coding and storage
Data encoding will be assured by the principal investigator of the study (FR). Data will be stored on DOXUlg (https://dox.uliege.be). DOXUlg is a platform for the University of Liège that is secure and confidential. Patients’ data will be accessible only to the principal investigator and promotor to maintain the confidentiality of data.
Statistical analysis
Normality will be investigated graphically by histogram and quantile-quantile plot and tested using the Shapiro-Wilk test. Continuous variables will be reported as mean (plus/minus standard deviation) or median (interquartile range) for skewed distributions, and qualitative variables as number and percentage. Homogeneity of the four groups will be assessed using the chi-squared test for qualitative and dichotomous variables and one-way (ANOVA-1) or the non-parametric Kruskal-Wallis test for quantitative variables. Repeated measures ANOVA will be used to compare the evolution of the parameters between day − 1 and day + 1 morning and afternoon, according to the groups. This analysis will be adjusted by the potential confounding factors. Calculations are always carried out on the maximum number of data available. Results will be considered as statistically significant at the 5% critical level (p < 0.05). Analyses will be performed using R 3.5.3 (R Core Team) and the package Rcommander (Rcmdr) and using SAS 9.4 (© SAS Institute Inc., Cary, NC, USA) [64].
Discussion
The aim of this study is to evaluate the feasibility of hypnosis, VR and VRH in increasing comfort (anxiety, pain and fatigue) in patients undergoing cardiac procedures, and to investigate the phenomenological experiences they undergo (absorption, dissociation, time perception, immersion and presence). For years, hypnosis and VR have been evaluated in different medical settings and have been shown to be efficient in decreasing perceptions of pain and anxiety [65,66,67,68]. More recently, a combination of these two techniques (VRH) was proposed to alleviate clinical symptoms, mainly anxiety and pain [54]. Until now there have been very few controlled studies comparing these techniques [47, 48]. Thereby, our study can potentially make a great contribution in the understanding both of the clinical impact of these approaches and of the mechanisms underlying them. The randomised controlled design is a particular strength of our study. Guidelines are important for tools like VR in terms of mechanisms and clinical benefits. Results of this study will inform us about the endpoint for future well-designed trials forhypnosis, VR and VRH.
There are some limitations to our study. The first limitation could be that some patients will drop out due to inability to participate on the day after surgery. We suspect that extreme fatigue and deep sedation due to surgery may be a barrier to properly following the hypnotic suggestions and the VR animation. The second limitation is that patients are assessed for 2 days and not for the entire period of their hospitalization.
In conclusion, our study will provide initial insight into the application of VR, hypnosis and VRH in the particular context of ICU care, by studying the specific population of patients undergoing cardiac surgery. We will able to measure the effects of VR, hypnosis and VRH on clinically relevant factors such as anxiety and pain. Others studies will then be developed to extend and adapt this protocol to other populations of patients in the ICU.
Trial status
Trial registration: ClinicalTrials.gov. Registration number: NCT03820700. Date registered: 29 January 2019 Study recruitment date: 6 October 2018. Study anticipated completion date: 28 December 2020. https://clinicaltrials.gov/ct2/show/NCT03820700
Availability of data and materials
The datasets used and/or analysed during the current study will be available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- ICU:
-
Intensive care unit
- VAS:
-
Visual analogue scale
- VR:
-
Virtual reality
- VRH:
-
Virtual reality hypnosis
References
Ramsay MA. A survey of pre-operative fear. Anaesthesia. 1972;27:396–402. https://doi.org/10.1111/j.1365-2044.1972.tb08244.x.
Hernández-Palazón J, Fuentes-García D, Falcón-Araña L, Roca-Calvo MJ, Burguillos-López S, Doménech-Asensi P, et al. Assessment of preoperative anxiety in cardiac surgery patients lacking a history of anxiety: contributing factors and postoperative morbidity. J Cardiothorac Vasc Anesth. 2018;32:236–44. https://doi.org/10.1053/j.jvca.2017.04.044.
Gonçalves KKN, Silva JI da, Gomes ET, Pinheiro LL de S, Figueiredo TR, Bezerra SMM da S, et al. Anxiety in the preoperative period of heart surgery. Revista Brasileira de Enfermagem. 2016;69:397–403. https://doi.org/10.1590/0034-7167.2016690225i.
Munafò MR, Stevenson J. Anxiety and surgical recovery: reinterpreting the literature. J Psychosom Res. 2001;51:589–96. https://doi.org/10.1016/S0022-3999(01)00258-6.
Stamenkovic DM, Rancic NK, Latas MB, Neskovic V, Rondovic GM, Wu JD, et al. Preoperative anxiety and implications on postoperative recovery: what can we do to change our history. Minerva Anestesiol. 2018;84:1307–17. https://doi.org/10.23736/S0375-9393.18.12520-X.
Langerud AK, Rustøen T, Småstuen MC, Kongsgaard U, Stubhaug A. Intensive care survivor-reported symptoms: a longitudinal study of survivors’ symptoms. Nurs Crit Care. 2018;23:48–54. https://doi.org/10.1111/nicc.12330.
Pandharipande PP, Patel MB, Barr J. Management of pain, agitation, and delirium in critically ill patients. Pol Arch Intern Med. 2014;124:114–23. https://doi.org/10.20452/pamw.2136.
Jones J, Hoggart B, Withey J, Donaghue K, Ellis BW. What the patients say: a study of reactions to an intensive care unit. Intensive Care Med. 1979;5:89–92. https://doi.org/10.1007/BF01686054.
Gélinas C. Management of pain in cardiac surgery ICU patients: have we improved over time? Intensive Crit Care Nurs. 2007;23:298–303. https://doi.org/10.1016/j.iccn.2007.03.002.
Granja C, Gomes E, Amaro A, Ribeiro O, Jones C, Carneiro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36:2801–9. https://doi.org/10.1097/CCM.0b013e318186a3e7.
Zeilani R, Seymour JE. Muslim women’s experiences of suffering in Jordanian intensive care units: a narrative study. Intensive Crit Care Nurs. 2010;26:175–84. https://doi.org/10.1016/j.iccn.2010.02.002.
Wong FYK, Arthur DG. Hong Kong patients’ experiences of intensive care after surgery: nurses’ and patients’ views. Intensive Crit Care Nurs. 2000;16:290–303. https://doi.org/10.1054/iccn.2000.1515.
Mattiussi E, Danielis M, Venuti L, Vidoni M, Palese A. Sleep deprivation determinants as perceived by intensive care unit patients: findings from a systematic review, meta-summary and meta-synthesis. Intensive Crit Care Nurs. 2019;53:43–53. https://doi.org/10.1016/j.iccn.2019.03.006.
Pathmanathan N, McClure J. Sedation and delirium in the intensive care unit. Anaesth Intensive Care Med. 2016;17:17–23. https://doi.org/10.1016/j.mpaic.2015.10.002.
Jannati M, Attar A. Analgesia and sedation post-coronary artery bypass graft surgery: a review of the literature. Ther Clin Risk Manag. 2019;15:773–81. https://doi.org/10.2147/TCRM.S195267.
Limandri BJ. Benzodiazepine use: the underbelly of the opioid epidemic. J Psychosoc Nurs Ment Health Serv. 2018;56:11–5. https://doi.org/10.3928/02793695-20180521-03.
Mijderwijk H, van Beek S, Klimek M, Duivenvoorden HJ, Grüne F, Stolker RJ. Lorazepam does not improve the quality of recovery in day-case surgery patients: a randomised placebo-controlled clinical trial. Eur J Anaesthesiol. 2013;30:743–51. https://doi.org/10.1097/EJA.0b013e328361d395.
White PF, Tufanogullari B, Taylor J, Klein K. The effect of pregabalin on preoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg. 2009;108:1140–5. https://doi.org/10.1213/ane.0b013e31818d40ce.
Stephens R, Dettmer M, Roberts B, Ablordeppey E, Fowler S, Kollef M, et al. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care Med. 2018;46:471–9. https://doi.org/10.1097/CCM.0000000000002885.
Pourmand A, Davis S, Lee D, Barber S, Sikka N. Emerging utility of virtual reality as a multidisciplinary tool in clinical medicine. Games Health J. 2017;6:263–70. https://doi.org/10.1089/g4h.2017.0046.
Scheffler M, Koranyi S, Meissner W, Strauß B, Rosendahl J. Efficacy of non-pharmacological interventions for procedural pain relief in adults undergoing burn wound care: a systematic review and meta-analysis of randomized controlled trials. Burns. 2018;44:1709–20. https://doi.org/10.1016/j.burns.2017.11.019.
Jensen MP, Jamieson GA, Lutz A, Mazzoni G, McGeown WJ, Santarcangelo EL, Demertzi A, De Pascalis V, Bányai EL, Rominger C, Vuilleumier P, Faymonville ME, Terhune DB. New directions in hypnosis research: strategies for advancing the cognitive and clinical neuroscience of hypnosis. Neurosci Conscious. 2017;3:2057-107. https://doi.org/10.1093/nc/nix004.
Elkins GR, Barabasz AF, Council JR, Spiegel D. Advancing research and practice: the revised APA Division 30 definition of hypnosis. Int J Clin Exp Hypn. 2015;63:1–9. https://doi.org/10.1080/00207144.2014.961870.
Spiegel D. Neurophysiological correlates of hypnosis and dissociation. J Neuropsychiatr Clin Neurosci. 1991;3:440–5. https://doi.org/10.1176/jnp.3.4.440.
Holroyd J. Hypnosis treatment of clinical pain: understanding why hypnosis is useful. Int J Clin Exp Hypn. 1996;44:33–51. https://doi.org/10.1080/00207149608416066.
Fisch S, Brinkhaus B, Teut M. Hypnosis in patients with perceived stress – a systematic review. BMC Complement Altern Med. 2017;17:323. https://doi.org/10.1186/s12906-017-1806-0.
McBride JJ, Vlieger AM, Anbar RD. Hypnosis in paediatric respiratory medicine. Paediatr Respir Rev. 2014;15:82–5. https://doi.org/10.1016/j.prrv.2013.09.002.
Valentine KE, Milling LS, Clark LJ, Moriarty CL. The efficacy of hypnosis as a treatment for anxiety: a meta-analysis. Int J Clin Exp Hypn. 2019;67:336–63. https://doi.org/10.1080/00207144.2019.1613863.
Akgul A, Guner B, Çırak M, Çelik D, Hergünsel O, Bedirhan S. The beneficial effect of hypnosis in elective cardiac surgery: a preliminary study. Thorac Cardiovasc Surg. 2016;64:581–8. https://doi.org/10.1055/s-0036-1580623.
Chen X, Yuan R, Chen X, Sun M, Lin S, Ye J, et al. Hypnosis intervention for the management of pain perception during cataract surgery. J Pain Res. 2018;11:1921–6. https://doi.org/10.2147/JPR.S174490.
Treggiari-Venzi MM, Suter PM, de Tonnac N, Romand J-A. Successful use of hypnosis as an adjunctive therapy for weaning from mechanical ventilation. Anesthesiol J Am Soc Anesthesiol. 2000;92:890.
Jafarizadeh H, Lotfi M, Ajoudani F, Kiani A, Alinejad V. Hypnosis for reduction of background pain and pain anxiety in men with burns: a blinded, randomised, placebo-controlled study. Burns J Int Soc Burn Inj. 2018;44:108–17. https://doi.org/10.1016/j.burns.2017.06.001.
Kendrick C, Sliwinski J, Yu Y, Johnson A, Fisher W, Kekecs Z, et al. Hypnosis for acute procedural pain: a critical review. Int J Clin Exp Hypn. 2016;64:75–115. https://doi.org/10.1080/00207144.2015.1099405.
Chamine I, Atchley R, Oken BS. Hypnosis intervention effects on sleep outcomes: a systematic review. J Clin Sleep Med. 2018;14:271–83. https://doi.org/10.5664/jcsm.6952.
Vanhaudenhuyse A, Gillet A, Malaise N, Salamun I, Grosdent S, Maquet D, et al. Psychological interventions influence patients’ attitudes and beliefs about their chronic pain. J Tradit Complement Med. 2018;8:296–302. https://doi.org/10.1016/j.jtcme.2016.09.001.
Vanhaudenhuyse A, Gillet A, Malaise N, Salamun I, Barsics C, Grosdent S, et al. Efficacy and cost-effectiveness: a study of different treatment approaches in a tertiary pain centre. Eur J Pain Lond Engl. 2015;19:1437–46. https://doi.org/10.1002/ejp.674.
Cordi MJ, Hirsiger S, Mérillat S, Rasch B. Improving sleep and cognition by hypnotic suggestion in the elderly. Neuropsychologia. 2015;69:176–82. https://doi.org/10.1016/j.neuropsychologia.2015.02.001.
Grégoire C, Bragard I, Jerusalem G, Etienne A-M, Coucke P, Dupuis G, et al. Group interventions to reduce emotional distress and fatigue in breast cancer patients: a 9-month follow-up pragmatic trial. Br J Cancer. 2017;117:1442–9. https://doi.org/10.1038/bjc.2017.326.
Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191:731–8. https://doi.org/10.1164/rccm.201411-2099CI.
Brito RA, do Nascimento Rebouças Viana SM, Beltrão BA, de Araújo Magalhães CB, de Bruin VMS, de Bruin PFC. Pharmacological and non-pharmacological interventions to promote sleep in intensive care units: a critical review. Sleep Breath. 2019. https://doi.org/10.1007/s11325-019-01902-7.
Lynn SJ, Laurence J-R, Kirsch I. Hypnosis, suggestion, and suggestibility: an integrative model. Am J Clin Hypn. 2015;57:314–29. https://doi.org/10.1080/00029157.2014.976783.
Rizzo AS, Koenig ST. Is clinical virtual reality ready for primetime? Neuropsychology. 2017;31:877–99. https://doi.org/10.1037/neu0000405.
Wiederhold BK, Wiederhold MD. A review of virtual reality as a psychotherapeutic tool. CyberPsychol Behav. 1998;1:45–52. https://doi.org/10.1089/cpb.1998.1.45.
Gupta A, Scott K, Dukewich M. Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it? Pain Med. 2018;19:151–9. https://doi.org/10.1093/pm/pnx109.
Baños RM, Botella C, García-Palacios A, Martín HV, Perpiñá C, Raya MA. Presence and reality judgment in virtual environments: a unitary construct? Cyberpsychol Behav Soc Netw. 2000;3:327–35. https://doi.org/10.1089/10949310050078760.
Patterson DR, Wiechman SA, Jensen M, Sharar SR. Hypnosis delivered through immersive virtual reality for burn pain: a clinical case series. Int J Clin Exp Hypn. 2006;54:130–42. https://doi.org/10.1080/00207140500528182.
Patterson DR, Hoffman HG, Palacios AG, Jensen MJ. Analgesic effects of posthypnotic suggestions and virtual reality distraction on thermal pain. J Abnorm Psychol. 2006;115:834–41. https://doi.org/10.1037/0021-843X.115.4.834.
Enea V, Dafinoiu I, Opriş D, David D. Effects of hypnotic analgesia and virtual reality on the reduction of experimental pain among high and low hypnotizables. Int J Clin Exp Hypn. 2014;62:360–77. https://doi.org/10.1080/00207144.2014.901087.
Tashjian VC, Mosadeghi S, Howard AR, Lopez M, Dupuy T, Reid M, et al. Virtual reality for management of pain in hospitalized patients: results of a controlled trial. JMIR Ment Health. 2017;4:e9. https://doi.org/10.2196/mental.7387.
Furman E, Jasinevicius TR, Bissada NF, Victoroff KZ, Skillicorn R, Buchner M. Virtual reality distraction for pain control during periodontal scaling and root planing procedures. J Am Dent Assoc. 2009;140:1508–16. https://doi.org/10.14219/jada.archive.2009.0102.
Cardoş RAI, David OA, David DO. Virtual reality exposure therapy in flight anxiety: a quantitative meta-analysis. Comput Hum Behav. 2017;72:371–80. https://doi.org/10.1016/j.chb.2017.03.007.
Chou H-Y, Chen S-C, Yen T-H, Han H-M. Effect of a virtual reality-based exercise program on fatigue in hospitalized Taiwanese end-stage renal disease patients undergoing hemodialysis. Clin Nurs Res. 2018. https://doi.org/10.1177/1054773818788511.
Patterson DR, Jensen MP, Wiechman SA, Sharar SR. Virtual reality hypnosis for pain associated with recovery from physical trauma. Int J Clin Exp Hypn. 2010;58:288–300. https://doi.org/10.1080/00207141003760595.
Patterson DR, Tininenko JR, Schmidt AE, Sharar SR. Virtual reality hypnosis: a case report. Int J Clin Exp Hypn. 2004;52:27–38. https://doi.org/10.1076/iceh.52.1.27.23925.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;304:81–4. https://doi.org/10.1016/S0140-6736(74)91639-0.
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. The Richmond agitation–sedation scale. Am J Respir Crit Care Med. 2002;166:1338–44. https://doi.org/10.1164/rccm.2107138.
Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8:1153–7. https://doi.org/10.1111/j.1553-2712.2001.tb01132.x.
Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis. 1986;174:727–35. https://doi.org/10.1097/00005053-198612000-00004.
Tellegen A, Atkinson G. Openness to absorbing and self-altering experiences (“absorption”), a trait related to hypnotic susceptibility. J Abnorm Psychol. 1974;83:268–77.
Vanhaudenhuyse A, Ledoux D, Gosseries O, Demertzi A, Laureys S, Faymonville M-E. Can subjective ratings of absorption, dissociation, and time perception during “neutral hypnosis” predict hypnotizability?: an exploratory study. Int J Clin Exp Hypn. 2019;67:28–38. https://doi.org/10.1080/00207144.2019.1553765.
Edmonds ZV, Mower WR, Lovato LM, Lomeli R. The reliability of vital sign measurements. Ann Emerg Med. 2002;39:233–7. https://doi.org/10.1067/mem.2002.122017.
Sharman JE, LaGerche A. Exercise blood pressure: clinical relevance and correct measurement. J Hum Hypertens. 2015;29:351–8. https://doi.org/10.1038/jhh.2014.84.
Rouche O, Wolak-Thierry A, Destoop Q, Milloncourt L, Floch T, Raclot P, et al. Evaluation of the depth of sedation in an intensive care unit based on the photo motor reflex variations measured by video pupillometry. Ann Intensive Care. 2013;3:5. https://doi.org/10.1186/2110-5820-3-5.
Fox J, Bouchet-Valat M, Andronic L, Ash M, Boye T, Calza S, et al. Rcmdr: R Commander. 2019.
Mallari B, Spaeth EK, Goh H, Boyd BS. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res. 2019;12:2053–85. https://doi.org/10.2147/JPR.S200498.
Oing T, Prescott J. Implementations of virtual reality for anxiety-related disorders: systematic review. JMIR Serious Games. 2018;6:e10965. https://doi.org/10.2196/10965.
Eijlers R, Utens EMWJ, Staals LM, de Nijs PFA, Berghmans JM, Wijnen RMH, et al. Systematic review and meta-analysis of virtual reality in pediatrics: effects on pain and anxiety. Anesth Analg. 2019;129:1344–53. https://doi.org/10.1213/ANE.0000000000004165.
Thompson T, Terhune DB, Oram C, Sharangparni J, Rouf R, Solmi M, et al. The effectiveness of hypnosis for pain relief: a systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev. 2019;99:298–310. https://doi.org/10.1016/j.neubiorev.2019.02.013.
Acknowledgements
The authors would like to thank the University and University Hospital of Liège, the Benoit Fundation (Belgium), the Non-Fria Grant (Belgium) and the Belgian Cancer Fundation (2017-064) for their financial contribution. Thanks to Oncomfort Society (www.oncomfort.com) (Wavre) and Musicotherapy and Counselling (www.musicotherapeute.be) (Liège) for the material. Thanks to all departments of the University Hospital of Liege involved in the project: Cardiac Surgery Department, Algology Department, Department of Intensive Care Units.
Funding
Funding covers fees of PhD student (FR) and post-doc researcher (AV). This work was supported by the University and University Hospital of Liège, the Benoit Foundation (Belgium), the Non-Fria Grant (Belgium) and the Belgian Cancer Foundation (2017–064).
Author information
Authors and Affiliations
Contributions
Study conception: FR, AV, ASN, MEF, DL, PBM. Acquisitions: FR. Analysis and interpretation data: FR, ND. Drafted, reviewed and finalized protocol: FR, DL, PBM, AV, MEF, ASN. Reviewed and approved the final version of this manuscript: AV, MEF, ASN. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Ethic Committee of the Faculty of Medicine and the Ethic Committee of the Faculty of Psychology, Speech Therapy and Educational Sciences of the University of Liège. This trial was registered on ClinicalTrials.gov with the trial identification number NCT03820700 in January 2019. The trial is currently ongoing and recruiting. Written informed consent templates authorized by the Committee of the Faculty of Medicine and the Ethic Committee of the Faculty of Psychology, Speech Therapy and Educational Sciences of the University of Liège is obtained from all individuals before initiation of study procedures.
Results of the trial will be reported in national and international meetings, as well as in scientific journals. There is no plan to individually notify participants regarding the results of this study. If the feasibility of implementing hypnosis, VR and VRH, during the preoperative or the postoperative periods is shown by this study, larger studies can be undertaken to study the effects of one of these tools on anxiety and pain in intensive care units.
Competing interests
Marie-Elisabeth Faymonville is part of a scientific board of Oncomfort society (www.oncomfort.com). Other authors report no conflicts of interest in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rousseaux, F., Faymonville, ME., Nyssen, AS. et al. Can hypnosis and virtual reality reduce anxiety, pain and fatigue among patients who undergo cardiac surgery: a randomised controlled trial. Trials 21, 330 (2020). https://doi.org/10.1186/s13063-020-4222-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-020-4222-6