Abstract
Background
A common complication after stroke is development of cognitive impairment and dementia. However, effective strategies for reducing the risk of developing these problems remain undefined. Potential strategies include intensive lowering of blood pressure (BP) and/or lipids. This paper summarises the baseline characteristics, statistical analysis plan and feasibility of a randomised control trial of blood pressure and lipid lowering in patients post-stroke with the primary objective of reducing cognitive impairment and dementia.
Methods
The Prevention Of Decline in Cognition After Stroke Trial (PODCAST) was a multi-centre prospective randomised open-label blinded-endpoint controlled partial-factorial internal pilot trial running in secondary and primary care. Participants without dementia were enrolled 3–7 months post ischaemic stroke or spontaneous intracerebral haemorrhage, and randomised to intensive versus guideline BP lowering (target systolic BP <125 mmHg versus <140 mmHg); patients with ischaemic stroke were also randomised to intensive or guideline lipid lowering (target LDL cholesterol <1.4 mmol/L versus <3 mmol/L). The primary outcome was the Addenbrooke’s Cognitive Examination-Revised; a key secondary outcome was to assess feasibility of performing a large trial of one or both interventions. Data are number (%) or mean (standard deviation). The trial was planned to last for 8 years with follow-up between 1 and 8 years. The plan for reporting the main results is included as Additional file 2.
Results
83 patients (of a planned 600) were recruited from 19 UK sites between 7 October 2010 and 31 January 2014. Delays, due to difficulties in the provision of excess treatment costs and to complexity of follow-up, led to few centres taking part and a much lower recruitment rate than planned. Patient characteristics at baseline were: age 74 (SD 7) years, male 64 (77 %), index stroke ischaemic 77 (93 %), stroke onset to randomisation 4.5 [SD 1.3] months, Addenbrooke’s Cognitive Examination-Revised 86 (of 100, SD 8), Montreal Cognitive Assessment 24 (of 30, SD 3), BP 147/82 (SD 19/11) mmHg, total cholesterol 4.0 (SD 0.8) mmol/L and LDL cholesterol 2.0 (SD 0.7) mmol/L, modified Rankin Scale 1.1 (SD 0.8).
Conclusion
Limited recruitment suggests that a large trial is not feasible using the current protocol. The effects of the interventions on BP, lipids, and cognition will be reported in the main publication.
Trial registration
ISRCTN85562386 registered on 23 September 2009
Similar content being viewed by others
Background
Post-stroke cognitive decline and dementia are common, and potentially devastating for both patients and carers. Although lowering blood pressure (BP) and lipids and the use of antithrombotic therapy are known to reduce recurrence after ischaemic stroke [1–6], the effect of these and other interventions on cognition is unclear [7]. The trial designated Prevention Of Decline in Cognition After Stroke Trial (PODCAST) was designed to assess the safety and tolerability of intensive versus guideline BP and lipid lowering in ischaemic stroke and intensive BP versus guideline in haemorrhagic stroke and the feasibility of performing a large trial on whether intensive treatment reduces cognitive decline and dementia post stroke (Protocol, Additional file 1) [8].
Aims
Start-up phase
-
To determine the initial safety and the tolerability of intensive versus guideline BP and lipid lowering therapy.
-
To determine the feasibility of recruiting and retaining sites and participants in a long-term dementia prevention trial involving patients with a previous stroke.
-
To determine the feasibility of reaching and maintaining target BP and lipid levels and identify any barriers to achieving and maintaining BP and lipid targets.
-
To determine the feasibility of performing recurrent cognitive assessment in clinic and by telephone.
Main phase
-
To determine if ‘intensive’ blood pressure lowering therapy and/or ‘intensive’ lipid lowering therapy after stroke reduces cognitive decline and dementia.
-
To determine if ‘intensive’ blood pressure lowering therapy and/or ‘intensive’ lipid lowering therapy after stroke reduces poor quality of life, poor function, depression, stroke recurrence, vascular events, and death.
Methods
PODCAST was a multi-centre prospective randomised open-label blinded-endpoint controlled partial-factorial phase IV trial in secondary and primary care. Participants from 30 UK Stroke Research Network sites who were post ischaemic stroke or intracerebral haemorrhage by 3–7 months were included. All patients gave informed consent.
Interventions
All patients were randomised (1:1) to intensive versus guideline blood pressure lowering (target systolic <125 mmHg versus <140 mmHg). Patients with an ischaemic stroke were also randomised (1:1) to intensive versus guideline lipid lowering (target low density lipoprotein-cholesterol <1.4 mmol/L versus <3 mmol/L). As a result, patients were randomised to one of six groups:
-
Intensive BP lowering and intensive lipid lowering (ischaemic stroke only)
-
Intensive BP lowering and guideline lipid lowering (ischaemic stroke only)
-
Guideline BP lowering and intensive lipid lowering (ischaemic stroke only)
-
Guideline BP lowering and guideline lipid lowering (ischaemic stroke only)
-
Intensive BP lowering only (intracerebral haemorrhage only)
-
Guideline BP lowering only (intracerebral haemorrhage only)
Intensive interventions were delivered in a secondary care/hospital research clinic; guideline interventions were delivered in primary care according to local practice.
Primary outcome
Addenbrooke’s Cognitive Examination-Revised. This was assessed at each clinic visit and its analysis and presentation is described in the outline data tables (Additional file 2). It is a brief cognitive screening tool that was selected because it has greater sensitivity in detecting Alzheimer’s disease and is more sensitive than the Mini-Mental State Examination (MMSE) [9] and has been shown to be sensitive at detecting mild cognitive impairment (MCI) in post-stroke populations [10]. It is good at detecting visuospatial, fluency and executive dysfunction [11].
Secondary outcomes
Feasibility of recruitment and retention of participants, tolerability and safety of the interventions, achieving and maintaining the blood pressure and lipid targets, maintaining differences in systolic blood pressure (>10 mmHg) and low density lipoprotein cholesterol (>1 mmol/L) between the treatment groups, and performing clinic and telephone follow-up of cognition measures. Additional tests of cognition were used:
-
Stroop, Trail making. Additional measures of executive function were added since vascular cognitive impairment is known to have a greater effect on these cognitive domains and they better predict development of dementia and mortality [12–15].
-
Montreal Cognitive Assessment (MoCA). This is widely used in post-stroke populations with reasonable sensitivity for detecting MCI and dementia [10, 16–18].
-
Telephone MMSE (t-MMSE). This has been compared to the MMSE and has shown strong correlation. A score of 16 on the t-MMSE equates to a score of 19 on the MMSE. A score of 26 on the MMSE equates to a score of 23 on the t-MMSE [19].
-
Telephone Interview for Cognitive Status (TICS). This has been validated in a post-stroke population [20].
Blinding
Participants received open-label management. Cognition was assessed both unblinded (in clinic) and blinded (by telephone) to treatment. Adjudication of events (dementia, vascular, serious adverse events) was blinded to management. Recruitment of 600 participants was planned (300/BP group, about 270/statin group) to be sufficient to demonstrate whether sufficient on-treatment differences in BP and lipids can be obtained and maintained, and whether cognition can be assessed satisfactorily. More details of methodology can be found at the published protocol [8].
The study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation of Good Clinical Practice. The study was approval by the national research ethics committee (NRES Committee East Midlands – Nottingham 1, approval 09/H0403/71, date 12/11/2009).
Eligibility
The full inclusion and exclusion criteria are given in the published protocol [8] and were designed to include a population who did not have known dementia but who were at higher risk of developing cognitive impairment and dementia on the basis of recent stroke and age, and who were likely to be able to attend follow-up for 5 years. Adult patients were eligible if they fulfilled all of the following criteria:
-
Age >70 years and telephone MMSE (t-MMSE) >16
-
° or age >60 and t-MMSE 17–20 (that is, indicator of impairment).
-
-
Previously independent (mRS 0–2)
-
Index event was ischaemic stroke (IS) or spontaneous intracerebral haemorrhage (ICH)
-
Systolic blood pressure between 125–170 mmHg
-
Total cholesterol 3–8 mmol/L
-
3–7 months post stroke onset
-
Ability to give written informed consent prior to enrolment
-
Had two informants who could support them if cognitive impairment developed
Consent
Informed consent was obtained to undertake a screening cognitive assessment at 8–26 weeks using a telephone version of the MMSE; initially this was done by telephone but was later delivered in clinic. If screened positive, informed consent for the trial was obtained and the clinician used the secure web-based randomisation system to enter a patient into the study between 12 and 30 months post stroke.
Randomisation
To reduce bias and optimise baseline matching between treatment groups, randomisation incorporated stratification (index event), minimisation (on baseline prognostic factors, as highlighted in Table 3), and simple randomisation (in 5 % of patients). Stratification and minimisation allow for improved matching at baseline, minimisation increases statistical power [21], and simple randomisation reduces predictability. The stratification and minimisation variables will be used for adjustment of the primary and secondary analyses. Following randomisation, the investigator was informed of the patient’s treatment allocation. Data entry during treatment used the same website and similar range and logic checks.
Trial governance
The trial was supervised by a Trial Steering Committee, and run by a Trial Management Committee (based in Nottingham UK). An independent Data Monitoring Committee met and assessed safety and efficacy on three occasions. Experts, who were blinded to treatment assignment, adjudicated cognitive and dementia outcomes, vascular events, brain scans, and serious adverse events.
Internet trial
The trial was designed as an electronic trial and was accessible via the following links:
-
Trial website: http://www.podcast-trial.org
-
Secure website for real-time data entry, validation and randomisation: https://www.nottingham.ac.uk/~nszwww/podcast/podcasttrialdb/podcast_login.php
-
Demo website for investigators to practise data entry: https://www.nottingham.ac.uk/~nszwww/podcast/podcasttrialdb-demo/podcast_login.php (log-in: demoinv1, password: nottingham; pin: 8888)
-
Resource website for investigators with all trial documents, including all protocol versions: http://www.podcast-trial.org/jevpybki.htm
-
Stroop test website: https://www.nottingham.ac.uk/~nszwww/stroop_demo
Data were entered via a secure Internet site. However, some NHS sites could not access certain features, in particular the Stroop application that ran as a Java applet. Problems arose because of hospital firewalls and issues with Java updates on PC and Mac computers.
Progress with the study and modifications to the design
The trial was designed to have two phases of recruitment and funding:
-
Start-up phase (36 months, 2010–2013): Recruitment of 600 patients from 30 sites over 24 months with minimum follow-up of at least 12 months. In reality, 83 patients were recruited with the target reduced to 100 patients.
-
Main phase (60 months): New funding to be sought for recruitment of 2,800 patients from 100 sites. Funding was never sought since the trial failed to recruit sufficient sites or patients at a rate that was feasible to support a large study.
Several protocol amendments were made, these covering the following issues:
August 2010
-
1.
Addition of twice yearly email reminders to investigators to highlight the need to achieve targets in BP and lipid lowering in patients randomised to intensive treatment. This was done because the difference between intensive and guideline BP and lipid levels was not reaching target (>1 mmol/L LDL cholesterol and >10 mmHg systolic).
May 2012
-
1.
Reduction in target lipid level: The level of target lipid concentration for intensive arm was reduced from LDL cholesterol <2.0 mmol/L to <1.3 mmol/L. This amendment was required because 50 % of patients were at or below the original target at baseline of 2.0 mmol/L, in part reflecting the high usage of statin therapy in this post-stroke population.
-
2.
Revision of suggested guideline lipid lowering therapy (to simvastatin 10–40 mg, pravastatin 10–40 mg, fluvastatin 10–80 mg), and intensive group statins (to atorvastatin > 20 mg, any dose of rosuvastatin). This was due to revised NICE guidelines on lipid management (CG 67, 2008).
-
3.
Addition of monitoring of serum glucose and HbA1c because some BP and lipid drugs may reduce, or cause, diabetes mellitus.
-
4.
Introduction of ‘floating’ clinic appointments to follow planned regular research appointments if a patient randomised to one or both intensive arms had BP and/or lipid readings above target levels. These floating appointments allowed earlier/more aggressive treatment escalation.
-
5.
Clarification that a standing blood pressure measurement should be done in clinic to detect postural hypotension.
-
6.
Addition of NYHA levels 3 or 4 as an exclusion criterion
-
7.
Removal of dementia-specific health-related Quality of Life (DEMQOL) as an outcome
-
8.
Addition/inclusion of patients with posterior circulation infarcts (POCI). POCI was an original exclusion criterion because this group was felt unlikely to develop cognitive impairment. However cerebellar and brain stem infarcts are known to cause cognitive impairment.
-
9.
Follow-up visits in clinic increased from once a year to every 6 months (with interval blinded telephone follow-up). Low recruitment meant that long-term follow-up in a large trial was unlikely, and so more and earlier visits were necessary to assess and escalate treatment for blood pressure and/or lipid.
June 2013
-
1.
Reduction in sample size for the start-up phase from 600 to 100 patients.
-
2.
Addition of an on-treatment CT scan at or after 1 year of treatment to assess potential changes in white matter disease and cerebral atrophy from pre-baseline.
Key milestones occurred as follows:
-
January 2008: Trial planning commenced.
-
July 2008: Joint funding application submitted to Alzheimer’s Society and Stroke Association.
-
January 2009: Funding awarded.
-
April 2009: Confirmation from UK Medicines and Healthcare products Regulatory Agency that trial is not a Clinical Trial of an Investigational Medical Product (CTIMP) and does not fall under the remit of the European Clinical trials Directive.
-
November 2009: Approval by UK National Research Ethics Service (Nottingham Research Ethics Committee 1).
-
September 2010: Commencement of funding.
-
October 2010: Recruitment of first patient.
-
September 2012: One year no-cost extension sought and obtained from funders because of low recruitment.
-
November 2013: Protocol published [8].
-
January 2014: Recruitment of last patient (that is, recruitment over 39 months).
-
October 2014: Final follow-up of last patient. End of funding.
Results
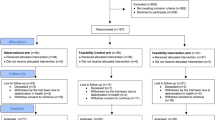
Only 83 participants were recruited (Fig. 1). The start-up phase did not recruit sufficient numbers to go to the full trial (planned recruitment of 600). A no-cost extension was requested with the aim of recruiting 100 participants. Funding for the main phase of the trial was never sought since the trial failed to recruit at a rate that was feasible to support a large study. Multiple reasons explain poor recruitment of both hospital sites and patients (Table 1). A key problem was the cost of intensive treatment, largely related to atorvastatin, which was proposed in the protocol for use in the intensive lipid control arm [8]. In many cases, the then primary care trusts (now clinical commissioning groups) refused to pay for atorvastatin. Nevertheless, none of these impediments damaged data integrity or validity.
Baseline characteristics
106 patients failed screening with some patients having multiple reasons for screen failure (Table 2). Between 1 October 2010 and 31 January 2014, 83 patients were recruited from 19 sites in the UK; a further 5 sites screened patients but did not recruit a patient.
Patient characteristics at baseline are shown in Table 3; key baseline data included, given as number (%) or mean (standard deviation): age 74.0 (6.8) years, male 64 (77.1 %), index stroke ischaemic 77 (92.8 %), stroke onset to randomisation 4.5 [1.3] months, Addenbrooke’s Cognitive Examination-Revised 86.1 (7.7, maximum score 100), Montreal Cognitive Assessment 24.0 (2.6, maximum score 30), modified Rankin Scale 1.1 (0.8), blood pressure 147.1/82.1 (18.6/11.1) mmHg, total cholesterol 4.0 (0.8) mmol/L and LDL cholesterol 2.0 (0.7) mmol/L. All but one participant had one informant and a majority had two informants (Table 4).
Reporting of results by allocated treatment
The database was locked and the trial unblinded and an interim analysis performed in November 2014, as per the SAP. Interim main results were reported at the UK Stroke Forum in early December 2014. Full results (presented in Additional file 2) will be submitted for publication once the final analysis has been performed. Summary and individual patient data from PODCAST will be shared with relevant Cochrane and other systematic reviews.
Discussion
Having recruited 83 patients, PODCAST did not achieve its adjusted recruitment target of 100 patients. As a result, the aim of assessing the feasibility of delivering the main phase was not met. Other aims, including the ability to obtain and then maintain differences in BP and lipid levels between intensive and guideline groups, ability to collect cognition scores during regular clinic and telephone follow-up, and incidence rates of cognitive impairment and dementia, will be reported in the main results.
Conclusions
Future trials of dementia prevention in post-stroke populations are required because 20–30 % of stroke survivors will have dementia [22], but >50 % of patients will have cognitive impairment [13, 14]. Cognitive impairment, especially executive dysfunction, results in impaired function, distress to patients and carers and is associated with worse prognosis [23–25]. However, the post-stroke population is very heterogeneous. Also rates of dementia at or before 1 year post-stroke range from 41.3 % in hospital series without excluding prior dementia to 7.4 % in community series of first ever stroke where prior dementia was excluded [23]. Importantly, participants appear to be keen to take part in this type of study, as post-stroke cognitive impairment is one of the silent unmet needs in >40 % of stroke survivors at 1 year [26].
Abbreviations
- ACE-R:
-
Addenbrooke’s Cognitive Examination-Revised
- BP:
-
blood pressure
- BPM:
-
beats per minute
- EQ-5D:
-
EuroQoL-5 dimensions
- EQ-VAS:
-
EuroQoL-Visual Analogue Scale
- HUS:
-
Health Utility Status (derived from EQ-5D)
- ICH:
-
intracerebral haemorrhage
- MMSE:
-
Mini-Mental State Examination
- MoCA:
-
Montreal Cognitive Assessment
- mRS:
-
Modified Rankin Scale
- NIHSS:
-
National Institutes of Health Stroke Scale
- PODCAST:
-
Prevention Of Decline in Cognition After Stroke Trial
- SAP:
-
statistical analysis plan
- SBP:
-
systolic BP
- TICS:
-
Telephone Interview Cognition Scale
- TOAST:
-
Trial of ORG 10172 in Acute Stroke Treatment
- ZDS:
-
Zung Depression Scale
References
Algra A, van Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1996;60(2):197–9.
EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after TIA or minor stroke. Lancet. 1993;342:1255–62.
Halkes PH, Gray LJ, Bath PM, Diener HC, Guiraud-Chaumeil B, Yatsu FM, et al. Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta-analysis by risk. J Neurol Neurosurg Psychiatry. 2008;79(11):1218–23.
Leonardi-Bee J, Bath PM, Bousser MG, Davalos A, Diener H-C, Guiraud-Chaumeil B, et al. Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke. 2005;36(1):162–8.
Manktelow BN, Potter JF. Interventions in the management of serum lipids for preventing stroke recurrence. Cochrane Database Syst Rev. 2009;8(3):CD002091.
Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and the secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–9.
Ankolekar S, Geeganage C, Anderton P, Hogg C, Bath PM. Clinical trials for preventing post stroke cognitive impairment. J Neurol Sci. 2010;299(1–2):168–74.
Blackburn D, Krishnan K, Fox L, Ballard C, Burns A, Ford G, et al. Prevention of Decline in Cognition after Stroke Trial (PODCAST): a study protocol for a factorial randomised controlled trial of intensive versus guideline lowering of blood pressure and lipids. Trials J. 2013;14:401.
Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21(11):1078–85.
Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke. 2012;43(2):464–9.
Morris K, Hacker V, Lincoln NB. The validity of the Addenbrooke's Cognitive Examination-Revised (ACE-R) in acute stroke. Disabil Rehabil. 2012;34(3):189–95.
Melkas S, Vataja R, Oksala NK, Jokinen H, Pohjasvaara T, Oksala A, et al. Depression-executive dysfunction syndrome relates to poor poststroke survival. Am J Geriatr Psychiatry. 2010;18(11):1007–16.
Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, Kappelle LJ, de Haan EH. Restrictions of the Mini-Mental State Examination in acute stroke. Arch Clin Neuropsychol. 2005;20(5):623–9.
Nys GM, van Zandvoort MJ, de Kort PL, van der Worp HB, Jansen BP, Algra A, et al. The prognostic value of domain-specific cognitive abilities in acute first-ever stroke. Neurology. 2005;64(5):821–7.
Wiberg B, Kilander L, Sundström J, Byberg L, Lind L. The relationship between executive dysfunction and post-stroke mortality: a population-based cohort study. BMJ Open. 2012;2:3.
Dong Y, Slavin MJ, Chan BP, Venketasubramanian N, Sharma VK, Collinson SL, et al. Improving screening for vascular cognitive impairment at three to six months after mild ischemic stroke and transient ischemic attack. Int Psychogeriatr. 2014;26(5):787–93.
Dong Y, Yean Lee W, Hilal S, Saini M, Wong TY, Chen CL, et al. Comparison of the Montreal Cognitive Assessment and the Mini-Mental State Examination in detecting multi-domain mild cognitive impairment in a Chinese sub-sample drawn from a population-based study. Int Psychogeriatr. 2013;25(11):1831–8.
Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. Impact of different operational definitions on mild cognitive impairment rate and MMSE and MoCA performance in transient ischaemic attack and stroke. Cerebrovasc Dis. 2013;36(5–6):355–62.
Newkirk LA, Kim JM, Thompson JM, Tinklenberg JR, Yesavage JA, Taylor JL. Validation of a 26-point telephone version of the Mini-Mental State Examination. J Geriatr Psychiatry Neurol. 2004;17(2):81–7.
Barber M, Stott DJ. Validity of the Telephone Interview for Cognitive Status (TICS) in post-stroke subjects. Int J Geriatr Psychiatry. 2004;19(1):75–9.
Weir CJ, Lees KR. Comparison of stratification and adaptive methods for treatment allocation in an acute stroke clinical trial. Stat Med. 2003;22:705–26.
Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–18.
Oksala NK, Jokinen H, Melkas S, Oksala A, Pohjasvaara T, Hietanen M, et al. Cognitive impairment predicts poststroke death in long-term follow-up. J Neurol Neurosurg Psychiatry. 2009;80(11):1230–5.
Tatemichi TK, Paik M, Bagiella E, Desmond DW, Pirro M, Hanzawa LK. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25(10):1915–9.
Wiberg B, Lind L, Kilander L, Zethelius B, Sundelof JE, Sundstrom J. Cognitive function and risk of stroke in elderly men. Neurology. 2010;74(5):379–85.
McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-Reported Long-Term Needs After Stroke. Stroke. 2011;42:1398-1403.
Acknowledgements
We thank all the patients, and their informants, for participating in the trial. We also thank the funders: the Alzheimer’s Society and the Stroke Association. The sponsor was the University of Nottingham. Finally, we thank the members of the independent Data Monitoring Committee who supervised safety from the beginning to end of the trial.
Chief Investigator(s)
P. M. W. Bath (trial, and lipid arm), G. Ford (BP arm)
Trial Steering Committee
Independent members: John O’Brien (Chair, Newcastle/Cambridge), Stephen Ball (Leeds)
Grant holders: Philip M. W. Bath (Chief Investigator, Nottingham), Gary Ford (Co-Chief Investigator, Newcastle/Oxford), Nikola Sprigg (Deputy Chief Investigator, Nottingham), Clive Ballard (London), Alistair Burns (Manchester), Jonathan Mant (Cambridge), Peter Passmore (Belfast), Stuart Pocock (Consultant Statistician, London), John Reckless (Bath), Rob Stewart (London), Joanna M Wardlaw (Neuroradiologist, Edinburgh).
Patient & Carer representatives: Dave Hanbury, John Murray, Shirley Nurock
Funders' representative: Suzanne Sorensen (Alzheimer’s Society, −2011), James Pickett (Alzheimer’s Society, 2011–), Peter Coleman (Stroke Association, 2010–14), Kate Holmes (2014–).
Sponsor representative: Angela Shone (University of Nottingham).
Independent Data Monitoring Committee
John Geddes (Oxford, Chair), Jan Staessen (Leuven), Chris Weir (Glasgow, Statistician).
Trial Management Committee
Trial Managers: S. Utton (−2014).
Assistant Trial Manager: H. Foster (2012–).
Medics: Sandeep Ankolekar (−2011), K. Krishnan (2012–).
Coordinators: L. Stokes (−2012), M. Mangoyana (2012–13), K. Whittamore (2013–14), M. Stringer (2014–).
Outcome Coordinators: J. Saunders (−2012), L. Wilkins (2011–12), L. Cobane (2012–13), J. Keeling (2013–).
Data managers: T. Jones (−2013), D. Hazle (2012–), M. Sampson (2013–).
Finance: W. Clarke.
Secretaries: Y. Smallwood, L. Dunn (2013–14).
Cognition/Dementia Adjudicators:
Alistair Burns (Chair, Manchester), Clive Ballard (London), Gary Ford (Newcastle).
Vascular Events Adjudicators:
Peter Passmore (Chair, Belfast), Amit Mistri (Leicester, 2013–14), Robert Henderson (Nottingham, 2013–14).
Serious Adverse Events Adjudicators
Tim England (Derby).
Neuroimaging Adjudicators
Joanna M. Wardlaw (Chair, Edinburgh), Dan Blackburn (Sheffield, 2014).
Health economics
Paul McCrone (London), Martin Knapp (London)
Statistical Analysis
L. Fox (2012–13), P. Scutt (2013–), Lisa Woodhouse (2014–).
Data Management and Programming
Liz Walker, L. Haywood (2011–), Richard Dooley (2013–14)
Recruiting sites (patients recruited/screened): PI, Investigators
Aberdeen Royal Infirmary (6/14): Dr M. J. MacLeod, A. Joyson, H. Gow, J. Furnace
Aintree Hospitals NHS Foundation Trust (5/9): Dr R. Durairaj, C. Cullen, D. Dickinson, M. Ashkar, T. Ingram, V. Sutton, Z. Mellor
Birmingham, Heart of England NHS Foundation Trust – Solihull Hospital (2/9): Dr K. Elfandi, U. Kahn, S. Stafford
Bournemouth, Royal & Christchurch Hospitals NHS Foundation Trust. (6/14): Dr D. Tiwari, A. Orpen, B. Longland, C. Ovington, E. Rogers, G. Hann, J. Bell, J. Kwan
Bradford Teaching Hospitals NHS Foundation Trust (1/5): Dr C. Patterson, B. Hairsine, L. Johnston, R. Bellfield
Chesterfield Royal Hospital NHS Foundation Trust (2/9): Dr M. Sajid, M. Ball
Cornwall, Royal Hospitals NHS Trust, Truro (9/17): Dr F. Harrington, A. Mate, C. Schofield, G. Courtauld, M. Drake
Doncaster & Bassetlaw Hospitals NHS Foundation Trust (1/2): Dr M. Kini, D. Walstow, M. Hanna
Inverness, Raigmore Hospital (1/3): Dr P. F. Findlay, A. Macaden, I. Shread
Margate, QEQM Hospital (1/1): Dr G. Thomas, S. A. Jones
Mid Cheshire Hospitals NHS Foundation Trust (3/10): Dr A. Kalathil, N. Gautam, R. Miller
Newcastle-upon-Tyne Hospitals NHS Foundation Trust (6/15): Dr S. Louw, A. Barkat, G. Kennedy, M. Fawcett, T. Thompson, V. Hogg
North Tees & Hartlepool NHS Foundation Trust (2/4): Dr B. Kumar, D. Bruce, M. Platton
Nottingham University Hospitals NHS Trust (22/27): Dr A. K. Shetty, Prof P. Bath, Dr K. Krishnan, F. Shelton, J. Clarke, L. Wilkins, Z. Rose, K. Whittamore
Scarborough General Hospital (1/1): Dr J. Paterson, K. Deighton
South Tyneside NHS Foundation Trust (1/4): Dr J. Scott, J. Graham, M. Duffy
West Cumberland Hospital, Whitehaven (1/1): Dr E. Orugun, H. Crowther, R. Jolly
Yeovil District Hospital NHS Foundation Trust (3/17): Dr K. Rashed, C. Buckley, C. Vickers, D. Hayward, E. Keeling, S. Board
York Hospitals NHS Foundation Trust (10/11): Dr J. Coyle, A-M Porteous, M. Keeling, N. Dyer, P. Willcoxson
Sites that screened but did not recruit:
Bath, Royal United Hospital NHS Trust (4): Dr J. Reckless, B. Madigan, D. Button
Derby Hospitals NHS Foundation Trust (1): Dr K. Muhiddin, M. Mangoyana
Leicester, University Hospitals (2): Dr D. Eveson, S. Khan
Poole Hospital NHS Foundation Trust (7): Dr S. Ragab, C. Dickson
South Tees Hospitals NHS Foundation Trust (2): Dr D. Broughton, K. Chapman
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The following authors report having received recent research or personal funding:
P. Bath – Lundbeck, Phagenesis, ReNeuron
R. Stewart - J&J, Lundbeck, Pfizer, Roche.
The remaining authors have no relevant declarations.
Authors’ contributions
PS performed statistical analysis. PS, DB and PB wrote the manuscript. JMW and DB analysed neuroimaging. AB, CB and GF advised on dementia management and diagnosis of neurodegenerative or vascular dementia. PB and GF designed the research, and PS and PB analysed the results. KK, JMW, PP, SP, JR, NS and RS helped design the study and analyse results. All authors read and approved the final manuscript.
Polly Scutt and Dan Blackburn contributed equally to this work.
Additional files
Additional file 1:
Trial protocol. (PDF 2304 kb)
Additional file 2:
Plan for reporting the main results. (DOCX 117 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Scutt, P., Blackburn, D., Krishnan, K. et al. Baseline characteristics, analysis plan and report on feasibility for the Prevention Of Decline in Cognition After Stroke Trial (PODCAST). Trials 16, 509 (2015). https://doi.org/10.1186/s13063-015-1033-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-015-1033-2