Abstract
Background
This study seeks to investigate the impacts of Lactobacillus reuteri (L. reuteri) on hepatic ischemia-reperfusion (I/R) injury and uncover the mechanisms involved.
Methods
Mice in the I/R groups were orally administered low and high doses of L.reuteri (L.reuteri-low and L. reuteri-hi; 1 × 1010 CFU/d and 1 × 1011 CFU/d), for 4 weeks prior to surgery. Following this, mice in the model group were treated with an Nrf2 inhibitor (ML-385), palmitoylcarnitine, or a combination of both.
Results
After treatment with L. reuteri, mice exhibited reduced levels of serum aminotransferase (ALT), aspartate aminotransferase (AST), and myeloperoxidase (MPO) activity, as well as a lower Suzuki score and apoptosis rate. L. reuteri effectively reversed the I/R-induced decrease in Bcl2 expression, and the significant increases in the levels of Bax, cleaved-Caspase3, p-p65/p65, p-IκB/IκB, p-p38/p38, p-JNK/JNK, and p-ERK/ERK. Furthermore, the administration of L. reuteri markedly reduced the inflammatory response and oxidative stress triggered by I/R. This treatment also facilitated the activation of the Nrf2/HO-1 pathway. L. reuteri effectively counteracted the decrease in levels of beneficial gut microbiota species (such as Blautia, Lachnospiraceae NK4A136, and Muribaculum) and metabolites (including palmitoylcarnitine) induced by I/R. Likewise, the introduction of exogenous palmitoylcarnitine demonstrated a beneficial impact in mitigating hepatic injury induced by I/R. However, when ML-385 was administered prior to palmitoylcarnitine treatment, the previously observed effects were reversed.
Conclusion
L. reuteri exerts protective effects against I/R-induced hepatic injury, and its mechanism may be related to the promotion of probiotic enrichment, differential metabolite homeostasis, and the Nrf2/HO-1 pathway, laying the foundation for future clinical applications.
Similar content being viewed by others
Introduction
Hepatic ischemia/reperfusion (I/R) injury is a significant complication commonly seen in hepatic transplantation, resection, and cases of hemorrhagic shock [1]. This type of injury occurs when there is an insufficient oxygen supply to the liver, followed by reperfusion, leading to inflammatory responses and oxidative stress that result in cell death [2]. Although pharmacotherapy has shown promise in preventing or mitigating I/R injury in experimental settings, there is still a lack of effective strategies and validated pharmacological targets for clinical application. This is due to a limited understanding of the complex signaling events triggered by I/R injury, as well as challenges such as short circulation time, poor solubility, and severe side effects associated with available treatments [3].
The gut microbiota and its metabolites play a crucial role in regulating the development of hepatic I/R injury [4, 5]. 3,4-dihydroxyphenylpropionic acid attenuates hepatic I/R injury via regulating inflammatory activity [5]. Metabolic reprogramming promoted by glutamine originating from gut microbiota helps decrease hepatic I/R injury [6]. Probiotics can alter the host’s microbial community and provide beneficial health effects. Lactobacillus reuteri (L. reuteri) has been recognized as a probiotic for improving hepatic health, and dietary L. reuteri prevents lipopolysaccharide (LPS)-mediated hepatocyte apoptosis by improving gut flora and bile acid metabolism [7, 8]. L. reuteri DSM 17938 improves gut microbiota to reduce d-galactosamine-induced hepatic failure in rats [9]. L. reuteri can reduce ischemic injury-induced cardiac damage [10]. Clostridium butyricum supplements prevent hepatic I/R injury by modulating gut microbial composition, which helps to reduce LPS and attenuate inflammation and oxidative stress [11]. The role of L. reuteri in hepatic I/R injury is currently unclear.
The development of hepatic I/R injury is largely due to oxidative stress and an exaggerated inflammatory response [12]. The Nrf2/HO-1 axis is a multi-organ protective chain against oxidative stress damage [13]. Senkyunolide I attenuates hepatic I/R injury in mice through the Nrf2/HO-1 pathway [14]. Curculigoside attenuates hepatic I/R injury-induced oxidative stress, inflammation, and apoptosis by activating the Nrf2/HO-1 pathway [15]. APS1 (a kind of Lactobacillus mali) ameliorates hepatic steatosis by modulating lipid metabolism through in vivo regulation of specific non-alcoholic fatty liver disease-associated gut microbiota [16]. However, the precise impacts of L. reuteri on hepatic I/R injury remain unclear. In this study, we investigated the effects and mechanisms of L. reuteri on hepatic I/R injury by constructing a mouse model with or without L. reuteri pretreatment. Our findings offer a novel approach to probiotic therapy for treating hepatic I/R injury.
Materials and methods
Culture and preparation of freeze-dried powder of L. reuteri
The bacterium L. reuteri was cultured under anaerobic conditions for 18 h in a sterile environment using the De Man Rogosa and Sharpe medium at a temperature of 37 °C. As soon as the culture reached the logarithmic growth phase, it was harvested and subsequently centrifuged at a rate of 4000×g for 5 min, maintained at 4 °C. The bacterial strain was then suspended in skimmed milk and freeze-dried to create a powder-like form using a vacuum over 14 h. The resultant strain powder was securely stored in sealed packaging at a temperature of 4 °C. The potency of the strain powder was quantified through the plate count method. L. reuteri was dissolved in phosphate buffered saline (PBS) for use.
Animal treatment
The study was approved by the Animal Ethical and Welfare Committee, The Second Xiangya Hospital, CSU (NO.20230479). C57BL/6J mice (8 ~ 10 weeks, males) were purchased from Hunan SJA Laboratory Animal Co., Ltd (China). All mice were housed in specific pathogen-free animal facilities and acclimatized for one week before starting the experiment. Hepatic I/R models were constructed based on previously described methods [5, 17]. Specifically, after being anesthetized, the blood supply to the left hepatic lobe and the middle lobe of the hepatic of mice was blocked by an arterial clip. Mice were ischemic for 90 min, after which the clamps were removed. Then, mice were reperfused for 1, 6, and 24 h. L. reuteri was purchased from Abiowell (Changsha, China). Mice in the Sham and I/R groups were orally administered vehicle (PBS), and low and high doses of L.reuteri (1 × 1010 CFU/d, L.reuteri-low; 1 × 1011 CFU/d, L. reuteri-hi), for 4 weeks before surgery [8]. Mice were euthanized at reperfusion for 1, 6, and 24 h, respectively. Figure S1 (top) illustrates the details of the experiment.
To further investigate the effect of the metabolite palmitoylcarnitine on hepatic I/R injury, mice were further divided into 5 groups, including Sham, I/R, ML-385, palmitoylcarnitine, and ML-385 + palmitoylcarnitine groups. The treatment steps for the I/R group were as described previously. Briefly, mice in the I/R group were reperfused for 6 h after being ischemic for 90 min. Palmitoylcarnitine (1 µM) was added to the perfusate at the beginning of reperfusion in both the palmitoylcarnitine group and ML-385 + palmitoylcarnitine group mice [18]. Mice in the Sham group underwent the same surgical procedures except for vascular occlusion. Mice in the ML-385 group and the ML-385 + palmitoylcarnitine group were injected with ML-385 (Nrf2 inhibitor, 30 mg/kg, 30 min before surgery) by intraperitoneal injection, respectively, prior to surgery [15]. Mice were euthanized after 6 h of reperfusion. Blood, hepatic, and colonic feces were collected. Since the time of surgery was closely related to the severity of hepatic injury, we completed the first surgery in mice between 8 and 9 a.m. In addition, mice were fasted for 1 h before surgery. Figure S1 (down) illustrates the details of the experiment.
Biochemical analysis and enzyme-linked immunosorbent assay (ELISA)
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and myeloperoxidase (MPO), as well as superoxide dismutase (SOD), malondialdehyde (MDA), glutathione (GSH), and glutathione peroxidase (GPx) activity levels were determined using biochemical kits (Nanjing Jiancheng Institute of Bioengineering, China) according to the manufacturer’s instructions. The biochemical kit item numbers for ALT, AST, MPO, SOD, MDA, GSH, and GPx were C009-2-1, C010-2-1, A044-1-1, A001-3-2, A003-1-2, A006-2-1, and A005-1-2, respectively.
Serum IL-1β, IL-6, TNF-α, and IL-10 concentrations were assessed by ELISA assay kits (CUSABIO, China). The ELISA kits for IL-1β, IL-6, TNF-α, and IL-10 were available under the item numbers CSB-E08054m, CSB-E04639m, CSB-E04594m, and CSB-E04741m, respectively.
Finally, a multifunctional enzyme labeling analyzer (MB-530, HEALES, China) was used to measure the optical density (OD450 nm) value.
Hematoxylin and eosin (H&E) staining and Suzuki score
As described previously [5], H&E was used to stain paraffin-embedded hepatic tissues. A light microscope (BA210T, Motic, China) was used to visualize the H&E-stained sections. As mentioned earlier [5], following the Suzuki scoring system, the histological damage score was calculated by adding up the scores for three distinct parameters: congestion, vacuolization, and necrosis. The scoring system assigns a range of 0 to 4 for each parameter, with a maximum score of 12.
TdT-mediated dUTP nick end labeling (TUNEL)
TUNEL kit (40306ES50, Shanghai Yeasen Biologicals) was used for fluorescence staining of paraffin sections. A fluorescence microscope (BA410T, Motic, China) was used to observe the sections after staining.
Western blot
In this process, we initially extracted the entire protein content. Subsequently, we utilized SDS-PAGE to separate the proteins, followed by transfer onto nitrocellulose membranes. Next, nitrocellulose membranes were incubated with primary antibodies (shown in Table S1) at 4 °C overnight. After that, nitrocellulose membranes were incubated with secondary antibodies (anti-mouse/rabbit IgG) for 90 min. A gel imaging system imaged protein bands after color development/exposure (ChemiScope6100, CLINX, China). PCNA (nucleus) and β-actin were internal references.
Quantitative real-time PCR (qRT-PCR)
Total cellular RNA was extracted and subsequently reverse-transcribed. Primer sequences for the Nrf2, HO-1, and β-actin genes are shown below. Nrf2 (143 bp), forward, 5’-GCTCCTATGCGTGAATCCCAA-3’, reverse, 5’-TTTGCCCTAAGCTCATCTCGT-3’; HO-1 (191 bp), forward, 5’-TCCATGTTGACTGACCACGACT-3’, reverse, 5’-CCCACCCCTCAAAAGATAGCC-3’; β-actin (223 bp), forward, 5’-ACATCCGTAAAGACCTCTATGCC-3’; reverse, 5’-TACTCCTGCTTGCTGATCCAC-3’. Relative expression of target genes was calculated using the 2−ΔΔCt method with β-actin as an internal reference, and each sample was analyzed in triplicate.
16S rRNA sequencing
Fecal DNA extraction was performed using the DNeasy PowerSoil Pro kit (Qiagen, USA). The V3 to V4 variable regions of the 16S rRNA gene were amplified by PCR using universal primers (341F: 5’-CCTACGGGGNGGCWGCAG-3’; 805R: 5’-GACTACHVGGGTATCTAATCC-3’). After amplification, purification and characterization, sequencing was performed on the Illumina NovaSeq6000 platform to construct gene libraries. Finally, representative reads were selected using the Qiime 2 software package. A linear discriminant analysis of effect sizes (LefSe) was used with LDA to identify feature taxa.
Immunofluorescent (IF) staining
IF staining was performed as described previously [19]. After being treated with heat-repair antigen and other routine treatments, the sections and primary antibodies (ZO-1, claudin-3, and occludin) (shown in Table S2) were incubated at 4 °C overnight. After that, the sections were incubated with the secondary antibody (anti-rabbit IgG(H + L)) at 37 °C for 90 min. Fluorescence microscopy was used to observe the expression of the target protein.
Liquid chromatography-mass spectrometry (LC-MS) analysis
The LC-MS analysis was conducted using an Agilent 1290 Infinity ultra-high performance liquid chromatography system coupled with an Agilent 6545 UHD and Accurate-Mass Q-TOF mass spectrometer. A Waters XSelect R HSS T3 column (2.5 μm, 100 × 2.1 mm) was utilized for chromatographic separation. The mobile phases comprised 0.1% formic acid in water solution (A) and 0.1% in acetonitrile (B). The flow rate and column temperature were set at 0.4 mL/min and 40 °C, respectively. The chromatographic gradient program involved the following steps: 0–3 min, 20% B; 3–9 min, 20–95% B; 9–13 min, 95% B; 13-13.1 min, 95 − 5% B; and 13.1–16 min, 5% B. Mass spectra were acquired in the range of 50-1500 m/z. Agilent Masshunter Qualitative Analysis B.08.00 software (Agilent Technologies, USA) and R software were employed for data analysis.
Statistical analysis
Statistical analysis was performed using Prism software (version 8.0). The data were presented as means ± standard deviation (SD). We used the Student’s t-test to analyze differences between the two groups, while the one-way analysis of variance (ANOVA) was implemented for comparisons across multiple groups. The level of significance was set at *P < 0.05.
Results
L. reuteri attenuates hepatic I/R injury
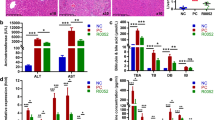
First, mice were utilized as a model to study hepatic I/R injury. The results showed significant increases in serum ALT, AST, and MPO activity levels, Suzuki score, and apoptosis rate in mice affected by I/R injury, with the most pronounced changes observed after 6 h of reperfusion. Subsequently, mice in the experimental group were administered L. reuteri treatment. The findings revealed that serum ALT, AST, MPO activity levels, Suzuki score, and apoptosis rate exhibited a concentration-dependent reduction in L. reuteri-treated mice, with a more pronounced impact observed in the L. reuteri-hi group (Fig. 1A, B, and C). L. reuteri administration demonstrated a mitigating effect on I/R-induced hepatic vacuolization and sinusoidal congestion, with a more pronounced impact observed in the L. reuteri-hi group (Fig. 1B). Following I/R injury, a significant decrease in Bcl2 expression was observed. Conversely, the Bax and cleaved-Caspase3 expressions were significantly elevated, with the most significant changes in the I/R-6 h group. Notably, L. reuteri treatment exhibited a reversal of these indicators, and the effect was more pronounced in the L. reuteri-hi group than in the L. reuteri-low group (Fig. 1D). The activation of JNK/p38, ERK, and NF-κB pathways has been closely implicated in hepatic I/R injury [20,21,22]. The ratios of p-p65/p65, p-IκB/IκB, p-p38/p38, p-JNK/JNK, and p-ERK/ERK levels were found to be significantly elevated in the I/R group compared to the Sham group. The peak values of these ratios were observed after 6 h of reperfusion. Conversely, in the presence of L. reuteri treatment, the ratios of p-p65/p65, p-IκB/IκB, p-p38/p38, p-JNK/JNK, and p-ERK/ERK were consistently lower in the L. reuteri-low and L. reuteri-hi groups when compared to the I/R group. Notably, the lowest levels of these indicators were observed in the L. reuteri-hi group (Fig. 1E). L. reuteri treatment effectively counteracted the I/R-6 h-induced elevation of IL-1β, IL-6, and TNF-α levels, as well as the corresponding decrease in IL-10 concentrations. Notably, these effects were more pronounced in the L. reuteri-hi group (Fig. 1F). Moreover, the administration of L. reuteri in low or high doses did not significantly affect these indices in the Sham group mice. These findings indicate that L. reuteri can suppress hepatic I/R-related tissue injury, apoptosis, inflammation, and the activation of JNK/p38, ERK, and NF-κB pathways.
L. reuteri inhibits hepatic I/R-induced tissue damage, apoptosis, and inflammation. (A) Serum ALT, AST, and MPO activity levels were measured by biochemical kits. (B) The degree of hepatic tissue damage was observed by H&E staining, and Suzuki scores were obtained. Scale bar: 25 μm (400×). (C) The level of cell apoptosis in hepatic tissue was assessed by TUNEL fluorescence staining. Scale bar: 25 μm (400×). (D) The expression of Bcl2, Bax, and cleaved-Caspase3 was detected by western blot. (E) The levels of p-p65/p65, p-IκB/IκB, p-p38/p38, p-JNK/JNK, and p-ERK/ERK were detected by western blot. (F) Concentrations of IL-1β, IL-6, TNF-α, and IL-10 were assessed by ELISA. n = 10 mice/group. #P < 0.05 vs. Sham + vehicle; *P < 0.05 I/R-1 h + vehicle, I/R-6 h + vehicle, or I/R-24 h + vehicle; &P < 0.05 I/R-1 h + L. reuteri-low, I/R-6 h + L. reuteri-low, or I/R-24 h + L. reuteri-low
L. reuteri relieves oxidative stress injury caused by hepatic I/R and activates the Nrf2/HO-1 pathway
To examine the effects of L. reuteri on hepatic I/R-induced oxidative stress injury, we measured the levels of SOD, GSH, GPx, and MDA. The data revealed that L. reuteri could reverse the I/R-induced decrease in SOD, GSH, and GPx levels while also reducing the elevated MDA levels, with a more pronounced impact observed in the L. reuteri-hi group (Fig. 2A). Notably, the Nrf2/HO-1 axis has been recognized as a vital protective mechanism against oxidative stress, including hepatic I/R injury [13, 14]. L. reuteri can enhance intestinal barrier function in rats with d-galactosamine-induced acute hepatic failure through modulation of the Nrf2/HO-1 pathway [23]. Hence, we hypothesized that the beneficial effects of L. reuteri on hepatic I/R injury might be associated with the Nrf2/HO-1 pathway. Therefore, we assessed the expression of Nrf2 and HO-1 proteins. Compared to the Sham + vehicle group, the expression of cytoplasmic Nrf2 (c-Nrf2) was significantly reduced in the Sham + L. reuteri-low and Sham + L. reuteri-hi groups. Conversely, nuclear Nrf2 (n-Nrf2) and HO-1 levels were significantly upregulated. Furthermore, the L. reuteri-treated groups exhibited lower levels of c-Nrf2 and higher levels of n-Nrf2 and HO-1 compared to the I/R-6 h + vehicle group. Remarkably, this trend was more pronounced in the L. reuteri-hi group, implying that L. reuteri facilitates the translocation of Nrf2 into the nucleus, promoting the activation of the Nrf2/HO-1 pathway (Fig. 2B and C). The above results elucidate that the alleviating effect of L. reuteri on hepatic I/R-induced oxidative stress injury may be associated with activating the Nrf2/HO-1 pathway.
L. reuteri alleviates hepatic I/R-induced oxidative stress injury and activates the Nrf2/HO-1 pathway. (A) SOD, MDA, GSH, and GPx levels were evaluated by biochemical kits. (B) The levels of Nrf2 and HO-1 were determined using qRT-PCR. (C) The levels of c-Nrf2, n-Nrf2, and HO-1 were assessed through western blot analysis. n = 10 mice/group. #P < 0.05 vs. Sham + vehicle; *P < 0.05 vs. I/R-6 h + vehicle. &P < 0.05 I/R-6 h + L. reuteri-low
L. reuteri remodeling gut microbiota in I/R mice
L. reuteri has been shown to protect against LPS-mediated hepatocyte apoptosis through the improvement of gut microbiota [7, 8]. To gain more understanding, we investigated the effect of L. reuteri on gut microbiota composition through 16S rRNA sequencing. The rank-abundance curves displayed a satisfactory species abundance and evenness distribution across all groups (Fig. 3A). Venn diagram demonstrating the number of intersecting amplicon sequence variants (ASVs) for the 4 groups was 237 (Fig. 3B). L. reuteri treatment counteracted the decreases in Chao1, ACE, Shannon, and Simpson indices induced by I/R injury, indicating that it enhances the number and diversity of microbial species (Fig. 3C). Beta diversity analysis revealed that the dissimilarities between different groups were significantly larger than the differences within each group (Fig. 3D). Furthermore, at the genus level, L. reuteri reversed the decline trend in Eubacterium xylanophilum, Blautia, Lachnospiraceae NK4A136, and Muribaculum levels induced by I/R injury (Fig. 3E). Lefse analysis demonstrated significant enrichment of Dubosiella, Faecalibaculum, and Bifidobacterium at the genus level in the I/R-6 h + L. reuteri-hi group (Fig. 3F). Subsequently, we investigated the impact of L. reuteri on hepatic I/R-induced intestinal barrier function. The findings indicated that L. reuteri reversed the decrease in the expression of key indicators of intestinal barrier function (ZO-1, claudin-3, and occludin) induced by hepatic I/R injury (Fig. 3G). L. reuteri may alleviate hepatic I/R injury by modulating gut microbiota and improving intestinal barrier integrity.
L. reuteri alters gut microbiota composition and alleviates intestinal barrier function impairment. (A) Rank-abundance curves. (B) Venn diagram. (C) Alpha diversity analysis including Chao1, ACE, Shannon, and Simpson indices. (D) Beta diversity analysis. (E) Genus level. (F) Lefse. (G) The expressions of ZO-1, claudin-3, and occludin were detected by IF staining. Scale bar: 25 μm (400×). n = 5 mice/group. #P < 0.05 vs. Sham + vehicle; *P < 0.05 vs. I/R-6 h + vehicle.
L. reuteri affects hepatic I/R-induced metabolic changes in mice
L. reuteri has been found to reduce the occurrence of cholestasis-associated microbiota and effectively prevent the inflammatory response and hepatocyte apoptosis in response to acute LPS stimulation [7]. We hypothesized that L. reuteri has an impact on hepatic I/R-induced metabolic changes in mice. The administration of L. reuteri effectively inhibited the increase in levels of metabolites such as Drotaverine, jubanine A assamsaponin B, PA (22:2(13Z,16Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)), and CL (8:0/8:0/8:0/19:0) induced by I/R injury. Additionally, L. reuteri reversed the decrease in levels of metabolites, such as Spinacoside C, Cob(I)yrinate a,c diamide, and palmitoylcarnitine caused by I/R injury (Fig. 4A and B). The enriched KEGG pathways observed were lipid metabolism, amino acid metabolism, metabolism of cofactors and vitamins, endocrine system, and cancer overview (Fig. 4C). These findings suggest that L. reuteri interventions partially restored microbial metabolic homeostasis in mice with hepatic I/R injury.
Pearson correlation coefficient analysis
We would like to explore further the beneficial metabolites of L. reuteri that alleviate hepatic I/R injury. Therefore, the differential metabolites, including spinacoside C, Cob(I)yrinate a,c diamide, and palmitoylcarnitine, were analyzed in correlation with differential gut microbiota, as well as levels of ALT, AST, MPO, Nrf2, and HO-1 using the Pearson correlation coefficient. The enrichment of spinacoside C displayed significant positive correlations with the levels of Eubacterium xylanophilum, Bifidobacterium, Dubosiella, Faecalibaculum, Parasutterella, Nrf2, and HO-1 (Figures S2A-2B). The enrichment of Cob(I)yrinate a,c diamide were significant positive correlations with the levels of Eubacterium xylanophilum, Bifidobacterium, Blautia, Dubosiella, Faecalibaculum, Muribaculum, Parasutterella, Nrf2, and HO-1 (Figures S3A-3B). However, there was no significant correlation between spinacoside C and Cob(I)yrinate a,c diamide enrichment and the levels of ALT, AST, and MPO (Figures S2B and S3B). The enrichment of palmitoylcarnitine displayed significant positive correlations with the levels of Eubacterium xylanophilum, Bifidobacterium, Blautia, Faecalibaculum, Lachnospiraceae NK4A136, Muribaculum, Nrf2, and HO-1. Moreover, it exhibited a negative correlation with ALT, AST, and MPO (Fig. 5A and B), demonstrating that palmitoylcarnitine is associated with gut microbiota levels and oxidative stress. The fatty acid-derived mitochondrial substrate palmitoylcarnitine selectively reduces the survival of colorectal and prostate cancer cells [24]. The intermediate fatty acid palmitoylcarnitine confers protection against myocardial I/R injury. Its mechanism of action involves inhibiting complex IV, promoting increased generation of ROS, and activating the RISK pathway [18, 25]. Therefore, we hypothesized a link between palmitoylcarnitine and the protective effects of L. reuteri in the liver I/R induction model. Subsequent studies have focused on palmitoylcarnitine.
Palmitoylcarnitine alleviates hepatic I/R injury through the Nrf2/HO-1 axis
Next, we investigated whether palmitoylcarnitine mitigates hepatic I/R injury through modulation of the Nrf2/HO-1 axis. Our findings demonstrated that palmitoylcarnitine effectively decreased the serum levels of ALT, AST, and MPO activity induced by I/R injury. Moreover, palmitoylcarnitine reduced the Suzuki score and inhibited the increased trend in apoptotic cell count/total cell count (Fig. 6A, B, and C). Palmitoylcarnitine demonstrated a significant decrease in the levels of Bax, cleaved Caspase3, p-p65/p65, p-IκB/IκB, p-p38/p38, p-JNK/JNK, and p-ERK/ERK induced by I/R injury. Additionally, palmitoylcarnitine significantly increased the levels of Bcl2, which were decreased by I/R injury (Fig. 6D and E). Palmitoylcarnitine effectively reversed the significant increase in IL-1β, IL-6, and TNF-α induced by I/R injury while reversing the significant decrease in IL-10 concentrations (Fig. 6F). Moreover, palmitoylcarnitine activated the expression of n-Nrf2 and HO-1 while inhibiting the expression of c-Nrf2 (Fig. 6G and H). Additionally, palmitoylcarnitine reversed the I/R-induced decrease in levels of SOD, GSH, and GPx, and attenuated the increase in MDA levels (Fig. 6I). Notably, apart from c-Nrf2, the effects of ML-385 on the aforementioned indicators were opposite to those of the palmitoylcarnitine group. Furthermore, the ML-385 + palmitoylcarnitine group effectively reversed the changes in the above indexes observed in the palmitoylcarnitine group. These results reveal that palmitoylcarnitine alleviates hepatic I/R injury by activating the Nrf2/HO-1 axis.
Palmitoylcarnitine alleviates hepatic I/R injury through the Nrf2/HO-1 axis. (A) Serum ALT, AST, and MPO activity levels were measured by biochemical kits. (B) The degree of hepatic tissue damage was observed by H&E staining, and Suzuki scores were obtained. Scale bar: 25 μm (400×). (C) The levels of apoptosis in hepatic tissue were assessed by TUNEL fluorescence staining. Scale bar: 25 μm (400×). (D) The expressions of Bcl2, Bax, and cleaved-Caspase3 were detected by western blot. (E) The levels of p-p65/p65, p-IκB/IκB, p-p38/p38, p-JNK/JNK, and p-ERK/ERK were detected by western blot. (F) IL-1β, IL-6, TNF-α, and IL-10 concentrations were assessed by ELISA. (G) The expressions of Nrf2 and HO-1 were detected by qRT-PCR. (H) The expressions of c-Nrf2, n-Nrf2, and HO-1 were detected by western blot. (I) SOD, MDA, GSH, and GPx levels were evaluated by biochemical kits. n = 10 mice/group. #P < 0.05 vs. Sham; *P < 0.05 vs. I/R; &P < 0.05 vs. palmitoylcarnitine
Discussion
I/R-induced hepatic injury is a key factor affecting the prognosis of hepatic surgery, which can lead to gut microbiota and extrahepatic metabolic disorders [26]. The present study revealed that L. reuteri inhibits I/R-induced apoptosis, inflammation, and oxidative stress damage in hepatic tissues. Its mechanism may be related to the promotion of nuclear translocation of Nrf2, the promotion of probiotic enrichment (e.g., Blautia and Lachnospiraceae NK4A136), and the adjustment of differential metabolite homeostasis (e.g., palmitoylcarnitine). In addition, exogenous palmitoylcarnitine supplementation was similar to the protective effect of L. reuteri on the I/R-induced model group, whereas Nrf2 inhibition partially reversed the above effects of palmitoylcarnitine, suggesting that palmitoylcarnitine inhibits I/R through the Nrf2/HO-1 pathway induced hepatic injury.
Disruption of normal gut microbiota is associated with I/R-induced damage to various tissues, such as intestinal and hepatic injury [27, 28]. A variety of probiotics can play a protective role in I/R-induced disease models. For example, Lactobacillus murinus mitigates intestinal I/R injury by modulating gut microbiota and macrophage IL-10 release [29]. VSL#3 ameliorates renal I/R injury by protecting intestinal barrier function, maintaining gut microbiota function, and modulating IL-10/GSK-3β/PTEN pathway-mediated macrophage phenotype [30]. Similarly, Bifidobacterium bifidum BGN4 inhibited I/R-induced renal tissue injury by modulating the colonic microenvironment and gut microbiota [31]. L. reuteri attenuated I/R-induced cardiac injury [10]. Similar to the above studies, the current study found that L. reuteri alleviated hepatic I/R-induced injury by a mechanism that may be related to the protection of intestinal barrier function and maintenance of gut microbiota homeostasis. Blautia, with potential probiotic properties, alleviates LPS-induced acute hepatic injury [32]. Lactobacillus acidophilus and Bifidobacterium may protect against myocardial ischemia-reperfusion [33]. Our study found that L. reuteri treatment increases the enrichment of probiotics (e.g., Bifidobacterium, Blautia, and Lachnospiraceae NK4A136), which might be one of the important reasons for the resistance of L. reuteri to hepatic I/R injury.
Metabolites of gut microbiota origin maintain intestinal and systemic homeostasis [34]. Bifidobacterium breve CCFM1025 attenuates major depression by modulating gut microbiota and tryptophan metabolism [35]. Dietary L. reuteri-derived metabolites contribute to immune checkpoint inhibitor therapy in melanoma [36]. Supplementation of L. reuteri with the ability to produce Aryl hydrocarbon receptor (AhR) ligands ameliorated E. coli-induced mastitis in an AhR-dependent manner [37]. In this study, L. reuteri was found to inhibit the I/R-induced elevation of metabolites drotaverine, jubanine A, assamsaponin B, PA (22:2(13Z,16Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)), and CL (8:0/8:0/8:0/19:0). L. reuteri reversed the I/R-induced decrease in the levels of the metabolites, such as spinacoside C, cob(I)yrinate a,c diamide, and palmitoylcarnitine. The regulation of hepatic I/R-induced metabolite homeostasis by L. reuteri might be important for its protective role. exhibited a negative correlation with the levels of ALT, AST, and MPO. Correlation analysis showed that only palmitoylcarnitine enrichment was negatively correlated with the levels of ALT, AST, and MPO. In addition, considering that spinacoside C and Cob(I)yrinate a,c diamide are less well studied, no commercial products are available for in vivo intervention in animals, and their function in other diseases has not been clarified. Therefore, we have not further explored the effects of Spinacoside C and Cob(I)yrinate a,c diamide on hepatic I/R injury in vivo at this time. The fatty acid-derived mitochondrial substrate palmitoylcarnitine selectively reduces the survival of colorectal and prostate cancer cells [24]. Palmitoylcarnitine protects against myocardial I/R injury, and its mechanisms involving inhibition of complex IV, increased ROS generation, and activation of the RISK pathway [18, 25]. In this study, we found for the first time that palmitoylcarnitine inhibites I/R-induced apoptosis, inflammation, and oxidative stress injury in hepatic tissue.
Up-regulation of downstream antioxidants upon Nrf2 activation can help promote hepatic recovery during I/R [38]. Yuan et al. reported that Gastrodin pretreatment protected the hepatic from I/R injury by activating Nrf2/HO-1 signaling [39]. Consistent with the above studies, the present study found a significant increase in the levels of ALT, AST, MPO, apoptosis, inflammatory factors, and MDA, and a significant decrease in the levels of SOD, GSH, and GPx after ML-385 treatment, exposing that inhibition of Nrf2 promotes I/R-induced hepatic injury. L. reuteri alleviates d-galactosamine-induced hepatic injury by promoting the Nrf2/HO-1 axis [23]. Similarly, L. reuteri and palmitoylcarnitine promote nuclear translocation of Nrf2, which further activates the downstream HO-1 pathway. L. reuteri and palmitoylcarnitine inhibited LPS-induced significant increases in the levels of ALT, AST, MPO, apoptosis, inflammatory factors, and MDA, and significant decreases in the levels of SOD, GSH, and GPx. ML-385 pretreatment reversed the above changes in the palmitoylcarnitine group, suggesting that palmitoylcarnitine alleviates I/R-induced hepatic injury by activating the Nrf2/HO-1 pathway. The above results suggest that the protective effect of L. reuteri against hepatic I/R injury may be related to promoting elevated levels of palmitoylcarnitine. JNK/p38, ERK, and NF-κB signaling activation are strongly associated with hepatic I/R injury [20,21,22]. In the present study, we found that L. reuteri and palmitoylcarnitine reverse the I/R-induced significant increase in the levels of p-p65/p65, p-IκB/IκB, p-p38/p38, p-JNK/JNK, and p-ERK/ERK.
L. reuteri was able to alter I/R-induced changes in other metabolites (e.g., spinacoside C). However, we did not delve further into the mechanism, which is a limitation of our study. In future studies, we plan to delve deeper into the impact and potential mechanisms of differential metabolites derived from L. reuteri on hepatic I/R injury.
Conclusion
L. reuteri exerts a beneficial effect in mitigating hepatic injury caused by I/R. This protective effect is possibly attributed to various reasons, including the enhancement of probiotic enrichment, such as Bifidobacterium and Lachnospiraceae NK4A136, the maintenance of metabolic balance characterized by differential metabolite homeostasis like palmitoylcarnitine, and the activation of the Nrf2/HO-1 pathway. These findings offer valuable insights into the possible clinical use of probiotics for treating hepatic I/R injury.
Data availability
The 16S rRNA sequencing data are deposited at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1036033. The other data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Pan WM, Wang H, Zhang XF, Xu P, Wang GL, Li YJ, Huang KP, Zhang YW, Zhao H, Du RL et al. miR-210 Participates in Hepatic Ischemia Reperfusion Injury by Forming a Negative Feedback Loop With SMAD4. Hepatology 2020, 72(6):2134–2148.
Huang Y, Xu Q, Zhang J, Yin Y, Pan Y, Zheng Y, Cai X, Xia Q, He K. Prussian blue scavenger ameliorates hepatic ischemia-reperfusion Injury by inhibiting inflammation and reducing oxidative stress. Front Immunol. 2022;13:891351.
Guan Y, Yao W, Yi K, Zheng C, Lv S, Tao Y, Hei Z, Li M. Nanotheranostics for the management of hepatic ischemia-reperfusion Injury. Small. 2021;17(23):e2007727.
Lu T, Li Q, Lin W, Zhao X, Li F, Ji J, Zhang Y, Xu N. Gut microbiota-derived glutamine attenuates Liver Ischemia/Reperfusion Injury via Macrophage metabolic reprogramming. Cell Mol Gastroenterol Hepatol. 2023;15(5):1255–75.
Li R, Xie L, Li L, Chen X, Yao T, Tian Y, Li Q, Wang K, Huang C, Li C, et al. The gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid, alleviates hepatic ischemia/reperfusion injury via mitigation of macrophage pro-inflammatory activity in mice. Acta Pharm Sin B. 2022;12(1):182–96.
Lu T, Li Q, Lin W, Zhao X, Li F, Ji J, Zhang Y, Xu N. Gut microbiota-derived glutamine attenuates Liver Ischemia/Reperfusion Injury via Macrophage metabolic reprogramming. Cell Mol Gastroenterol Hepatol 2023.
Lin Z, Wu J, Wang J, Levesque CL, Ma X. Dietary Lactobacillus reuteri prevent from inflammation mediated apoptosis of liver via improving intestinal microbiota and bile acid metabolism. Food Chem. 2023;404(Pt B):134643.
Zhu T, Mao J, Zhong Y, Huang C, Deng Z, Cui Y, Liu J, Wang H. L. Reuteri ZJ617 inhibits inflammatory and autophagy signaling pathways in gut-liver axis in piglet induced by lipopolysaccharide. J Anim Sci Biotechnol. 2021;12(1):110.
Jiang H, Yan R, Wang K, Wang Q, Chen X, Chen L, Li L, Lv L. Lactobacillus reuteri DSM 17938 alleviates d-galactosamine-induced liver failure in rats. Biomed Pharmacother. 2021;133:111000.
Koppinger MP, Lopez-Pier MA, Skaria R, Harris PR, Konhilas JP. Lactobacillus reuteri attenuates cardiac injury without lowering cholesterol in low-density lipoprotein receptor-deficient mice fed standard chow. Am J Physiol Heart Circ Physiol. 2020;319(1):H32–h41.
Yang X, Yu H, Wei J, Wei Q, Huang H, Chen J, Li J, Yu S. The protective effects of dietary Clostridium butyricum supplementation on hepatic ischemia reperfusion injury in rats. Acta Cir Bras. 2022;37(9):e370904.
Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L, Zhang J, Liu C, et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019;20:296–306.
Zhang X, Ding M, Zhu P, Huang H, Zhuang Q, Shen J, Cai Y, Zhao M, He Q. New Insights into the Nrf-2/HO-1 Signaling Axis and Its Application in Pediatric Respiratory Diseases. Oxid Med Cell Longev 2019, 2019:3214196.
Yang Q, Zhao ZZ, Xie J, Wang YP, Yang K, Guo Y, Wang JF, Deng XM. Senkyunolide I attenuates hepatic ischemia/reperfusion injury in mice via anti-oxidative, anti-inflammatory and anti-apoptotic pathways. Int Immunopharmacol. 2021;97:107717.
Du P, Zhang X, Luo K, Li Y, Fu C, Xiao J, Xiao Q. Curculigoside mitigates hepatic ischemia/reperfusion-induced oxidative stress, inflammation, and apoptosis via activation of the Nrf-2/HO-1 pathway. Hum Exp Toxicol. 2022;41:9603271221087146.
Chen YT, Lin YC, Lin JS, Yang NS, Chen MJ. Sugary kefir strain Lactobacillus mali APS1 ameliorated hepatic steatosis by regulation of SIRT-1/Nrf-2 and gut microbiota in rats. Mol Nutr Food Res. 2018;62(8):e1700903.
Ren J, Hu D, Mao Y, Yang H, Liao W, Xu W, Ge P, Zhang H, Sang X, Lu X, et al. Alteration in gut microbiota caused by time-restricted feeding alleviate hepatic ischaemia reperfusion injury in mice. J Cell Mol Med. 2019;23(3):1714–22.
Lou PH, Lucchinetti E, Zhang L, Affolter A, Schaub MC, Gandhi M, Hersberger M, Warren BE, Lemieux H, Sobhi HF, et al. The mechanism of Intralipid®-mediated cardioprotection complex IV inhibition by the active metabolite, palmitoylcarnitine, generates reactive oxygen species and activates reperfusion injury salvage kinases. PLoS ONE. 2014;9(1):e87205.
Liu L, Guo S, Shi W, Liu Q, Huo F, Wu Y, Tian W. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote Periodontal Regeneration. Tissue Eng Part A. 2021;27(13–14):962–76.
Ding MJ, Fang HR, Zhang JK, Shi JH, Yu X, Wen PH, Wang ZH, Cao SL, Zhang Y, Shi XY, et al. E3 ubiquitin ligase ring finger protein 5 protects against hepatic ischemia reperfusion injury by mediating phosphoglycerate mutase family member 5 ubiquitination. Hepatology. 2022;76(1):94–111.
Zhou J, Guo L, Ma T, Qiu T, Wang S, Tian S, Zhang L, Hu F, Li W, Liu Z, et al. N-acetylgalactosaminyltransferase-4 protects against hepatic ischemia/reperfusion injury by blocking apoptosis signal-regulating kinase 1 N-terminal dimerization. Hepatology. 2022;75(6):1446–60.
Yu Q, Wu L, Liu T, Li S, Feng J, Mao Y, Fan X, Guo C, Wu J. Protective effects of levo-tetrahydropalmatine on hepatic ischemia/reperfusion injury are mediated by inhibition of the ERK/NF-κB pathway. Int Immunopharmacol. 2019;70:435–45.
Zhou Q, Wu F, Chen S, Cen P, Yang Q, Guan J, Cen L, Zhang T, Zhu H, Chen Z. Lactobacillus reuteri improves function of the intestinal barrier in rats with acute liver failure through Nrf-2/HO-1 pathway. Nutrition 2022, 99–100:111673.
Turnbull PC, Hughes MC, Perry CGR. The fatty acid derivative palmitoylcarnitine abrogates colorectal cancer cell survival by depleting glutathione. Am J Physiol Cell Physiol. 2019;317(6):C1278–c1288.
Zhu SC, Chen C, Wu YN, Ahmed M, Kitmitto A, Greenstein AS, Kim SJ, Shao YF, Zhang YH. Cardiac complex II activity is enhanced by fat and mediates greater mitochondrial oxygen consumption following hypoxic re-oxygenation. Pflugers Arch. 2020;472(3):367–74.
He Z, Liu Y, Li Z, Sun T, Li Z, Manyande A, Xiang H, Xiong J. Gut microbiota regulates circadian oscillation in hepatic ischemia-reperfusion injury-induced cognitive impairment by interfering with hippocampal lipid metabolism in mice. Hepatol Int 2023.
Chen J, Wang Y, Shi Y, Liu Y, Wu C, Luo Y. Association of Gut Microbiota with Intestinal Ischemia/Reperfusion Injury. Front Cell Infect Microbiol. 2022;12:962782.
Cornide-Petronio ME, Álvarez-Mercado AI, Jiménez-Castro MB, Peralta C. Current knowledge about the Effect of Nutritional Status, Supplemented Nutrition Diet, and gut microbiota on hepatic ischemia-reperfusion and regeneration in liver surgery. Nutrients 2020, 12(2).
Hu J, Deng F, Zhao B, Lin Z, Sun Q, Yang X, Wu M, Qiu S, Chen Y, Yan Z, et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via toll-like receptor 2 signaling. Microbiome. 2022;10(1):38.
Ding C, Han F, Xiang H, Wang Y, Li Y, Zheng J, Xue W, Ding X, Tian P. Probiotics ameliorate renal ischemia-reperfusion injury by modulating the phenotype of macrophages through the IL-10/GSK-3β/PTEN signaling pathway. Pflugers Arch. 2019;471(4):573–81.
Yang J, Ji GE, Park MS, Seong YJ, Go YS, Lee HY, Fang Y, Kim MG, Oh SW, Cho WY, et al. Probiotics partially attenuate the severity of acute kidney injury through an immunomodulatory effect. Kidney Res Clin Pract. 2021;40(4):620–33.
Mao B, Guo W, Liu X, Cui S, Zhang Q, Zhao J, Tang X, Zhang H. Potential Probiotic properties of Blautia producta against Lipopolysaccharide-Induced Acute Liver Injury. Probiotics Antimicrob Proteins. 2023;15(3):785–96.
Borshchev YY, Burovenko IY, Karaseva AB, Minasian SM, Protsak ES, Borshchev VY, Semenova NY, Borshcheva OV, Suvorov AN, Galagudza MM. Probiotic therapy with Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis results in infarct size limitation in rats with obesity and chemically Induced Colitis. Microorganisms 2022, 10(11).
Su X, Gao Y, Yang R. Gut microbiota-derived tryptophan metabolites maintain gut and systemic homeostasis. Cells 2022, 11(15).
Tian P, Chen Y, Zhu H, Wang L, Qian X, Zou R, Zhao J, Zhang H, Qian L, Wang Q, et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: a randomized clinical trial. Brain Behav Immun. 2022;100:233–41.
Bender MJ, McPherson AC, Phelps CM, Pandey SP, Laughlin CR, Shapira JH, Medina Sanchez L, Rana M, Richie TG, Mims TS, et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186(9):1846–1862e1826.
Zhao C, Hu X, Bao L, Wu K, Feng L, Qiu M, Hao H, Fu Y, Zhang N. Aryl hydrocarbon receptor activation by Lactobacillus reuteri tryptophan metabolism alleviates Escherichia coli-induced mastitis in mice. PLoS Pathog. 2021;17(7):e1009774.
Panisello-Roselló RGB, Sanchez-Nuno A, Alva S, Roselló-Catafau N, Carbonell J. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. Febs j. 2022;289(18):5463–79.
Yuan B, Huang H, Qu S, Zhang H, Lin J, Jin L, Yang S, Zeng Z. Gastrodin pretreatment protects Liver Against Ischemia-Reperfusion Injury via activation of the Nrf2/HO-1 pathway. Am J Chin Med. 2020;48(5):1159–78.
Funding
This work was supported by the Natural Science Foundation of Hunan Province, China (No. 2022JJ30866).
Author information
Authors and Affiliations
Contributions
LZ: Conceptualization, Data curation, Writing-original draft; XG: Methodology, Data curation, Writing-original draft; JT: Data curation, Formal analysis; RZ: Methodology, Data curation; ML: Formal analysis, Visualization; CL: Data curation; CW: Data curation; XL: Project administration, Supervision, Writing-review & editing. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Animal Ethical and Welfare Committee, The Second Xiangya Hospital, CSU (NO.20230479).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13062_2024_462_MOESM1_ESM.xlsx
Supplementary Material 1: Table S1 Antibody information for western blot. Table S2 Antibody information for IF staining.
13062_2024_462_MOESM4_ESM.tif
Supplementary Material 4: Figure S2. Pearson correlation coefficient analysis (A) Correlation analysis of spinacoside C with differential gut microbiota. (B) Correlation analysis of spinacoside C and ALT, AST, MPO, Nrf2, and HO-1 levels.
13062_2024_462_MOESM5_ESM.tif
Supplementary Material 5: Figure S3. Pearson correlation coefficient analysis (A) Correlation analysis of Cob(I)yrinate a,c diamide with differential gut microbiota. (B) Correlation analysis of Cob(I)yrinate a,c diamide and ALT, AST, MPO, Nrf2, and HO-1 levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Gong, X., Tan, J. et al. Lactobacillus reuteri mitigates hepatic ischemia/reperfusion injury by modulating gut microbiota and metabolism through the Nrf2/HO-1 signaling. Biol Direct 19, 23 (2024). https://doi.org/10.1186/s13062-024-00462-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13062-024-00462-5