Abstract
Amphiregulin (AREG) is a ligand of epidermal growth factor receptor and participates in the fibrosis of multiple organs. However, whether AREG can regulate hypertrophic cardiomyopathy is not well known. This research aims to explore the effect of AREG on cardiac hypertrophy, and whether the oxidative stress and apoptosis was involved in the influence of AREG on cardiac hypertrophy. Angiotensin (Ang) II induced cardiac hypertrophy in mice and neonatal rat cardiomyocytes (NRCMs) or HL-1 cells in vitro. AREG expressions raised in the heart of mice. After AREG downregulation, the increases of Ang II induced cardiac weight and cardiomyocytes area were inhibited. Down-regulation of AREG could inhibit Ang II induced the increases of atrial natriuretic peptide, brain natriuretic peptide, beta-myosin heavy chain in the heart of mice, and NRCMs and HL-1 cells. The enhancement of oxidative stress in mice heart with Ang II treatment was alleviated by AREG knockdown. The raises of Ang II induced Bax and cleaved caspase3 in mice heart were inhibited by AREG downregulation. AREG downregulation reduced myocardial hypertrophy via inhibition of oxidative and apoptosis. AREG may be a target for future cardiac hypertrophy treatment.
Similar content being viewed by others
Introduction
Myocardial hypertrophy can cause congestive heart failure, and is the main cause of incidence rate and mortality worldwide [1]. Cardiac hypertrophy is related to the adverse consequences of various states of cardiovascular disease. It is an important risk factor of cardiovascular disease, including heart failure, arrhythmia and sudden death [2]. Pathological cardiac hypertrophy begins with an adaptive response to increased workload. However, continuous hemodynamic pressure can lead to maladjustment and eventually lead to heart failure [3]. Increased cardiomyocyte size and heart weight [4,5,6], as well as increased expression of genes, such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and beta-myosin heavy chain (β-MHC) are the features of cardiac hypertrophy [7].
Amphiregulin (AREG) is a ligand of epidermal growth factor receptor (EGFR), which is widely expressed in cardiomyocytes and fibroblasts [8]. Under physiological conditions, the activation of EGFR in the heart induces major intracellular signal cascades and control fibroblast proliferation, migration and collagen synthesis. However, under the chronic stress state characterized by the continuous increase of AREG, the long-term activation of EGFR can enhance the activation, proliferation, myofibroblast differentiation, migration and collagen synthesis of cardiac fibroblasts [9]. However, whether AREG participated in the regulation of cardiac hypertrophy is still unclear.
It is well known that oxidative stress promotes protein oxidation, lipid peroxidation, protease activation, DNA fragmentation and gene expression changes, resulting in cardiomyocyte damage and cardiomyocyte loss in patients with different types of heart disease [10]. Oxidative stress was enhanced in the disease of cardiac hypertrophy [11]. It is still unclear whether AREG modulate oxidative stress in hypertrophic cardiomyopathy.
Apoptosis, also known as programmed cell death, plays an important role in the physiological development and pathological process of a variety of cells and tissues. Increased diastolic function and decreased systolic function determine pathological hypertrophy, which usually causes heart failure and is related to increased cardiomyocyte death [12]. The phenotypic variability and heterogeneous remodeling observed in hypertrophic cardiomyopathy is the end-result of many factors to include apoptosis [13]. Angiotensin (Ang) II, a member of renin-angiotensin system, traditionally viewed as a regulator of apoptosis in cardiovascular system [14, 15]. The purpose of this research was to explore the role of AREG on cardiomyocytes apoptosis of hypertrophic cardiomyopathy. In summary, this present study would probe the influences of AREG on cardiac hypertrophy and fibrosis, and quest whether AREG regulates cardiac hypertrophy through affecting oxidative stress and apoptosis.
Results
Expression of AREG
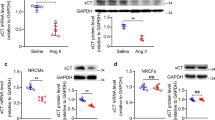
AREG mRNA level raised in the mice heart treated with Ang II (Fig. 1a). AREG protein level also increased in the mice heart with Ang II treatment detected by western blotting (Fig. 1b) or immunofluorescence staining (Fig. 1c).
AREG knockdown alleviated cardiac hypertrophy of mice
Ang II administration increased heart weight (HW), HW/body weight (BW), HW/tibial length (TL), left ventricle weight (LW)/BW, interventricular septal thickness at end diastole (IVSd) and interventricular septal thickness at end systole (IVSs) of mice, which were inhibited by AREG knockdown (Fig. 2a). The levels of ANP, BNP and β-MHC were elevated in mice heart with Ang II treatment. These increases were suppressed after downregulation of AREG (Fig. 2b). The area of cardiomyocytes raised in mice with Ang II treatment, and was attenuated by AREG knockdown (Fig. 2c).
AREG knockdown alleviated cardiac hypertrophy of mice. a, The enhancements of Ang II induced heart weight, HW/BW, HW/TL, LW/BW, IVSd andIVSs of mice were alleviated by AREG downregulation. b, The enhancements of Ang II induced ANP, BNP and β-MHC in mice heart were alleviated by AREG downregulation. c, The enhancement of Ang II induced cardiomyocytes area of mice was alleviated by AREG downregulation. n = 8 for each group. Express the results as mean ± SEM
AREG knockdown alleviated cardiac fibrosis of mice
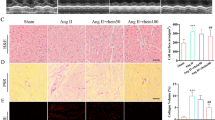
Cardiac fibrosis was detected by masson staining (blue). AREG knockout can reduce AngIIinduced myocardial fibrosis in mice (Fig. 3a). The collagen I, collagen III and TGF-β levels raised in mice heart with Ang II treatment, which were inhibited after AREG downregulation (Fig. 3b).
AREG knockdown alleviated cardiac fibrosis of mice. a, The Ang II induced cardiac fibrosis of mice was alleviated by AREG downregulation. b, The enhancements of Ang II induced collagen I, collagen III and TGF-β in mice heart were alleviated by AREG downregulation. n = 8 for each group. Express the results as mean ± SEM
Effects of AREG on cardiomyocytes hypertrophy
Four doses of AREG were used to search the influence of AREG on cardiomyocytes hypertrophy. Neonatal rat cardiomyocytes (NRCMs) was treated with Ang II to induce hypertrophic model [16, 17]. The doses of 1 and 10 ng/ml have no effect on the hypertrophy of NRCMs. The doses of 100 and 1000 ng/ml enhanced the expressions of ANP, BNP and β-MHC in NRCMs (Fig. 4a). The doses of 1 and 10 ng/ml have no effect on the hypertrophy of HL-1 cells. The doses of 100 and 1000 ng/ml enhanced the expressions of ANP, BNP and β-MHC in HL-1 cells (Fig. 4b). The findings indicated that AREG induced cardiomyocytes hypertrophy. Knockdown of AREG restrained the enhancements of Ang II induced ANP, BNP and β-MHC in NRCMs (Fig. 4c). The enhancements of Ang II induced ANP, BNP and β-MHC in HL-1 were also suppressed after AREG knockdown (Fig. 4d).
Effects of AREG on cardiomyocytes hypertrophy. a, The ANP, BNP and β-MHC levels raised in the NRCMs after treating with AREG. b, The ANP, BNP and β-MHC levels raised in the HL-1 cells after treating with AREG. c, The enhancemnets of Ang II induced ANP, BNP and β-MHC in the NRCMs were alleviated by AREG downregulation. d, The enhancements of Ang II induced ANP, BNP and β-MHC in the HL-1 cells were alleviated by AREG downregulation. n = 6 for each group. Express the results as mean ± SEM
Effects of AREG on oxidative stress
The 8-hydroxy-2′ -deoxyguanosine (8-OHdG) positive cells number was increased in mice heart with Ang II treatment, and was suppressed after AREG knockdown (Fig. 5a). The superoxide anions, NADPH oxidase (Nox) activity and malondialdehyde (MDA) levels raised in mice heart with Ang II treatment, and were attenuated by AREG knockdown (Fig. 5b). Ang II elevated the superoxide anions, Nox activity and MDA levels in NRCMs, and were reversed after AREG downregulation (Fig. 5c). Downregulating of AREG inhibited the enhancements of superoxide anions, Nox activity and MDA induced by Ang II in the HL-1 cells (Fig. 5d).
Effects of AREG on oxidative stress. a, The increase of Ang II induced 8-OHdG positive cells in mice heart was alleviated by AREG downregulation. Magnification 400X. b, The enhancements of Ang II induced superoxide anions, Nox activity and MDA in mice heart were alleviated by AREG downregulation. c, The enhancements of Ang II induced superoxide anions, Nox activity and MDA in the NRCMs were alleviated by AREG downregulation. d, The enhancements of Ang II induced superoxide anions, Nox activity and MDA in the HL-1 cells were alleviated by AREG downregulation. n = 8 (a and b) or 10 (c and d) for each group. Express the results as mean ± SEM. Magnification
Effects of AREG on apoptosis
The number of cleaved caspase3 positive cell raised in mice heart with Ang II treatment, which was suppressed after AREG knockdown (Fig. 6a). The level of caspase3/caspase3 was increased in the heart of Ang II-treated mice, and this increase was suppressed by AREG knockdown (Fig. 6b). The Bax positive cell number raised in mice heart with Ang II treatment, which was attenuated by AREG knockdown (Fig. 6c). The TUNEL positive cell number raised in mice heart with Ang II treatment, which was inhibited after AREG knockdown (Fig. 6d).
Effects of AREG on apoptosis. a, The enhancement of Ang II induced cleaved caspase3 positive cells in mice heart was alleviated by AREG downregulation. b, The enhancement of Ang II induced cleaved caspase3/caspase3 in mice heart was alleviated by AREG downregulation. c, The enhancement of Ang II induced Bax positive cells in mice heart was alleviated by AREG downregulation. d, The enhancement of Ang II induced TUNEL positive cells in mice heart was alleviated by AREG downregulation. n = 8 for each group. Express the results as mean ± SEM. Magnification 400X
Discussion
It was found that AREG expression enhanced in mice heart with Ang II treatment. Knockdown of AREG alleviated cardiac hypertrophy and fibrosis in mice heart with Ang II treatment. AREG downregulation attenuated the increases of oxidative stress and apoptosis induced by Ang II in mice heart.
AREG is a member of epidermal growth factor family, which plays a key role in cardiovascular diseases [18]. AREG expression is upregulated in many disease models. For example, AREG expression was significantly enhanced in acute lung injury induced by lipopolysaccharide and pulmonary fibrosis induced by bleomycin [19, 20]. AREG expression also raised in MI mice heart [9]. We present found that AREG expression raised in mice heart with Ang II treatment. In addition, AREG expressions were also elevated in NRCMs and HL-1 cells treated with Ang II. These outcomes showed that the level of AREG increased in hypertrophic cardiomyopathy. It remained to be further explored that AREG may be involved in the regulation of myocardial hypertrophy.
Previous researches have also showed that AREG was involved in several organ fibrosis diseases such as lung and liver fibrosis [21, 22]. Similarly, AREG administration enhanced fibrosis of heart, while AREG knockdown relieved cardiac fibrosis of myocardial infarction [9, 23]. Our current results showed that AREG knockdown alleviated Ang II induced cardiac fibrosis in hypertrophic cardiomyopathy, which supported the influence of AREG on promoting cardiac fibrosis reported as previous studies. We further found that the enhancements of Ang II induced heart weight and cardiomyocytes area were attenuated after downregulation of AREG. Moreover, the ANP, BNP and β-MHC expressions raised in the NRCMs and HL-1 cells after AREG administration, and the enhancements of Ang II induced ANP, BNP and β-MHC in the NRCMs and HL-1 cells were suppressed by AREG knockdown. Our outcomes demonstrated that AREG enhanced cardiac hypertrophy, and targeting of AREG could alleviate hypertrophic cardiomyopathy.
Oxidative stress is the accumulation of free radicals due to the imbalance between the production of reactive oxygen species and antioxidant defense. In the heart, reactive oxygen species activate signaling pathways involved in cardiomyocyte hypertrophy and interstitial fibrosis [24]. NADPH oxidases are transmembrane enzymes, which play an important role in mediating cardiac dysfunction by transferring electrons from NADPH to molecular oxygen to produce superoxide and promote superoxide production. [25]. MDA and 8-OHdG have been widely used as biomarkers of oxidative stress [26, 27]. Cardiac hypertrophy may contribute to heart failure and is closely related to oxidative stress [28]. In current research, we found that AREG knockdown significantly weakened the enhancement of Ang II induced 8-OHdG positive cells in mice heart. The increases of Ang II induced superoxide anions, Nox activity and MDA in mice heart and cultured cardiomyocytes were suppressed after downregulating of AREG. These outcomes showed that AREG downregulation weakened cardiac hypertrophy via attenuation of oxidative stress.
In physiology, apoptosis plays an important role in maintaining homeostasis through destroying unnecessary cells. However, excessive apoptosis can lead to pathological state and eventually organ failure. The raise of cardiac oxidative stress plays a key role in mediating cardiac hypertrophy, fibrosis and apoptosis, as well as heart failure. [29]. Cardiomyocyte apoptosis raised in hypertrophic cardiomyopathy of mice, and this increase resulted in fibrosis interstitial fibrosis at later stages [30]. We present found that the increases of Ang II induced Bax, cleaved caspase3 and TUNEL positive cells in mice heart were inhibited by downregulation of AREG. These results indicated that AREG downregulation mitigated the apoptosis to improve hypertrophic cardiomyopathy.
In conclusions, the AREG expression raised in the heart of hypertrophy. The down-regulation of AREG could reduce myocardial hypertrophy by reducing oxidative stress and apoptosis. AREG targeted therapy may be a strategy for hypertrophic cardiomyopathy in the future.
Materials and methods
Animals and treatment
Kept the 8–10 week old male C57/BL6/J mice (Vital River Biological Co., Ltd, Beijing, China) in a 12 h-light dark cycle, and was free to standard chow and tap water. All experiments were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University (Nanjing, China) and followed the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, 1996). With osmotic minipumps (model 2004; ALZET, CA, USA) surgically placed below the neck, the mice were infused with Ang II (1.44 mg/kg/day, Sigma, MO, USA) or saline (solvent control) at a rate of 0.25 μl/h for four weeks. Meanwhile, AREG was downregulated by adeno-associated virus (shAREG) injection via tail vein (2 * 1011 in 200 μl saline; OBIO, Shanghai, China). shNC was used as a control.
Echocardiography
Four weeks after Ang II infusion, transthoracic echocardiography was performed under isoflurane (1.5–2.0%) anesthesia using an ultrasound system (VisualSonics, Toronto, Canada) with a 21 MHz probe. The mean value of three consecutive cardiac cycles was measured. Measured the LW, IVSd and IVSs. After echocardiography, the mice were sacrificed and the HW, HW/ BW, HW/ TL and LW/BW were measured.
Wheat germ agglutinin staining
The heart samples were collected via euthanasia after echocardiography. Stained the heart sections with FITC-conjugated wheat germ agglutinin (WGA; Invitrogen Inc., CA, USA) to measure the cross-sectional area of cardiomyocytes. Selected three to five random fields (around 30–50 cells per field) from three slices of each animal, observed under confocal microscope (Carl Zeiss GmbH, Oberkochen, Germany) and analyzed with Zeiss software.
Masson staining
Using Masson staining method (Service Biological Technology Co., Ltd, Wuhan, China) to observe the 5 µm thick heart section and determine the degree of cardiac fibrosis (blue). Obtained the tissue sections from the mouse heart with a light microscope (Carl Zeiss GmbH, Oberkochen, Germany). Analyzed images with Image-Pro Plus software (Media Cybernetics, Inc., MD, USA).
Culture of cardiomyocytes isolated from neonatal rat and treatment
NRCMs were isolated from neonatal Sprague Dawley rats aged 1–2 days (Vital River Biological Co.). Hearts were removed and digested in PBS containing type II collagenase (Worthington Biochemical Corp., NJ, USA) and trypsin (Sigma, MO, USA). Discarded the atria and great vessels. Cut the ventricles into small pieces and digested with type II collagenase and trypsin. In order to reduce fibroblasts and enrich for cardiomyocytes, collected and cultured cells from digestion in Complete Dulbecco's modified Eagle's medium (DMEM; GIBCO, Invitrogen Inc.) for 2–4 h. Cultured the cardiomyocytes with 5% CO2 at 37 °C. Firstly, divided the NRCMs into PBS and four doses of AREG groups (1, 10, 100 and 1000 pg/ml; R&D, Shanghai, China) for 24 h. Secondly, NRCMs were treated with adenovirus-control (shNC) or adenovirus-mediated AREG knockdown (shAREG; Genechem, Shanghai, China). Meanwhile, Ang II (10–6 M) was added into the medium for 24 h.
Culture of HL-1 cells and treatment
Mice cardiomyocytes HL-1 cells were cultured in intact Dulbecco modified Eagle medium, and 10% fetal bovine serum (Nanjing Bio-Channel Biotechnology Co., Ltd, Nanjing, China) was added at 37 °C with 5% CO2 and 95% air. Treated HL-1 cells the same as NRCMs.
Quantitative real-time PCR
Used quantitative real-time PCR (qRT-PCR) to determine the mRNA level. Isolated total RNA with Trizol reagent (Invitrogen, USA). Obtained the mRNA concentration with NANODROP ONE (Thermo, Shanghai, China), and 0.5 μg of total RNA was reverse transcribed to cDNA with 10 μl of random primers and PrimeScript™ RT Master mix (Takara Biotechnology Co., Ltd.). Performed qRT-PCR with the ABI Prism 7900 sequence detection system (Applied Biosystems, CA, USA). The primers were shown in Table 1. All samples were amplified three times in 384-well plates. The mean Ct value was normalized to endogenous control (GAPDH) values and then calculated Δcycle threshold (ΔCT) value to determine the relative mRNA expression level. Then calculated the 2−ΔΔCt values.
Western blotting
Total protein from heart and cell samples was extracted in RIPA lysis buffer and their concentrations were measured by the BCA method (Beyotime Biotechnology, Shanghai, China). The protein samples were electrophoresed and then transferred to PVDF membranes. After blocking the non-specific antigen in 5% skim milk, immunoblotted membranes with primary antibodies of AREG reacted with mouse (Santa, Shnaghai, China) or GAPDH (Abcam). Band exposure was achieved by chemiluminescence.
Immunofluorescence
Fixed the heart samples with 4% paraformaldehyde 24 h at room temperature. Afterwards, incubated the samples with the primary antibody against AREG (Santa, TX, USA), Bax (Abcam), cleaved caspase3 (CST, MA, USA), caspase3 (CST) or 8-OHdG (Santa) a whole night at 4 °C, and then incubated with the corresponding secondary antibody (Jackson ImmunoResearch, PA, USA) at room temperature for 2 h. Then, the nucleus were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; Life Technologies Co., Grand Island, NY, USA). Took the images of fluorescent cell with a fluorescence microscope (Carl Zeiss GmbH, Oberkochen, Germany).
Measurement of superoxide anions
Measured superoxide anion levels of the samples with fluorescein derived chemiluminescence. Detected the protein consistence with a BCA kit (Beyotime Biotechnology). Added dark adapted fluorescein (5 μM) to each sample to generate photon emission, and measured it once a minute with a microplate reader (BioTek) for 10 min. The level of superoxide anion was expressed as mean light units (MLU) per milligram per minute.
Measurement of NADPH oxidase activity
Measured the activity of Nox in the sample with enhanced fluorescein chemiluminescence. As a substrate, NADPH (100 μM) was added into the media, and reacted with Nox to generate superoxide anions. Determined the light emissionproduced by the reaction of lucigenin (5 μM) and superoxide anions with a microplate reader (BioTek) once a minute for 10 min. The Nox activity level was presented as the MLU per milligram protein per minute.
Determination of MDA level
Homogenized the samples in cracking buffer (Thermo Fisher Scientific, MA, USA). The protein concentration was detected by a BCA kit (Beyotime Biotechnology). The level of MDA was measured by ELISA kit (USCN Business Co., Ltd., Wuhan, China).
Statistical analyses
Data were presented as mean ± SEM. The statistical significance between groups was assessed by one-way analysis of variance (ANOVA) of Bonferroni posttest using GraphPad Prism 6.0 (GraphPad software Inc., CA, USA), and a two-tailed P-value < 0.05 was defined as statistically significant.
Availability of data and materials
Available upon requests.
Abbreviations
- 8-OHdG:
-
8-Hydroxy-2′ –deoxyguanosine
- ANP:
-
Atrial natriuretic peptide
- AREG:
-
Amphiregulin
- BNP:
-
Brain natriuretic peptide
- β-MHC:
-
Beta-myosin heavy chain
- BW:
-
Body weight
- HW:
-
Heart weight
- IVSd:
-
Interventricular septal thickness at end diastole
- IVSs:
-
Interventricular septal thickness at end systole
- LW:
-
Left ventricle weight
- MDA:
-
Malondialdehyde
- Nox:
-
NADPH oxidase
- NRCMs:
-
Neonatal rat cardiomyocytes
- TL:
-
Tibial length
References
Hajializadeh Z, Khaksari M. The protective effects of 17-beta estradiol and SIRT1 against cardiac hypertrophy: a review. Heart Fail Rev. 2022;27:725–38.
Lei H, Hu J, Sun K, Xu D. The role and molecular mechanism of epigenetics in cardiac hypertrophy. Heart Fail Rev. 2021;26:1505–14.
Aung LHH, Jumbo JCC, Wang Y, Li P. Therapeutic potential and recent advances on targeting mitochondrial dynamics in cardiac hypertrophy: a concise review. Mol Ther Nucl Acids. 2021;25:416–43.
Liu C, Yang CX, Chen XR, et al. Alamandine attenuates hypertension and cardiac hypertrophy in hypertensive rats. Amino Acids. 2018;50:1071–81.
Shen T, Liu Y, Dong S, et al. Alarin moderated myocardial hypertrophy via inhibiting cyclic adenosine monophosphate/protein kinase a signaling pathway to attenuate autophagy. Peptides. 2021;146: 170669.
Vanhoutte D, Schips TG, Vo A, et al. Thbs1 induces lethal cardiac atrophy through PERK-ATF4 regulated autophagy. Nat Commun. 2021;12:3928.
Li R, Qi Y, Yuan Q, et al. Protective effects of dioscin against isoproterenol-induced cardiac hypertrophy via adjusting PKCepsilon/ERK-mediated oxidative stress. Eur J Pharmacol. 2021;907: 174277.
Adu-Amankwaah J, Adzika GK, Adekunle AO, et al. ADAM17, a key player of cardiac inflammation and fibrosis in heart failure development during chronic catecholamine stress. Front Cell Dev Biol. 2021;9: 732952.
Liu L, Jin X, Hu CF, et al. Amphiregulin enhances cardiac fibrosis and aggravates cardiac dysfunction in mice with experimental myocardial infarction partly through activating EGFR-dependent pathway. Basic Res Cardiol. 2018;113:12.
Dhalla NS, Elimban V, Bartekova M, Adameova A. Involvement of oxidative stress in the development of subcellular defects and heart disease. Biomedicines. 2022;10(2):393.
Ji M, Zuo Z, Zhang M, Xu Z, Hu G. Osteocrin alleviates cardiac hypertrophy via attenuating oxidative stress. Peptides. 2022;152: 170773.
McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–62.
Becker RC, Owens AP 3rd, Sadayappan S. Tissue-level inflammation and ventricular remodeling in hypertrophic cardiomyopathy. J Thromb Thrombolysis. 2020;49:177–83.
Cui YK, Hong YX, Wu WY, et al. Acacetin ameliorates cardiac hypertrophy by activating Sirt1/AMPK/PGC-1alpha pathway. Eur J Pharmacol. 2022;920: 174858.
Du X, Liu T, Shen C, et al. Anti-fibrotic mechanism of SPP1 knockdown in atrial fibrosis associates with inhibited mitochondrial DNA damage and TGF-beta/SREBP2/PCSK9 signaling. Cell Death Discov. 2022;8:246.
Yue R, Zheng Z, Luo Y, et al. NLRP3-mediated pyroptosis aggravates pressure overload-induced cardiac hypertrophy, fibrosis, and dysfunction in mice: cardioprotective role of irisin. Cell Death Discov. 2021;7:50.
Zhao K, Li Y, Zhou Z, et al. Ginkgolide A alleviates cardiac remodeling in mice with myocardial infarction via binding to matrix metalloproteinase-9 to attenuate inflammation. Eur J Pharmacol. 2022;923: 174932.
Sugita J, Fujiu K, Nakayama Y, et al. Cardiac macrophages prevent sudden death during heart stress. Nat Commun. 2021;12:1910.
Xu Y, Meng C, Liu G, et al. Classically activated macrophages protect against lipopolysaccharide-induced acute lung injury by expressing amphiregulin in mice. Anesthesiology. 2016;124:1086–99.
Ding L, Liu T, Wu Z, et al. Bone marrow CD11c+ cell-derived amphiregulin promotes pulmonary fibrosis. J Immunol. 2016;197:303–12.
Madtes DK, Busby HK, Strandjord TP, Clark JG. Expression of transforming growth factor-alpha and epidermal growth factor receptor is increased following bleomycin-induced lung injury in rats. Am J Respir Cell Mol Biol. 1994;11:540–51.
Perugorria MJ, Latasa MU, Nicou A, et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 2008;48:1251–61.
Liu L, Song S, Zhang YP, et al. Amphiregulin promotes cardiac fibrosis post myocardial infarction by inducing the endothelial-mesenchymal transition via the EGFR pathway in endothelial cells. Exp Cell Res. 2020;390: 111950.
Pagan LU, Gomes MJ, Gatto M, Mota GAF, Okoshi K, Okoshi MP. The role of oxidative stress in the aging heart. Antioxidants (Basel). 2022;11(2):336.
Kuroda J, Sadoshima J. NADPH oxidase and cardiac failure. J Cardiovasc Transl Res. 2010;3:314–20.
Rani N, Arya DS. Chrysin rescues rat myocardium from ischemia-reperfusion injury via PPAR-gamma/Nrf2 activation. Eur J Pharmacol. 2020;883: 173389.
Sheikholeslami MA, Ghafghazi S, Pouriran R, Mortazavi SE, Parvardeh S. Attenuating effect of paroxetine on memory impairment following cerebral ischemia-reperfusion injury in rat: the involvement of BDNF and antioxidant capacity. Eur J Pharmacol. 2021;893: 173821.
Yang H, Bai J, Zhan C, et al. Hydrogen attenuates thyroid hormone-induced cardiac hypertrophy in rats by regulating angiotensin II type 1 receptor and NADPH oxidase 2 mediated oxidative stress. Eur J Pharmacol. 2022;922: 174917.
Prandi FR, Evangelista I, Sergi D, Palazzuoli A, Romeo F. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants. Heart Fail Rev. 2022. https://doi.org/10.1007/s10741-021-10200-y.
Toischer K, Rokita AG, Unsold B, et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122:993–1003.
Acknowledgements
Not applicable.
Funding
The study was supported by Jiangsu Province Postdoctoral Fund (2021K444C), Sources of Funding National Natural Science Foundation of China (81570237; 81870197), and The Top-level Clinical Discipline Project of Shanghai Pudong (PWYgf2021-01).
Author information
Authors and Affiliations
Contributions
MYJ and YL performed the research and wrote the manuscript. ZZ and CX analyzed the data. LL and YL wrote and edited the manuscript, and provided funding. All authors accepted the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal procedures were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ji, M., Liu, Y., Zuo, Z. et al. Downregulation of amphiregulin improves cardiac hypertrophy via attenuating oxidative stress and apoptosis. Biol Direct 17, 21 (2022). https://doi.org/10.1186/s13062-022-00334-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13062-022-00334-w