Abstract
Patient-derived organoid models of estrogen receptor-positive (ER+) breast cancer would provide a much-needed tool to understand drug resistance and disease progression better. However, the establishment and long-term maintenance of ER expression, function, and response in vitro remains a significant challenge. Here, we report the development of an ER+ breast tumor organoid medium (BTOM-ER) that conserves ER expression, estrogen responsiveness, and dependence, as well as sensitivity to endocrine therapy of ER+ patient-derived xenograft organoids (PDXO). Our findings demonstrate the utility of subtype-specific culture conditions that better mimic the characteristics of the breast epithelial biology and microenvironment, providing a powerful platform for investigating therapy response and disease progression of ER+ breast cancer.
Similar content being viewed by others
Introduction
Breast cancer remains one of the most frequently diagnosed cancers and causes of cancer deaths among women worldwide [23]. Despite estrogen receptor-positive (ER+) breast cancer being the most prevalent subtype, the establishment of clinically relevant models remains a challenge. More recently, three-dimensional (3D) organoid models established from patient tumors bridge the gap between cell culture and patient-derived xenograft platforms [4, 9, 21]. Previous studies have shown that organoid media formulations are critical for the establishment, growth, and maintenance of patient-specific characteristics of ER+ breast cancer organoid models [9, 12, 21]. However, consistent establishment, decreased estrogen receptor (ER) expression, and loss of ER-dependent transcription after extended culturing continues to be a challenge for the generation and use of organoid models from ER+ breast tumors.
Here, we report the development of a simplified media considering breast tissue-relevant cytokines and growth factors to establish and expand ER+ organoids that retain ER expression over long-term culture. In addition, they retain responsiveness to estrogen and sensitivity to the anti-hormone therapeutic agent, fulvestrant, identifying a new approach for generating ER+ organoid models for breast cancer.
Results
Optimization of media conditions that support robust growth of ER+ PDX organoids
In designing the media, we excluded serum to prevent the proliferation of fibroblasts and stem cell factors to prevent reprogramming or the expansion of undifferentiated cells at the expense of differentiated epithelial cells. In particular, R-spondin-1 was not included due to its roles in promoting stem and basal epithelial cell differentiation and proliferation [14]. Noggin was not included for its role in promoting stemness by interfering with ER transcriptional responses [18]. Bovine pituitary extract (BPE) and B27 is used as a serum-free mitogenic supplement [10] and to protect against oxidative stress, a common challenge that occurs in 3D cultures [15]. We included growth factors implicated in ER biology in developmental biology studies. For example, prolactin stimulates ER expression and works in concert with progesterone receptor to promote autocrine secretion-mediated mammary epithelial cell and breast cancer proliferation [6, 17]. Amphiregulin supports ER expression and estrogen signaling in the mammary gland [3]. IL6 expression is positively correlated with hormone receptor-positive tumors and has been shown to coordinate estrogen expression in vivo [5, 22, 24]. The above factors were added to DMEM/F12 media in conjunction with other growth factors frequently used to support the proliferation of mammary epithelial cells in culture and in vivo, including FGF2, FGF10, EGF, insulin, and hydrocortisone (See Additional file 1 for details of media recipe and preparation). Supplementation of estradiol to the media caused varying effects on cell growth; some organoid cultures showed no difference, whereas others showed a non-significant increase in growth (Additional file 1: Fig. S1). Furthermore, estrogen receptor expression and activity can be negatively regulated in both normal and cancer contexts during prolonged exposure to E2 in culture and in vivo ([1, 11]; hence no E2 supplementation was used for routine culture.
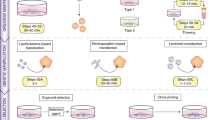
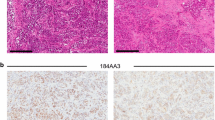
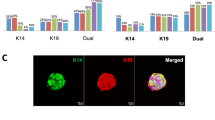
We used two independent ER+ patient-derived xenograft tumors [9] (Table 1) to find the optimal concentrations of the various growth factors to define a Breast Tumor Organoid Media for ER+ breast cancers (BTOM-ER) that support growth and expansion (Fig. 1a, Table 2). The PDXOs achieved robust growth with an average doubling time of 2.042 days (Fig. 1b) and remained stable in later passages. These data are consistent with the most commonly used ER+ breast cancer cell lines (T47D and MCF7), which have an average doubling time of 1–1.5 days [20, 25]. The phase and histomorphology of PDXOs were heterogeneous within cultures consisting of solid and hollow organoids with the formation of budding structures between 10 and 14 days post-plating (Fig. 1c). Both proliferation rates and phase morphological characteristics were relatively consistent over 15 passages, as determined by phase morphology and Incucyte live-cell imaging (data not shown). Importantly, immunofluorescence analysis for cytokeratin 8/18 and estrogen receptor demonstrated the presence of ER+ luminal tumor epithelia (Fig. 1d). Collectively, these data suggest that BTOM-ER allows for the establishment and maintenance of ER+ luminal epithelial organoid cultures that maintain proliferative capacity during long-term culture.
BTOM promotes robust growth and ER expression of ER+ PDXOs. A Schematic describing establishment of PDXOs. B A representative experiment of growth over time of PDX011 and PDX5 organoids, as measured by 3D Cell Titer Glo (Promega). Doubling time was calculated using GraphPad Prism Software. C H&E and brightfield images to show the morphology of established PDXOs. Scale bars: 100 μm. D Immunofluorescence of PDXOs displaying CK8/18 and ER-alpha expression. DAPI was used to stain nuclei
PDXOs maintain estrogen receptor expression, are estrogen-responsive, and are sensitive to endocrine therapy
Consistent with ER expression detected in the PDX tumors in vivo, analysis of cell lysates from early (passage 2) and late passage (passage 20) organoid cultures expressed detectable ERalpha (Fig. 2a). Additionally, treatment of PDXOs with physiological levels of estrogen resulted in increased proliferation compared to vehicle control (Fig. 2b). In contrast, culturing the organoids in phenol red-free media to exclude the weak estrogenic activity of phenol red reduced cell proliferation in PDX5 tumor-derived cultures that are known to express mutant ER [9] (Fig. 2c). These results are consistent with previous studies that showed these PDXs are responsive to estrogen stimulation [8, 9].Organoid lines from PDX011 tumors expressing wild-type ER showed only a weaker but significant decrease in proliferation. Finally, we treated PDXOs with the selective estrogen receptor degrader (SERD) fulvestrant, which is a widely used treatment for ER+ breast cancer. As expected, fulvestrant treatment resulted in a significant decrease in proliferation compared to vehicle control, suggesting that PDXOs, like ER+ breast tumors, require ER function to proliferate (Fig. 2d). Thus, we demonstrate that ER+ PDXOs maintain ER expression, estrogen dependence, and responsiveness and retain endocrine sensitivity during long-term culture.
PDXOs grown in BTOM maintain estrogen-dependent phenotypes of ER+ breast cancer. A Western blot showing Estrogen Receptor alpha expression in PDX011 and PDX5 organoids. VINCULIN was used as a loading control. B Luminescence levels in PDX011 and PDX5 organoids after 10 days of treatment with b-estradiol or vehicle control (EtOH). N = 3. C Representative brightfield images of Day 10 PDX011 and PDX5 grown in either BTOM or phenol red-free BTOM (left). Luminescence values for PDX011 and PDX5 organoids 10 days after being grown in either BTOM or phenol red-free BTOM (N = 3). D Luminescence values for PDX011 and PDX5 after being treated for 5 days with increasing doses of fulvestrant or vehicle control (N = 3)
Discussion
We report the development of an organoid culture media for the generation and maintenance of ER+ breast tumor organoids. We considered growth factors and cytokines with established roles in ER-mediated signaling and growth, as well as maintenance of ER expression, leading to the inclusion of components such as prolactin, amphiregulin, IL6, and bovine pituitary extract. We excluded regulators of stemness, including WNT, R-Spondin-1, and Noggin, to avoid the selection of less differentiated epithelial lineages at the expense of differentiated lineages. Given the consideration of growth factors that impact ER biology, it is likely BTOM-ER will be efficient in supporting the establishment and maintenance of ER+ luminal epithelial organoids from normal breast or mouse mammary glands; however, further studies will be required to investigate this possibility. We also recognize that the BTOM-ER we report here has multiple factors that could introduce confounding variables. However, we note that the factors used in the media were required to maintain ER expression and hence constitute an essential media for long-term expression of ER expression in primary breast tumor cells. It is possible that future efforts can identify strategies to simplify the media formulation. Nevertheless, we believe that, BTOM-ER represents a major advance for developing new clinically relevant models for ER+ breast cancer models that can be used for developing new therapies.
Similar to many other tumor organoid platforms, the organoid culture conditions outlined here do not contain fibroblasts or immune cells, which have been shown to play a significant role in ER-mediated transcription and endocrine therapy response [2, 19]. However, the culture conditions reported here are suitable for co-cultures, as we demonstrated recently for the culture of T cells with mouse or rat mammary tumor organoid models to investigate T-cell mediated killing of tumor epithelia [7, 16] to study the interaction between tumor-epithelial and stroma [12]. Thus, we believe that the culture conditions reported here can serve as a new platform for understanding ER biology in primary breast tumor-derived epithelia and for the development of ways to overcome drug resistance in ER+ breast cancer.
Methods
ER+ breast tumor organoid media (BTOM-ER)
For BTOM-ER growth media, 2.145 ml of Reagent A, 50 ml of Reagent B, and 1.0% penicillin–streptomycin were added to 100 ml of DMEM/F-12. Please see Table 1 for components of Reagent A and Reagent B and Additional file 1 for further details on components and media recipes.
Establishment of PDX organoid cultures
PDX tumor tissue was placed in a 10 cm dish, and 1–5 ml of resuspension media (DMEM-F12, 1.0% Penicillin–streptomycin, and 1.0% BSA) was added to preserve the viability of the cells during processing. Tissue was then minced using sterile surgical scalpels into fragments ranging from 0.5 to 1.0 mm. Minced tissue was then transferred to a 15 ml conical tube and pelleted by centrifugation at 1500 rpm for 5 min at 4 degrees centigrade. After the supernatant was carefully removed, 2–10 ml of digestion media (DMEM-F12, 0.1 mg/ml Collagenase/Dispase, and 1.0% Penicillin–streptomycin) was added, and the tube was placed in a 37 degrees centigrade shaker for 30–90 min, with checking every 15 min to check progress and ensure proper digestion of the tissue. The tissue is then pelleted again and resuspended in Accutase for 15–30 min to digest tissue into organoid fragments further. The tissue is then put through a 250 mm strainer to remove debris or large tissue fragments while allowing organoids to pass through. Organoids are pelleted and gently resuspended in BTOM-ER containing 5% Matrigel and ROCK-inhibitor (10 mM Y267632, Tocris), and then plated on top of a solidified Matrigel-coated well. Media was changed every 3–4 days.
Passaging of PDXOs
After the removal of BTOM-ER, digestion media is added to each well, and the plate is incubated at 37 degrees centigrade for 1.5 h. When the Matrigel has become a slurry and organoids are dispersed into small clumps or clusters, cold resuspension media is added at a 1:1 volume ratio to each well, and the suspension is transferred to a 15 ml conical tube. After centrifugation at 1500 rpm for 5 min, the supernatant is removed, and the pellet is resuspended in Accutase and incubated for 20–30 min at 37 degrees centigrade. After incubation, resuspension media is added to the suspension. After centrifugation and removal of the supernatant, the pellet is resuspended in BTOM-ER and plated as done during the establishment of cultures.
Immunohistochemistry (IHC) and imaging of PDXOs
Immunohistochemistry was performed as previously described [13]. Briefly, organoid tissue sections were generated by plating 20,000 cells/well of an 8-chamber slide. After the formation of mature organoid structures (typically 7–10 days post-plating), organoids were fixed in 4% PFA for 2 h. After treatment with hematoxylin solution for 15 min, organoids were washed twice with water. The organoids were then scraped onto a solidified layer of histogel, with additional histogel added on top to create a sandwich within a cryomold. Once solidified, the histogel sandwich was transferred to a tissue cassette and fixed in 10% formalin for 16–24 h, followed by a brief wash in 70% EtOH. The cassettes were stored in 70% EtOH until submitted for sectioning. To obtain brightfield images of organoids in culture, cells were plated at a density of 25,000 cells/well, and images were taken at 20 ×, 10 ×, and 4 × magnification using Spot Imaging software.
Proliferation and doubling time of PDXOs
Organoids were digested as above and treated with TrypLE instead of Accutase to generate single cells. Each assay well in a 96-well plate was pre-coated with 30 ml of Matrigel and was allowed to solidify in a 37 degrees centigrade incubator for at least 10 min before plating cell suspensions on top. Cells were resuspended in BTOM-ER at a density of 50,000 cells/ml, and 100 ml was added to each well in triplicate. On the indicated days, corresponding wells were assessed for viability using 3D Cell Titer Glo (Promega). Viability measurements were normalized to Day 0, and doubling time was calculated using GraphPad Prism software.
Western blot analysis
Organoids were grown in six-well plates at a density of 250,000 cells/ml. Once organoids reached confluency, each well was washed with ice-cold PBS. Matrigel was broken up by pipetting, and the slurry was transferred into a 15 ml conical tube. An additional 1.0 ml of PBS was added to the well to collect any remaining organoids. Organoids were pelleted by centrifugation at 3000 rpm for 5 min, and the supernatant was removed. The pellet was resuspended with 1.0 ml of Cell Recovery Solution (Corning) and incubated for one hour on ice. The released organoids were pelleted by centrifugation, and the supernatant was removed. After washing the pellet once with ice-cold PBS, the cells were resuspended with RIPA buffer, and western blotting analysis was performed. Signals were detected using Amersham Imager System via chemiluminescence.
Estrogen responsiveness and dependence of PDXOs
For both estrogen responsiveness and dependence, organoids were digested and plated as above 40–50,000 cells/ml, depending on the organoid line. Three days after plating, media was refreshed, and wells were treated with either 1.0 nM of b-estradiol or vehicle control (EtOH) using the Tecan D300e drug dispenser. For dependence, media was replaced with fresh BTOM-ER or phenol red-free BTOM-ER. Media was refreshed every 2–3 days. After 10 days, organoids were assessed for viability using 3D Cell Titer Glo (Promega). Viability measurements were analyzed using GraphPad Prism software.
Endocrine therapy treatment of PDXOs
Organoids were prepared as above, and on day three, media was refreshed, and wells were treated with either vehicle control or indicated concentrations of fulvestrant (Selleckchem) using the Tecan D300e drug dispenser. Media was refreshed, and plates were retreated every 2–3 days. After 5 days of treatment, organoids were assessed for viability using 3D Cell Titer Glo (Promega).
Statistics and reproducibility
Error bars were generated by Standard Error Mean (SEM) calculations. For experiments with two conditions, an unpaired one-tailed Student’s T-test was performed. For experiments with three or more conditions, one-way ANOVA followed by a Bonferroni comparison was used. Doubling time was calculated by applying a non-linear regression for exponential growth for each condition.
Availability of data and materials
Organoid lines generated will distributed freely upon publication of this manuscript.
References
Borrás M, Hardy L, Lempereur F, El Khissiin AH, Legros N, Gol-Winkler R, Leclercq G. Estradiol-induced down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. J Steroid Biochem Mol Biol. 1994;48(4):325–36. https://doi.org/10.1016/0960-0760(94)90072-8.
Brechbuhl HM, Finlay-Schultz J, Yamamoto TM, Gillen AE, Cittelly DM, Tan AC, Sams SB, Pillai MM, Elias AD, Robinson WA, Sartorius CA, Kabos P. Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clin Cancer Res. 2017. https://doi.org/10.1158/1078-0432.CCR-15-2851.
Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci USA. 2007;104(13):5455–60. https://doi.org/10.1073/PNAS.0611647104/ASSET/B244EB86-6343-4501-B738-30EAB63DB227/ASSETS/GRAPHIC/ZPQ0060752610005.JPEG.
Dekkers JF, van Vliet EJ, Sachs N, Rosenbluth JM, Kopper O, Rebel HG, Wehrens EJ, Piani C, Visvader JE, Verissimo CS, Boj SF, Brugge JS, Clevers H, Rios AC. Long-term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat Protoc. 2021;16(4):1936–65. https://doi.org/10.1038/s41596-020-00474-1.
Fontanini G, Campani D, Roncella M, Cecchetti D, Calvo S, Toniolo A, Basolo F. Expression of interleukin 6 (IL-6) correlates with oestrogen receptor in human breast carcinoma. Br J Cancer. 1999. https://doi.org/10.1038/sj.bjc.6690394.
Frasor J, Gibori G. Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab. 2003;14(3):118–23. https://doi.org/10.1016/S1043-2760(03)00030-4.
Gil Del Alcazar CR, Trinh A, Alečković M, Rojas Jimenez E, Harper NW, Oliphant MUJ, Xie S, Krop ED, Lulseged B, Murphy KC, Keenan TE, Van Allen EM, Tolaney SM, Freeman GJ, Dillon DA, Muthuswamy SK, Polyak K. Insights into immune escape during tumor evolution and response to immunotherapy using a rat model of breast cancer. Cancer Immunol Res. 2022. https://doi.org/10.1158/2326-6066.CIR-21-0804.
Gou X, Anurag M, Lei JT, Kim BJ, Singh P, Seker S, Fandino D, Han A, Rehman S, Hu J, Korchina V, Doddapaneni H, Dobrolecki LE, Mitsiades N, Lewis MT, Welm AL, Li S, Lee AV, Robinson DR, Ellis MJ. Transcriptional reprogramming differentiates active from inactive ESR1 fusions in endocrine therapy-refractory metastatic breast cancer. Can Res. 2021;81(24):6259–72. https://doi.org/10.1158/0008-5472.CAN-21-1256/674074/AM/TRANSCRIPTIONAL-REPROGRAMMING-DIFFERENTIATES.
Guillen KP, Fujita M, Butterfield AJ, Scherer SD, Bailey MH, Chu Z, DeRose YS, Zhao L, Cortes-Sanchez E, Yang CH, Toner J, Wang G, Qiao Y, Huang X, Greenland JA, Vahrenkamp JM, Lum DH, Factor RE, Nelson EW, Welm AL. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 2022;3(2):232–50. https://doi.org/10.1038/S43018-022-00337-6.
Hammond SL, Ham RG, Stampfer MR. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Nat Acad Sci. 1984;81(17):5435–9. https://doi.org/10.1073/pnas.81.17.5435.
Hatsumi T, Yamamuro Y. Downregulation of estrogen receptor gene expression by exogenous 17β-estradiol in the mammary glands of lactating mice. Exp Biol Med. 2006;231(3):311–6. https://doi.org/10.1177/153537020623100311.
Hogstrom JM, Cruz KA, Selfors LM, Ward MN, Mehta TS, Kanarek N, Philips J, Dialani V, Wulf G, Collins LC, Patel JM, Muranen T. Simultaneous isolation of hormone receptor positive breast cancer organoids and fibroblasts reveals stroma-mediated resistance mechanisms. J Biol Chem. 2023. https://doi.org/10.1016/J.JBC.2023.105021.
Huang L, Bockorny B, Paul I, Akshinthala D, Frappart PO, Gandarilla O, Bose A, Sanchez-Gonzalez V, Rouse EE, Lehoux SD, Pandell N, Lim CM, Clohessy JG, Grossman J, Gonzalez R, Del Pino SP, Daaboul G, Sawhney MS, Freedman SD, Muthuswamy SK. PDX-derived organoids model in vivo drug response and secrete biomarkers. JCI Insight. 2020. https://doi.org/10.1172/JCI.INSIGHT.135544.
Jardé T, Lloyd-Lewis B, Thomas M, Kendrick H, Melchor L, Bougaret L, Watson PD, Ewan K, Smalley MJ, Dale TC. Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat Commun. 2016;7(1):1–14. https://doi.org/10.1038/ncomms13207.
Kent KD & Bomser JA. Bovine pituitary extract provides remarkable protection against oxidative stress in human prostate epithelial cells In Vitro. Cell Develop Biol--Animal 2003. https://doi.org/10.1290/0311082.
Meng Q, Xie S, Gray GK, Dezfulian MH, Li W, Huang L, Akshinthala D, Ferrer E, Conahan C, Perea Del Pino S, Grossman J, Elledge SJ, Hidalgo M, Muthuswamy SK. Empirical identification and validation of tumor-targeting T cell receptors from circulation using autologous pancreatic tumor organoids. J Immunother Cancer. 2021;9(11):e003213. https://doi.org/10.1136/JITC-2021-003213.
Naylor MJ, Lockefeer JA, Horseman ND, Ormandy CJ. Prolactin regulates mammary epithelial cell proliferation via autocrine/paracrine mechanism. Endocrine. 2003;20(2):111–4.
Páez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grübler Y, Chervin A, Goldberg V, Goya R, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proc Natl Acad Sci USA. 2003;100(3):1034–9. https://doi.org/10.1073/PNAS.0237312100/ASSET/45393DF0-EFEF-4C71-B668-A4E88854768E/ASSETS/GRAPHIC/PQ0237312005.JPEG.
Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 2011. https://doi.org/10.1186/bcr2912.
Risinger AL, Dybdal-Hargreaves NF, Mooberry SL. Breast cancer cell lines exhibit differential sensitivities to microtubule-targeting drugs independent of doubling time. Anticancer Res. 2015;35(11):5845.
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, Korving J, van Boxtel R, Duarte AA, Lelieveld D, van Hoeck A, Ernst RF, Blokzijl F, Nijman IJ, Hoogstraat M, Clevers H. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1–2):373-386.e10. https://doi.org/10.1016/J.CELL.2017.11.010.
Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM, Sasser AK, Sullivan NJ, Studebaker AW. Interleukin-6 is a potent growth factor for ER-α-positive human breast cancer. FASEB J. 2007;21(13):3763–70. https://doi.org/10.1096/FJ.07-8832COM.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022. https://doi.org/10.3322/caac.21708.
Speirs V, Kerin MJ, Walton DS, Newton CJ, Desai SB, Atkin SL. Direct activation of oestrogen receptor- α by interleukin-6 in primary cultures of breast cancer epithelial cells. Br J Cancer. 2000;82(7):1312. https://doi.org/10.1054/BJOC.1999.1097.
Sutherland RL, Hall RE, Taylor IW. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells1; 1983. http://aacrjournals.org/cancerres/article-pdf/43/9/3998/2417028/cr0430093998.pdf.
Acknowledgements
We thank the PDXNet Consortium, especially Dr. Alana Welm and Dr. Michael T. Lewis, for providing the PDX models used for this study. The authors would also like to thank the Beth Israel Deaconess Medical Center Histology Core for sectioning and staining of the PDXO samples. Lastly, the authors would like to thank Ridhdhi Desai, Weilin Li, Ling Huang, and Almudena de Gregorio for their technical assistance, constructive feedback, and helpful discussions.
Funding
This work was supported by the National Institutes of Health Grant 5K00CA223023 (MUJO), the Breast Cancer Research Foundation (SKM), and the NCI Intramural program (SKM) and the Ludwig Center at Harvard (SKM).
Author information
Authors and Affiliations
Contributions
MO and DA co-developed the media conditions and initiated the studies. MO performed experiments related to ER requirements and dependence. MO, DA, and SKM co-wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The materials used are exempt IRB review because they were de-identified materials propagated in xenograft models and provided as frozen tissue chunks. No animal studies were performed as part of this study.
Competing interests
The authors declare that have no competing interests in relation to the results reported here. SKM is an Editor for Breast Cancer Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Oliphant, M.U.J., Akshinthala, D. & Muthuswamy, S.K. Establishing conditions for the generation and maintenance of estrogen receptor-positive organoid models of breast cancer. Breast Cancer Res 26, 56 (2024). https://doi.org/10.1186/s13058-024-01798-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-024-01798-6