Abstract
Background
Intraoperative electron radiotherapy (IOERT) can be used to treat early breast cancer during the conservative surgery thus enabling shorter overall treatment times and reduced irradiation of organs at risk. We report on our first 996 patients enrolled prospectively in a registry trial.
Methods
At Jules Bordet Institute, from February 2010 onwards, patients underwent partial IOERT of the breast. Women with unifocal invasive ductal carcinoma, aged 40 years or older, with a clinical tumour size ≤ 20 mm and tumour-free sentinel lymph node (on frozen section and immunohistochemical analysis). A 21 Gy dose was prescribed on the 90% isodose line in the tumour bed with the energy of 6 to 12 MeV (Mobetron®-IntraOp Medical).
Results
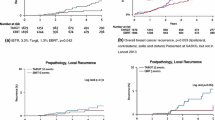
Thirty-seven ipsilateral tumour relapses occurred. Sixteen of those were in the same breast quadrant. Sixty patients died, and among those, 12 deaths were due to breast cancer. With 71.9 months of median follow-up, the 5-year Kaplan–Meier estimate of local recurrence was 2.7%.
Conclusions
The rate of breast cancer local recurrence after IOERT is low and comparable to published results for IORT and APBI. IOERT is highly operator-dependent, and appropriate applicator sizing according to tumour size is critical. When used in a selected patient population, IOERT achieves a good balance between tumour control and late radiotherapy-mediated toxicity morbidity and mortality thanks to insignificant irradiation of organs at risk.
Similar content being viewed by others
Introduction
The current standard of care for early stage breast cancer is breast conserving surgery (BCS) followed by adjuvant whole breast irradiation (WBI) [1]. This strategy extends the treatment time to about 1–3 weeks with classic treatment modalities [2]. This treatment time can be a cause of non-compliance to radiotherapy treatment (RT). Badakhshi et al. found that in a German cohort at a reference centre for breast cancer treatment, about 5% of patients were not compliant to adjuvant external radiotherapy treatment [3]. Half of those patients claimed that accessing the treatment centre every day of the week was logistically too difficult. Non-compliance increases dramatically in emerging and developing countries where radiotherapy centres are further apart and harder to access [4]. Yet to do without radiotherapy after BCS is not acceptable, as a number of randomized control trials (RCTs) has shown [5,6,7,8]. Multiple options are becoming available to reduce treatment duration. For example, hypofractionated WBI radiotherapy that reduces the total treatment time appears to be quickly becoming the new standard in early breast cancer [9, 10]. Accelerated partial breast irradiation (APBI) is also emerging as another option [11,12,13,14,15,16].
In the case of this study, we explore partial breast intraoperative radiotherapy (IORT), drastically reducing treatment time to a one-shot, radiotherapy treatment in the operating room.
The other major advantage of IORT is that the procedure reduces the size of irradiated breast tissue, thus significantly lowering the radiotherapy dose delivered to organs at risks (OAR). WBI, which is used as the current standard of care, is directly responsible for an increase in morbidity and non-breast cancer-related deaths mediated by an increase in the incidence of lung and heart disease proportionate to the radiotherapy dose delivered to those organs [17,18,19,20]. The intraoperative electron radiotherapy (IOERT) used in our hospital allows for the delivery of RT while entirely sparing the lungs and heart by placing a shield on the pectoral wall during the procedure.
This registry trial brings us high quality real-world data and evidence to support IOERT use in selected patients for early breast cancer. It also brings possible explanations for the disappointing results of the ELIOT IOERT RCT [21, 22].
Methods
Patient selection and preoperative workup
Within an ongoing prospective registry trial, patients who received IOERT as APBI were evaluated according to breast cancer survival and recurrence as primary endpoints at Institut Jules Bordet hospital in Brussels, Belgium. This study was approved by an institutional review board and included eligible patients from February 2010 until October 2019.
Female patients aged 40 years or over with biopsy proven unifocal invasive ductal carcinoma of clinical size ≤ 20 mm, and no lymph node involvement upon sentinel lymph node frozen section pathological examination were considered eligible for study inclusion. Furthermore, no restrictions were made towards molecular breast cancer subtypes as defined by histological grading, hormonal or HER2 receptor status.
On the contrary, if sentinel lymph node examination was deemed unfeasible on preoperative lymphoscintigraphy, as is the case with sole internal mammary lymph drainage, patients were excluded from our study. Patients with lymphovascular involvement and invasive lobular carcinoma on preoperative biopsy specimen were excluded. Likewise patients with a suspicion of extensive intraductal disease on preoperative MRI or core biopsy were excluded.
Every patient that presented at our institution fulfilling the above-mentioned criteria was offered IORT APBI. Every patient that undertook this treatment while fulfilling the criteria was included in the registry.
Eligible patients underwent a preoperative workup consisting of conventional mammography and echography as well as breast MRI to rule out multifocal disease. Additionally, an echography-guided and/or MRI-guided biopsy was performed as deemed necessary. All patients underwent a metastatic work-up including a chest X-ray, liver ultrasound, bone radionuclide scan and blood tests.
Surgery and IOERT
All patients underwent a lumpectomy with sentinel lymph node excision (SLN) following our previously published protocol (Additional file 1: Appendix A). Once a first frozen section analysis of the surgically removed tumour and lymph node had confirmed a tumour-free margin ≥ 1 mm and no lymph node involvement (pN0), patients were cleared for intraoperative irradiation. In the case of a tumour bordering the pectoral aponeurosis, the aponeurosis was removed to ensure safe margins.
In some patients, final pathology revealed a positive sentinel lymph node despite consistently negative SLN results on frozen sections. If patients presented with a macro metastasis in the SLN final pathology, complete axillary node dissection was performed. In cases of pN1mic, this was deemed unwarranted. Patients presenting with micro- or macroscopic spread to the sentinel lymph node were not given additional external radiation therapy. Additionally, if there was invasive tumour on the final pathology margin, patients were offered a re-excision whenever deemed feasible.
Similarly, despite our best efforts to exclude patients with multifocal breast tumours from this study, final pathology analysis sometimes revealed patients with multifocal lesions, i.e. a second tumour adjacent to the first in the same lumpectomy piece. These patients were included in this study and reported as multifocal breast cancers.
All patients were treated with electrons generated by an IntraOp (Mobetron) dedicated mobile accelerator as detailed in our previous protocol (Additional file 1: Appendix A). The tumour was removed in one piece with a 1–2 cm safety margin. A shield was inserted on the pectoral aponeurosis. The breast tissue surrounding the tumour dissection was then sutured over the shield. A cylindrical applicator is then placed over the breast tissue. As a rule, the field diameter used was at least 40 mm bigger than the pathological tumour diameter (thus a 10 mm tumour would be treated with a 50 mm applicator diameter). The dose delivered was 21 Gy, prescribed over the 90% isodose line.
Systemic treatments
Systemic treatments were determined according to institutional guidelines taking into consideration the tumour’s molecular subtype and the patients’ comorbidities.
Follow-up and results assessment
All patients were seen at the clinic 10 days after surgery to assess for acute treatment toxicity. Subsequent follow-up consisted of regular check-ups and imaging according to our institutional guidelines. At the very least, all patients were scheduled for an annual mammogram, bilateral breast echography, blood sample and clinician consult. When possible, cosmetic results were assessed by the physician during the consult according to the RTOG, CTCAE v3.0 and LENT SOMA scales [23, 24].
Overall survival and local recurrence
Follow-up time ran from the operation date to the patient’s death or last known follow-up. We systematically attempted to contact and reschedule patients that had missed appointments to minimize patients lost to follow-up.
Patients were considered to have local recurrence or distant disease as soon as pathological confirmation was obtained. Biopsy to confirm local recurrence or metastasis was always sought when necessary to guide systemic therapy.
Time to recurrence or metastasis represents the time from the IOERT to the reported event. All end-point variables reported are defined according to the DATECAN guidelines [25]. Molecular subtypes were defined according to the St-Gallen 2013 guidelines [26].
Statistical analysis
The reference date for analysis was 01/08/2021, insofar as all patient data up to that date were included for analysis. Five-year event rates were estimated using the Kaplan–Meier estimator. Multivariate competing risk regression was used to assess for independent factors associated with local recurrence.
Only four patients had significant missing data insofar as histopathological data were missing. They were subsequently excluded from the final analysis. There are no recurrences in these four patients at the time of publication.
Statistical analysis was performed using STATA statistical software (version 16.0, STATA Corp) for data compilation, validation and analysis. Data analysis was performed between 01/08/2021 and 01/10/2021.
Results
Out of 1000 patients, 996 evaluable patients who received full dose APBI through IOERT between 23 February 2010 and 08/10/2019 were identified. Patient demographics, tumour characteristics and treatment are summarized in Table 1. The median age was 61.5 (range 40–89) years. Median follow-up was 71.9 months according to the reverse Kaplan–Meier method. As of 1 August 2020, this study has a 97.0% complete follow-up using the completeness measure proposed by Clark et al. [27]
During follow-up, 37 ipsilateral breast tumour recurrences (IBTR) occurred with an incidence rate estimated at 2.7% (95% CI 1.8–4.0) at five years and 3.5% (95% CI 2.4–5.2) at median follow-up. Of those 37 recurrences, 16 were in the same breast quadrant. Additionally, there were 22 distant metastases (of which six had prior local recurrence) and five lymph node metastases (of which one had prior local recurrence). Overall relapse-free survival was estimated to be 92.3% (95% CI 90.8–94.4) at five years and 90.3% (95% CI 87.9–92.2) at median follow-up. The rate of distant metastasis occurrence was 1.7% (95% CI 1.0–2.9) and 2.2% (95% CI 1.3–3.5), respectively, at the five-year mark and at median follow-up. Likewise, overall survival was as high as 95.9% (95% CI 94.3–97.1) at five years and 94.4% (95% CI 92.4–95.9) at median follow-up.
There were 60 (6.0%) deaths among our cohort. Of those, 12 (1.2%) were attributable to breast cancer.
Table 2 summarizes the results of our multivariate competing risk analysis of factors associated with IBTR. Patients meeting institutional criteria for BRCA genetic assessment (these criteria are close to the 2010 NCCN guidelines [28]) and with a multifocal breast cancer were associated with a significantly increased risk of IBTR. Tumour size is on the verge of statistical significance for increased risk of IBTR. Age, lymphovascular involvement, perineural invasion, tumour grade and molecular subtypes were not associated with IBTR.
Acute treatment toxicity remained low, with 15 (1.5%) hematomas (none requiring subsequent surgery), eight (0.8%) infections, nine (0.9%) wound dehiscence, six (0.6%) patients having both an infection and wound dehiscence, ten (1%) patients with delayed uncomplicated wound closure, and one (0.1%) case of wound necrosis. Late toxicities and cosmetic results are reported in Table 3. Late toxicities are not given for the entire set of patients given that those assessments were progressively discontinued.
Discussion
First, a significant part of our patient population is aged 65 or older. These patients would also fit all criteria for completely omitting breast radiotherapy as attempted in clinical trials [29,30,31]. These trials showed that omitting WBI resulted in no difference in overall survival in this select population. Yet, a significant increase in IBTR was demonstrated in all trials with a risk difference of about 6% at 10 years in favour of WBI. Thereafter, some guidelines were modified to include RT omission in elderly patients [4, 32]. Some might thus think that RT for elderly patients with early stage breast cancer is no longer needed. However, it should be noted that while WBI is most likely an overtreatment for most of this patient population, APBI was never tested as a comparator in these clinical trials. We would argue that APBI and IORT techniques in particular could well become the best treatment modality for these patients. Indeed, these immediate, one-shot RT treatments still allow for a strong reduction in treatment burden while yielding strong IBTR risk reduction as discussed below.
Our results are aligned with those of the main external and brachytherapy APBI trials [11, 13,14,15, 33,34,35]. While IMPORT LOW has lower five-year IBTR rates than our study, the populations are not similar thus precluding any direct comparison. For example, 23% of our trial population received chemotherapy, 20% had grade 3 tumours against only 5% and 10%, respectively, in IMPORT LOW. The same goes for the GEC-ESTRO trial population. Additionally, we have a relatively similar IBTR rate to both RAPID and the Florence trial, which had populations that more closely resembled our study population. This is particularly significant given that contrary to external APBI, IOERT has insignificant irradiation of organs at risk.
Compared to the populations of the TARGIT-A and ELIOT trials, our study population has far fewer node positive tumours of a size greater than 2 cm or lobular breast cancers [22, 36, 37]. This was expected given the inclusion criteria of this study. Population grade was comparable to both trials. There were more Luminal B-like tumours in ELIOT than in our study. Although there is no molecular subtype data available for TARGIT, surrogate markers (such as ER/PR positivity and tumour grade) are somewhat similar. We included younger patients than both studies given enrolment was open to patients as young as 40.
Interestingly, the results of this study closely resemble those of the TARGIT-A clinical trial IORT arm, one of the two main trials to have compared IORT to WBI. TARGIT-A outlined an IBTR 5-year recurrence risk of 3.3% and overall survival of 96.1%. These results are comparable to the 2.7% and 95.9% rates found by this study [38]. Although the intraoperative radiotherapeutic technique used in our study is different to the one used in TARGIT-A and any comparison should therefore be made carefully, it is interesting to note that similar IBTR and OS outcomes speak in favour of our technique being as safe and efficacious as that of the TARGIT trial when applied to selected populations. It should also be noted that a 2.7% IBTR rate at five years is well within the acceptable non-inferiority margin used in clinical trials comparing APBI with WBI.
Our study also seems to have yielded better IBTR rates than those found in the ELIOT trial (4.4% vs 2.7%). As the IORT technique used in this study is the same as the one in used in the ELIOT trial (IOERT), this is of particular interest. Crucially, differences in lymph node metastasis (1% vs 0.2% in our study) and in distant metastasis (respectively 4.5% vs 1.7%), 5-year rates can also be noted.
We have multiple hypotheses to explain these differences in outcome:
First, the ELIOT trial began enrolment more than a decade before the first patients in our study were treated. During that period, chemotherapy treatment guidelines changed, adopting, among others, taxanes and Trastuzumab into regular chemotherapy regimens for breast cancer. The additional efficacy of these new chemotherapy regimens might have contributed to better results in more aggressive breast cancer subtypes/grade and brought IBTR risk closer to baseline favourable subtype/low grade breast cancer in our study.
Second, investigators in the ELIOT trial enrolled patients with more advanced tumours than in our cohort. Most notably, ELIOT enrolled larger size (up to 2.5 cm), node positive cancers in their trial. Out-of-quadrant dissemination and subsequent IBTR could therefore be more likely. Similarly, lobular breast cancers were enrolled although they are more likely to be multifocal and therefore again risk increasing the IBTR rate [39].
Third, although our radiotherapy technique is similar to ELIOT’s, it is highly operator-dependent. Many variables account for a successful IOERT treatment. Most notably, the treatment critically relies on applicator size and surgical technique to bring the bordering breast tissue inside the irradiation field [40, 41]. The median applicator size in this study is 55 mm compared to 40 mm in ELIOT [42]. In fact, given that the median tumour size in this study is smaller than in the ELIOT trial, the difference should be even greater. Table 4 summarizes the different applicator sizes of both ELIOT and our study. Systematic applicator under sizing has a profound impact on radiotherapy treatment. Out of foci recurrence, for example, has been associated with smaller applicator sizes [43, 44]. In the case of this study, a 15 mm increase in applicator size increases the treated surface by a factor of about 1.89. We would argue this is probably the most important difference between the two studies, and it probably explains most of the difference between ELIOT’s higher IBTR rate and this study.
Much thought had gone into creating a protocol for choosing an appropriate applicator size for patients. We based ours on studies by Holland and Faverly et al. [45, 46] These studies focused on post-mastectomy specimens in the 1980s and showed that a significant number of patients had residual tumour at more than 1 cm from tumour edge. The number of patients having residual tumour tissue dropped by almost half if the surgical margin was increased to 3 cm. We therefore chose—albeit somewhat arbitrarily—to have a total tumour margin of at least 3 cm. This was usually composed of a 1 cm surgical microscopic tumour-free margin and a radiotherapy margin of at least 2 cm. We also introduced a proportional increase in the radiotherapy margin size according to tumour size (Additional file 1: Appendix A). For a 5-mm tumour, for example, the radiotherapy margin was at 2.25 cm and the total margin (surgical + radiotherapy) at 3.25 cm. In the case of a 20-mm tumour, however, these margins would increase to 3 and 4 cm, respectively (Additional file 1: Appendix A).
ELIOT trialists had undertaken a stratified post hoc analysis of factors associated with IBTR [21]. The trial showed on univariate analysis that among others, Luminal B and triple negative molecular subtypes and tumour grade were very strong factors associated with IBTR. Our study, however, has found that while IBTR is strongly associated with patients meeting genetic testing criteria and presenting with plurifocal breast cancer (factors we therefore consider to be relative contraindication to IOERT), grade and molecular subtype were not significantly related to IBTR. In all likelihood, there probably is a slightly increased risk of IBTR with more aggressive cancer subtypes, with increased tumour grade, or with proliferative index, but all these factors are probably also responsible for increased tumour size, and this multicollinearity would perhaps explain part of the non-significance of those variables. As bigger tumours are more likely to have out-of-quadrant foci, tumour size being on the verge of significance in our multivariate analysis therefore seems quite self-explanatory.
Given the results of this study, we hypothesize that tumour size is still a very significant factor of IBTR and that rather than contraindicating some aggressive molecular subtypes or tumour grades, it would be more beneficial to take into account both tumour size and then secondary tumour characteristics in potential guidelines [47, 48]. It should be noted that the post-hoc analyses used in the ELIOT trial to try to find factors associated with IBTR were just exploratory in nature, as were those underpinning our study. Moreover, factors found to be linked to IBTR are very inconsistent across different APBI trials with some finding no link between molecular types or grades and IBTR [16, 49]. Thus, guidelines based on these analyses should always be prospectively assessed.
One main study limitation is that cosmetic evaluations were progressively discontinued given the difficulty in collecting quality data. In total, about 855 patients benefited from a summary long-term skin toxicity and cosmesis assessment. Overall treatment for evaluated patients was demonstrated to be well-tolerated for acute and late toxicity as well as breast cosmesis in keeping with the previously published literature [17, 50,51,52].
This study provides real-life data proving that IOERT using our inclusion criteria yields a low IBTR rate similar to the successful TARGIT IORT clinical trial. It is therefore the conclusion of this study that IOERT guidelines are probably too restrictive and that they could be opened up to allow inclusion of all molecular subtypes/tumour grade, with some restrictions based on tumour size.
Most importantly, this study seeks to highlight the fact that IOERT results in extremely low irradiation to organs at risk due to pectoral wall shielding. Given the significant improvements in breast cancer survival in the past decades, the authors share Vaidya et al.’s opinion that reducing treatment toxicity is now of the upmost importance [18]. Since radiotherapy-related morbidity and mortality is mediated by the irradiation of organs at risk, it is understood that drastically reducing the dose given to those organs with IOERT will reduce non-subcutaneous tissue-related toxicity [19, 20]. It follows that when comparing IOERT to WBI, additional local recurrences can be tolerated, if the patient wills it, as long as they do not affect breast cancer associated mortality. This is especially true as local recurrence does not seem to be as strong an indicator of poor prognosis in IORT as it has been in WBI [53]. Such a trade-off is therefore justified in light of the net gain in overall mortality that can be expected on the basis of lower non-breast cancer mortality [18]. We are of the opinion that overall we should take care not to harm patients by overtreating them with WBI when other IORT treatments are available and have demonstrably no negative impact on breast-cancer mortality.
Nevertheless, we must stress the importance of appropriate and exhaustive patient workup with MRI imaging by a specialized breast radiologist, timely pathological examination of the core biopsy and tumour frozen section as key elements contributing to these good results. If, at any point during patient workup, there was some lingering doubt about IOERT suitability, we would not proceed with the technique.
Availability of data and materials
The datasets analysed during the current study are not publicly available as of yet but are available from the corresponding author on reasonable request.
References
Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–30. https://doi.org/10.1093/annonc/mdv298.
Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16. https://doi.org/10.1016/S0140-6736(11)61629-2.
Badakhshi H, Gruen A, Sehouli J, Budach V, Boehmer D. The impact of patient compliance with adjuvant radiotherapy: a comprehensive cohort study. Cancer Med. 2013;2(5):712–7. https://doi.org/10.1002/cam4.114.
Gupta S, Rastogi K, Bhatnagar A, Singh D, Gupta K, Choudhary A. Compliance to radiotherapy: a tertiary care center experience. Indian J Cancer. 2018;55(2):166. https://doi.org/10.4103/ijc.ijc_517_17.
Abe O, Abe R, Enomoto K, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. https://doi.org/10.1016/S0140-6736(05)67887-7.
Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–20. https://doi.org/10.1056/NEJMoa0906260.
Forrest AP, Stewart HJ, Everington D, et al. Randomized controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial. Lancet. 1996;348(9029):708–13. https://doi.org/10.1016/S0140-6736(96)02133-2.
Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328(22):1587–91. https://doi.org/10.1056/NEJM199306033282202.
Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395(10237):1613–26. https://doi.org/10.1016/S0140-6736(20)30932-6.
Meattini I, Becherini C, Boersma L, et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022;23(1):e21–31. https://doi.org/10.1016/S1470-2045(21)00539-8.
Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer. 2015;51(4):451–63. https://doi.org/10.1016/j.ejca.2014.12.013.
Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a ran. Lancet. 2016;387(10015):229–38. https://doi.org/10.1016/S0140-6736(15)00471-7.
Polgár C, Fodor J, Major T, Sulyok Z, Kásler M. Breast-conserving therapy with partial or whole breast irradiation: ten-year results of the Budapest randomized trial. Radiother Oncol. 2013;108(2):197–202. https://doi.org/10.1016/j.radonc.2013.05.008.
Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet. 2019;394(10215):2165–72. https://doi.org/10.1016/S0140-6736(19)32515-2.
Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390(10099):1048–60. https://doi.org/10.1016/S0140-6736(17)31145-5.
Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol. 2020;38(35):4175–83. https://doi.org/10.1200/JCO.20.00650.
Korzets Y, Fyles A, Shepshelovich D, Amir E, Goldvaser H. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;175(3):531–45. https://doi.org/10.1007/s10549-019-05209-9.
Vaidya JS, Bulsara M, Wenz F, et al. Reduced mortality with partial-breast irradiation for early breast cancer: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2016;96(2):259–65. https://doi.org/10.1016/j.ijrobp.2016.05.008.
Simonetto C, Wollschläger D, Kundrát P, et al. Estimating long-term health risks after breast cancer radiotherapy: merging evidence from low and high doses. Radiat Environ Biophys. 2021;60(3):459. https://doi.org/10.1007/S00411-021-00924-8.
Taylor C, Duane FK, Dodwell D, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–9. https://doi.org/10.1200/JCO.2016.72.0722.
Orecchia R, Veronesi U, Maisonneuve P, et al. Intraoperative irradiation for early breast cancer (ELIOT): long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021;22(5):597–608. https://doi.org/10.1016/S1470-2045(21)00080-2.
Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14(13):1269–77. https://doi.org/10.1016/S1470-2045(13)70497-2.
LENT Soma. LENT SOMA scales for all anatomic sites. Int J Radiat Oncol Biol Phys. 1995;31(5):1049–91. https://doi.org/10.1016/0360-3016(95)90159-0.
Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–81. https://doi.org/10.1016/S1053-4296(03)00031-6.
Gourgou-Bourgade S, Cameron D, Poortmans P, et al. Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials)†. Ann Oncol. 2015;26(5):873–9. https://doi.org/10.1093/ANNONC/MDV106.
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(9):2206–23. https://doi.org/10.1093/ANNONC/MDT303.
Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet (London, England). 2002;359(9314):1309–10. https://doi.org/10.1016/s0140-6736(02)08272-7.
Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Cancer Netw. 2010;8(5):562–94. https://doi.org/10.6004/JNCCN.2010.0043.
Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–73. https://doi.org/10.1016/S1470-2045(14)71221-5.
Chesney TR, Yin JX, Rajaee N, et al. Tamoxifen with radiotherapy compared with Tamoxifen alone in elderly women with early-stage breast cancer treated with breast conserving surgery: a systematic review and meta-analysis. Radiother Oncol. 2017;123(1):1–9. https://doi.org/10.1016/j.radonc.2017.02.019.
Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–7. https://doi.org/10.1200/JCO.2012.45.2615.
Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, Version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(6):691–722. https://doi.org/10.6004/jnccn.2022.0030.
Philippson C, Simon S, Vandekerkhove C, et al. Early invasive cancer and partial intraoperative electron radiation therapy of the breast: experience of the Jules Bordet Institute. Int J Breast Cancer. 2014;2014:1–6. https://doi.org/10.1155/2014/627352.
Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019;394(10215):2155–64. https://doi.org/10.1016/S0140-6736(19)32514-0.
Ott OJ, Strnad V, Hildebrandt G, et al. GEC-ESTRO multicenter phase 3-trial: accelerated partial breast irradiation with interstitial multicatheter brachytherapy versus external beam whole breast irradiation: early toxicity and patient compliance. Radiother Oncol. 2016;120(1):119–23. https://doi.org/10.1016/J.RADONC.2016.06.019.
Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet (London, England). 2014;383(9917):603–13. https://doi.org/10.1016/S0140-6736(13)61950-9.
Vaidya JS, Bulsara M, Baum M, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ. 2020. https://doi.org/10.1136/BMJ.M2836.
Vaidya JS, Wenz F, Bulsara M, et al. An international randomised controlled trial to compare TARGeted Intraoperative radioTherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technol Assess (Rockv). 2016;20(73):1–188. https://doi.org/10.3310/HTA20730.
Mejdahl MK, Wohlfahrt J, Holm M, et al. Synchronous bilateral breast cancer: a nationwide study on histopathology and etiology. Breast Cancer Res Treat. 2020;182(1):229–38. https://doi.org/10.1007/S10549-020-05689-0.
Intra M, Luini A, Gatti G, et al. Surgical technique of intraoperative radiation therapy with electrons (ELIOT) in breast cancer: a lesson learned by over 1000 procedures. Surgery. 2006;140(3):467–71. https://doi.org/10.1016/J.SURG.2006.03.019.
Veronesi U, Gatti G, Luini A, et al. Intraoperative radiation therapy for breast cancer: technical notes. Breast J. 2003;9(2):106–12. https://doi.org/10.1046/J.1524-4741.2003.09208.X.
Orecchia R. Milan-Eliot randomized trial results: first analysis. Madrid: International Society of Intraoperative Radiation Therapy; 2008.
Hensley FW. Present state and issues in IORT Physics. Radiat Oncol. 2017;12(1):1–30. https://doi.org/10.1186/S13014-016-0754-Z.
Leonardi MC, Maisonneuve P, Mastropasqua MG, et al. Accelerated partial breast irradiation with intraoperative electrons: using GEC-ESTRO recommendations as guidance for patient selection. Radiother Oncol. 2013;106(1):21–7. https://doi.org/10.1016/J.RADONC.2012.10.018.
Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol. 1990;8(1):113–8. https://doi.org/10.1200/JCO.1990.8.1.113.
Faverly DRG, Hendriks JHCL, Holland R, Faverly D. Breast carcinomas of limited extent frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer. 2001;91(4):647–59. https://doi.org/10.1002/1097-0142(20010215)91:4%3c647::aid-cncr1053%3e3.0.co;2-z.
Correa C, Harris EE, Leonardi MC, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol. 2017;7(2):73–9. https://doi.org/10.1016/J.PRRO.2016.09.007/ATTACHMENT/44C2751F-DB2E-47DE-9180-685C894DD136/MMC1.PDF.
Strnad V, Krug D, Sedlmayer F, et al. DEGRO practical guideline for partial-breast irradiation. Strahlentherapie und Onkol. 2020;196(9):749–63. https://doi.org/10.1007/S00066-020-01613-Z/TABLES/3.
Goulding A, Asmar L, Wang Y, et al. Outcomes after accelerated partial breast irradiation in women with triple negative subtype and other “high risk” variables categorized as cautionary in the ASTRO guidelines. Front Oncol. 2021;11:428. https://doi.org/10.3389/FONC.2021.617439/BIBTEX.
Palubicka A, Jaworski R, Wekwejt M, et al. Surgical site infection after breast surgery: a retrospective analysis of 5-year postoperative data from a single center in Poland. Medicina (B Aires). 2019. https://doi.org/10.3390/MEDICINA55090512.
Gallagher M, Jones DJ, Bell-Syer SV. Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD005360.PUB5/MEDIA/CDSR/CD005360/IMAGE_N/NCD005360-CMP-002-01.PNG.
Polgár C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year resul. Lancet Oncol. 2017;18(2):259–68. https://doi.org/10.1016/S1470-2045(17)30011-6.
Vaidya JS, Bulsara M, Baum M, et al. New clinical and biological insights from the international TARGIT-A randomised trial of targeted intraoperative radiotherapy during lumpectomy for breast cancer. Br J Cancer. 2021;125(3):380. https://doi.org/10.1038/S41416-021-01440-8.
Acknowledgements
We wish to thank every member of the Jules Bordet Institute who participated in patient care and “L’Association Jules Bordet”.
Funding
This research benefited from a research grant by IntraOp Medical Corporation. The funding source had no involvement in study design, data collection and analysis or in the writing of the report.
Author information
Authors and Affiliations
Contributions
CP and J-MN designed the research, all authors performed the research, CP and SL wrote the manuscript, CP, SL and SS analysed the data. Critical revision of the manuscript was done by ADC and AD. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of the Institut Jules Bordet hospital approved this study. The ethics committee waived the requirement for an informed consent given that the study was entirely non-interventional. Additionally, as of now, most patients enrolled in our study have signed an institution-wide broad consent for research. Whenever a patient consults us, we strive to discuss and seek broad consent for research if that has not been done before.
Consent for publication
Not applicable.
Competing interests
Catherine Philippson, Stéphane Simon, Christophe Vandekerkhove, Alex De Caluwe, Dirk Van Gestel, Antoine Desmet received research grants from Intraop Medical Corporation. The other authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary information on the surgical and radiotherapy techniques.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Philippson, C., Larsen, S., Simon, S. et al. Intraoperative electron radiotherapy in early invasive ductal breast cancer: 6-year median follow-up results of a prospective monocentric registry. Breast Cancer Res 24, 83 (2022). https://doi.org/10.1186/s13058-022-01582-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-022-01582-4