Abstract
Background
The purpose was to evaluate glial fibrillary acidic protein (GFAP) and total-tau in plasma as predictors of poor neurological outcome after out-of-hospital (OHCA) and in-hospital cardiac arrest (IHCA), including comparisons with neurofilament light (NFL) and neuron-specific enolase (NSE).
Methods
Retrospective multicentre observational study of patients admitted to an intensive care unit (ICU) in three hospitals in Sweden 2014–2018. Blood samples were collected at ICU admission, 12 h, and 48 h post-cardiac arrest. Poor neurological outcome was defined as Cerebral Performance Category 3–5 at 2–6 months after cardiac arrest. Plasma samples were retrospectively analysed for GFAP, tau, and NFL. Serum NSE was analysed in clinical care. Prognostic performances were tested with the area under the receiver operating characteristics curve (AUC).
Results
Of the 428 included patients, 328 were OHCA, and 100 were IHCA. At ICU admission, 12 h and 48 h post-cardiac arrest, GFAP predicted neurological outcome after OHCA with AUC (95% CI) 0.76 (0.70–0.82), 0.86 (0.81–0.90) and 0.91 (0.87–0.96), and after IHCA with AUC (95% CI) 0.77 (0.66–0.87), 0.83 (0.74–0.92) and 0.83 (0.71–0.95). At the same time points, tau predicted outcome after OHCA with AUC (95% CI) 0.72 (0.66–0.79), 0.75 (0.69–0.81), and 0.93 (0.89–0.96) and after IHCA with AUC (95% CI) 0.61 (0.49–0.74), 0.68 (0.56–0.79), and 0.77 (0.65–0.90). Adding the change in biomarker levels between time points did not improve predictive accuracy compared to the last time point. In a subset of patients, GFAP at 12 h and 48 h, as well as tau at 48 h, offered similar predictive value as NSE at 48 h (the earliest time point NSE is recommended in guidelines) after both OHCA and IHCA. The predictive performance of NFL was similar or superior to GFAP and tau at all time points after OHCA and IHCA.
Conclusion
GFAP and tau are promising biomarkers for neuroprognostication, with the highest predictive performance at 48 h after OHCA, but not superior to NFL. The predictive ability of GFAP may be sufficiently high for clinical use at 12 h after cardiac arrest.
Similar content being viewed by others
Background
Among patients admitted to intensive care after cardiac arrest, the most common cause of death is related to brain injury, preceded by withdrawal of life-sustaining therapies (WLST) after the prediction of a poor neurological outcome [1]. The 2021 ERC/ESICM guidelines recommend multimodal neuroprognostication from 72 h after a cardiac arrest based on a combination of clinical examination, biomarkers of brain injury, neurophysiological investigations, and neuroimaging [2]. The majority of studies on post-cardiac arrest care, including neuroprognostication and biomarkers of brain injury, have been performed after out-of-hospital cardiac arrest (OHCA), with results incorporated into guidelines also informing care after in-hospital cardiac arrest (IHCA) [2]. Biomarkers in the blood are suitable tools for prognostication since they are easily obtained, results are quantitative and objective, and they are not known to be affected by sedatives or muscle relaxants. The current ERC/ESICM guidelines recommend one biomarker of brain injury—neuron-specific enolase (NSE), with a predictive value 48-72 h after cardiac arrest [2]. Novel biomarkers, including neurofilament light (NFL), glial fibrillary acidic protein (GFAP), total-tau, and phosphorylated tau, have shown promising prognostic value after cardiac arrest [3,4,5,6,7,8,9,10,11,12]. GFAP is an intermediate-filament component in the astrocyte cytoskeleton and is released from damaged or activated astrocytes [3, 13]. GFAP is elevated in serum or plasma after cardiac arrest, intracerebral haemorrhage, head trauma, and ischemic stroke [14,15,16,17,18]. Elevated GFAP may also be seen in healthy individuals with developing Alzheimer's pathology, but levels are lower than those seen after cardiac arrest [19]. Tau protein is a microtubule-stabilising structure found in smaller unmyelinated axons and is released non-specifically after brain injury. Elevated tau concentrations in serum or plasma have been described after ischemic stroke and cardiac arrest [3, 4, 13]. In selected groups of comatose survivors after OHCA, tau has an excellent ability (AUC 0.91–0.93) to predict poor outcomes at 48 h. [4, 5]. The predictive ability of GFAP has differed between studies [4, 7,8,9, 11, 20, 21]. Excellent prediction (AUC 0.83–0.91) between 24-72 h post-cardiac arrest has been reported in selected OHCA, whereas AUC as low as 0.59–0.67 were reported in a small study on OHCA and a small mixed sample of OHCA and IHCA.
This study aimed to evaluate the predictive ability of GFAP and tau to predict neurological outcome in OHCA and IHCA at multiple time points. We also compared the predictive ability of GFAP and tau to NSE, the only biomarker of post-hypoxic brain injury used in clinical practice, and to NFL (which has shown promising predictive abilities). Additionally, we wanted to test if a combination of biomarkers originating from astroglial cells and neurons improved prediction compared to individual biomarkers. We, therefore, tested a combination of GFAP and tau and a combination of GFAP and NFL, considering previously described very high predictive values of NFL at 12 h in the same patients [22].
Methods
Study setting
Retrospective multicentre observational study of post-cardiac arrest patients admitted to three intensive care units (ICU) in Skåne, Sweden, between 2014–2018. The study was a part of the SWECRIT biobank project (https://clinicaltrials.gov/ct2/show/NCT04974775), which aimed to include all adults admitted to the involved ICUs to study biomarkers in critically ill patients [22]. The Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines were followed [23].
Study participants and outcomes
Blood samples were collected at ICU admission and 12 h and 48 h post-cardiac arrest. Cardiac arrest patients were identified by ICD-10 in the local intensive care registry. Patients with only an admission sample (drawn < 6 h after cardiac arrest) were excluded. Samples drawn within six hours of the specified time points were included for statistical analysis. Patient data were collected from medical records, the International Cardiac Arrest Registry (INTCAR), the local ICU registry, and the Swedish population register. During the study period, post-cardiac arrest care was given according to current guidelines, including target temperature management (TTM) at 36 °C for 28 h and multimodal neuroprognostication at ≥ 72 h for all unconscious patients. Withdrawal of life-sustaining therapies (WLST) was practised, and information on time to WLST and reasons for WLST (neurological, circulatory failure/multi-organ failure, comorbidity, or ethical reasons) were collected [24].
Long-term neurological outcome was assessed by any healthcare professional at 2–6 months after cardiac arrest according to the Cerebral Performance Category (CPC) scale. Good outcome was defined as CPC 1–2 (good cerebral performance or moderate cerebral disability), and poor outcome as CPC 3–5 (severe cerebral disability, coma, or brain death).
Biomarker analysis
The local laboratory centrifuged, aliquoted, and froze plasma samples at each site before they were sent to the biobank at Region Skåne, Sweden (BD-47, SC-1922) for long-term storage until analysis. GFAP, total-tau, and NFL levels were analysed using commercially available Single molecule array (Simoa) assays on an HD-X instrument (Quanterix, Billerica, MA) at the Clinical Neurochemistry Laboratory at the University of Gothenburg by staff blinded to all clinical data. NSE was prospectively analysed 24 h and 48 h after cardiac arrest, using an electrochemiluminescence immunoassay on cobas e601/e602 instruments (Roche Diagnostics, Mannheim, Germany). NSE levels were available to treating physicians and employed in multimodal neuroprognostication in clinical practice. NSE levels were not reported for samples with significant haemolysis [25].
Statistics
Patient characteristics are presented as median and interquartile range (IQR) for continuous variables and as counts and percentages for categorical variables. Categorical variables were compared using the Chi-squared test and continuous variables with the Mann–Whitney U test or logistic regression. The diagnostic performance of the biomarkers was assessed by calculating the area under the receiver operating characteristic curve (AUC) from the logistic regression models. The DeLong method was used to compare AUCs. We examined if the change in GFAP and tau levels between time points (delta) improved the performance compared to individual time points using logistic regression and AUC. We determined the cut-off values of GFAP and tau for poor prognosis at low false-positive rates (FPR) and good prognosis at low false-negative rates (FNR) based on the receiver operating characteristic curve (ROC) coordinate points. To reduce the skewness of the biomarker measurements, we used log10-transformed data. The false discovery rate (FDR) was used to adjust p-values for multiplicity. Significance was set at p < 0.05. Statistical analyses were performed with R, version 4.1.2 (The R Foundation for Statistical Computing).
Ethics and consent
The Regional Ethical Review Board in Lund, Sweden, approved the study protocol (registration no. 2014/47 and 2022-02681-01). For patients who regained consciousness, written informed consent was obtained.
Results
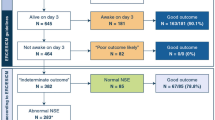
Four hundred twenty-eight patients were included, 328 OHCA and 100 IHCA (Fig. 1). Characteristics of included compared to missed and excluded patients have previously been published for the cohort used in this study [22]. Cardiac arrest characteristics differed between OHCA and IHCA (Table 1). In OHCA, the time to ROSC was longer, a shockable first recorded rhythm and a cardiac cause were more common; patients who suffered IHCA had a higher Glasgow Coma Scale Motor Score (GCS-M) on admission, a good neurological outcome was more common, and IHCA were less often subject to WLST, particularly WLST due to poor neurological prognosis (Additional file 1: eTable 1). After sample collection, plasma samples were frozen within a median of 1.25 h (IQR 1.0–2.1 h).
Predictive performance of GFAP
GFAP levels are shown in Fig. 2A, B at ICU admission, 12 h, and 48 h, and Fig. 2C for all available data. GFAP levels were higher in patients with poor outcome compared to patients with good outcome at all pre-defined time points in OHCA (p < 0.001) and in IHCA (p < 0.003) (Fig. 2A, B). The predictive accuracy expressed as ROC curves is shown in Fig. 3A, B. At admission after OHCA, GFAP had an AUC of 0.76 (95% CI 0.70–0.82), at 12 h, an AUC of 0.86 (95% CI 0.81–0.90), and at 48 h, the AUC was 0.91 (95% CI 0.87–0.96). After IHCA predictive performance was AUC 0.77 (95% CI 0.66–0.87) at admission, AUC 0.83 (95% CI 0.74–0.92) at 12 h and 0.83 (95% CI 0.71–0.95) at 48 h post-cardiac arrest. The prognostic performance remained similar after excluding patients who obeyed commands (GCS-M 6) on admission (Additional file 1: eTable 2). The cause of arrest (cardiac versus non-cardiac) did not significantly affect the predictive ability of GFAP in OHCA or IHCA (Additional file 1: eTable 3 and eFigure 1). The cut-off for prediction of poor outcome with FPR ≤ 2% after OHCA was at 12 h 1626 pg/mL with sensitivity 0.34 (95% CI 0.27–0.41), and at 48 h 2376 pg/ml with sensitivity 0.32 (95% CI 0.24–0.40) (Additional file 1: eTable 4). The cut-off for prediction of good outcome with FNR ≤ 5% was after OHCA at 12 h 111 pg/mL with specificity 0.47 (95% CI (0.38–0.56), and at 48 h 183 pg/ml with specificity 0.64 (95% CI (0.52–0.76) (Additional file 1: eTable 5).
A–F Plasma GFAP and tau by time point, cardiac arrest group, and outcome. Boxplots are shown for GFAP in panels A, B and tau levels in panels D, E at ICU admission, 12 and 48 h post cardiac arrest for OHCA and IHCA, respectively. The boxes show the median and interquartile range (IQR). The bold lines in panels C and F show the trajectory trend, smoothed via generalised additive models, of GFAP and tau with 95% CI. The dots represent samples and the lines patients. Additional samples (n = 60) collected outside the defined time points (admission [0–6 h], 12 ± 6 h, and 48 ± 6 h post-arrest) were included. GFAP glial fibrillary protein, OHCA out‑of‑hospital cardiac arrest, IHCA in‑hospital cardiac arrest, CPC Cerebral Performance Category
A–D Prediction of poor vs good outcome using plasma GFAP and tau. The area under the receiver operating characteristic curve (AUC), with 95% confidence intervals, is shown for GFAP and tau at ICU admission, 12 and 48 h after OHCA and IHCA. GFAP glial fibrillary protein, OHCA out‑of‑hospital cardiac arrest, IHCA in‑hospital cardiac arrest
Predictive performance of tau
Tau levels at ICU admission, 12 h, and 48 h are shown in Fig. 2D–E and in Fig. 2F for all available longitudinal data. Tau levels were higher in patients with poor outcome compared to patients with good outcome at admission, 12 h and 48 h after OHCA (p < 0.001) and at all time points after IHCA (p < 0.02) except at admission (p = 0.08) (Fig. 2D, E). In all patients, irrespective of outcome, tau levels had a pattern with an initial release following cardiac arrest with a subsequent decrease (Fig. 2F). In patients with poor outcome, tau levels rose again toward 48 h (Fig. 2F). The predictive ability of tau was highest at 48 h post-cardiac arrest after both OHCA (AUC 0.93 (95% CI 0.89–0.96)) and IHCA (AUC 0.77 (95% CI 0.65–0.90)) (Fig. 3C, D). The predictive ability was similar after excluding patients with GCS-M 6 on admission (Additional file 1: eTable 2). The cause of cardiac arrest (cardiac versus non-cardiac) did not significantly affect the prognostic ability of tau in OHCA or IHCA (Additional file 1: eTable 3 and eFigure 1). At 48 h after OHCA, the cut-off for prediction of poor outcome with FPR ≤ 2% was 35.5 pg/ml with sensitivity 0.76 (95% CI 0.69–0.83) (Additional file 1: eTable 6) and for prediction of good outcome with FNR ≤ 5% 3.5 pg/ml with specificity 0.45 (95% CI (0.33–0.58) (Additional file 1: eTable 7).
Serial measures of GFAP and tau
For each biomarker, we tested if adding the change of levels between time points (delta) improved the predictive accuracy compared to using only the last time point. At no time point did the delta improve the predictive accuracies compared to the last single time point (Additional file 1: eTable 8 and 9).
Plasma GFAP versus tau
The predictive ability of GFAP versus tau and the combination of GFAP and tau versus either biomarker alone is presented in Additional file 1: eTable 10. GFAP was superior to tau at 12 h after both OHCA (p = 0.003) and IHCA (p = 0.04), and its predictive value was not improved by adding tau at any time after OHCA or IHCA. The predictive value of tau was improved by the addition of GFAP at admission and 12 h after both OHCA (p = 0.02, p < 0.001) and IHCA (p = 0.04, p = 0.03) but not at 48 h.
Plasma GFAP versus NSE
The predictive abilities of GFAP and NSE are shown in Fig. 4A, B and Additional file 1: eTable 11. Comparisons between NSE and GFAP were made in subsets of patients with available biomarker levels at the compared time points. After both OHCA and IHCA, the predictive ability of GFAP at 12 h was similar to NSE at 48 h after both OHCA (p = 0.08) and IHCA (p = 0.08). At 48 h, the predictive values of GFAP and NSE were similar after OHCA (p = 0.86) and IHCA (p = 0.53).
A–D Classification of poor versus good outcome using NSE, GFAP, and tau. Receiver operatic characteristic curves (ROC) and the area under the curve (AUC), with 95% confidence intervals, are shown in a subgroup of patients with NSE levels at 48 h and 24 h post arrest and GFAP or tau at 48 h and 12 h, respectively. Only participants with both NSE and GFAP or tau levels were included (at 48 h; 190 OHCA and 41 IHCA, at 12/24 h; 232 OHCA and 59 IHCA), and therefore, the results for GFAP and tau differ slightly from those in Fig. 3. NSE neuron-specific enolase, GFAP glial fibrillary protein, OHCA out‑of‑hospital cardiac arrest, IHCA in‑hospital cardiac arrest
Plasma tau versus NSE
The predictive abilities of tau and NSE in patients with available NSE levels are shown in Fig. 4C, D and Additional file 1: eTable 12. At 48 h, tau and NSE had similar predictive values after OHCA (p = 0.59) and IHCA (p = 0.62).
Plasma GFAP versus NFL
GFAP and NFL had a similar predictive performance at all time points after IHCA and admission after OHCA (Additional file 1: eTable 13). After OHCA, NFL was superior to GFAP at 12 h and 48 h (p = 0.01, p = 0.02). Compared to GFAP alone, adding NFL to GFAP improved predictive accuracy at 12 h and 48 h after OHCA (p < 0.001, p = 0.005) but not at admission or after IHCA.
Plasma tau versus NFL
Tau and NFL had a similar predictive performance at all time points after IHCA and admission after OHCA (Additional file 1: eTable 14). NFL was superior to tau at 12 h and 48 h after OHCA (p < 0.001, p = 0.004). Compared to tau alone, adding NFL to tau improved the predictive accuracy at all time points after OHCA (p = 0.004, p < 0.001, p < 0.003) and 12 h after IHCA (p = 0.04).
Discussion
In this multicentre observational study, we found that GFAP and tau are reliable predictors of neurological outcome at 48 h after OHCA. Additionally, GFAP displayed excellent performance already 12 h post-cardiac arrest, regardless of the location (OHCA or IHCA) or cause of the arrest (cardiac vs non-cardiac), but was not a reliable predictor of outcome at ICU admission. The predictive value of tau was low at 12 h after cardiac arrest and at ICU admission.
The predictive performance of GFAP at 48 h after OHCA in our study was similar to that found in other studies at 24 h and 48 h after OHCA, including one cohort where WLST was not practised [4, 7,8,9]. Forty-eight hours after IHCA, GFAP showed considerably better predictive performance than earlier reported in a small mixed population of OHCA and IHCA, possibly explained by case mix, different laboratory methods, or sample size [21]. The major novel finding of our study is the high predictive value of GFAP already at 12 h after both OHCA and IHCA. The release pattern of GFAP into the blood after cardiac arrest included an early and continuous rise in patients with poor neurological outcomes, likely explaining the predictive value at 12 h. In addition, low GFAP levels reliably predicted good neurological outcomes. The predictive value of GFAP at admission was not clinically useful, as observed in previous studies [4, 21].
The only biomarker recommended by guidelines is NSE from 48 h after cardiac arrest. In the present study, GFAP at 12 h displayed a predictive value similar to that previously reported for NSE 48 h after OHCA, suggesting that GFAP at 12 h may be as good a neuroprognostic marker as NSE 36 h later [25,26,27]. However, interpretations are limited as NSE was used in patient care, impacting decisions on WLST and patient outcome. Additionally, NSE was analysed with an assay with 4% between-run imprecision, whereas the Simoa has been reported to have a larger variability (14%) [25, 28].
For tau, we found the highest predictive ability 48 h after OHCA, and two previous studies have reported similar predictive values after OHCA [4, 5]. In one of the studies, tau outperformed NSE at 48 h and 24 h in a select cohort of OHCA, a finding not replicated in the current study in a broader population of OHCA [5]. Both studies employed the same laboratory analysis methods for tau and NSE but differed in how haemolysis was handled in reporting NSE values. The same study reported a high predictive value of tau at 24 h after cardiac arrest [5]. This, in combination with our results, suggests that the earliest time point of clinically useful predictive value may be from around 24 h after cardiac arrest. We noted a temporal pattern in plasma tau levels where all patients, irrespective of outcome, appear to have an initial release with elevated tau, with decreasing levels in patients with good outcomes but a second burst in patients with poor outcomes. We speculate that the initial burst may reflect the opening of the blood–brain barrier or simply the release of tau from the peripheral nervous system directly into the bloodstream. This release pattern and association with outcome have been described previously in smaller studies [4, 6, 29]. Despite this pattern, employing multiple time points for outcome prediction was not superior to the last individual sample.
The present study included unselected OHCA and IHCA patients, rarely studied in post-cardiac arrest care. We found that prognostic abilities for all investigated biomarkers were generally lower after IHCA than OHCA, a finding also reported for NFL [22]. This is an important observation since evidence from OHCA is often extrapolated to inform the care of IHCA. Biomarkers of brain injury will fail to identify patients with poor outcome after cardiac arrest without significant post-hypoxic brain injury (e.g., haemodynamic collapse), possibly explaining the lower predictive value after IHCA. Withdrawal of life-support was more common after OHCA than IHCA. After OHCA WLST due to a poor neurological prognosis was more common whereas after IHCA WLST due to co-morbidity was more common, suggesting differences in degree of brain injury. Additionally, the number of patients in the IHCA group was small.
The results of the present study may be another step towards earlier and safe multimodal neuroprognostication. Biomarkers of brain injury in the blood may have an advantage early during post-cardiac arrest care when sedative agents are frequently administered and confound clinical examination and electroencephalography. We recently showed that NFL offers excellent outcome prediction as early as 12 h after OHCA in the same cohort used in the present study [22]. Additionally, the novel brain injury biomarker p-tau181 has been studied 24 h after OHCA and showed excellent outcome prediction but remains unstudied at earlier time points [10]. It may be that some biomarkers of brain injury, including GFAP and NFL, can provide useful neuroprognostic information even in patients affected by sedation 12 h after cardiac arrest. However, our studies on GFAP and NFL at 12 h after cardiac arrest were performed in the same patients and require validation. A combination of biomarkers may further improve neuroprognostication if the biomarker has different cellular origins. In the present study, the different combinations of GFAP, tau and NFL did not significantly improve prognostic performance compared to the most predictive biomarker alone. However, multiple biomarkers all suggest a similar outcome, improving the clinical safety of prognostication and reducing the risk of confounders or laboratory errors affecting decisions on WLST. Implementing novel biomarkers with 24/7 availability in clinical practice requires commercial assays, larger and more diverse studies to establish and validate outcome prediction cut-offs, and integrating the new biomarkers into routine clinical workflows. Collaborative efforts between researchers, healthcare institutions, and commercial partners are necessary to accelerate the translation of these biomarkers from research to clinical practice and ultimately improve the prediction of patient outcomes after cardiac arrest. Available commercial assays are Abbott Alinity (GFAP), Lumipulse (NFL) and Roche Elecsys (NFL, GFAP, tau). Still, they all require further validation of cut-offs for use in neuroprognostication after cardiac arrest.
Strengths and limitations
This study is strengthened by its multicentre design, large sample size, prospective and standardised blood sampling and handling, comparison of IHCA and OHCA and batch analysis of biomarkers. Study limitations include the retrospective study design, where the data quality depends on the quality of medical records, the smaller sample size of IHCA, particularly at 48 h and the rate of WLST. Another limitation, particularly in the IHCA subgroup, is that the analyses include patients in which neuroprognostication may not be necessary due to awakening and those who die from other reasons than hypoxic brain injury. Due to the consent process, there may be missing data among survivors (but not non-survivors), which may bias the results towards patients with poor prognoses.
Conclusion
GFAP and tau are promising novel biomarkers for predicting neurological outcome after cardiac arrest, with the highest predictive performance at 48 h after OHCA. The predictive ability of GFAP may be sufficiently high for clinical use at 12 h after cardiac arrest. NFL had similar or superior predictive performance at all time points.
Availability of data and materials
The protocol and the datasets analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AUC:
-
Area under the receiver operation curve
- CI:
-
Confidence interval
- CPC:
-
Cerebral performance category
- CPR:
-
Cardiopulmonary resuscitation
- ERC:
-
European Resuscitation Council
- ESICM:
-
European Society of Intensive Care Medicine
- GCS-M:
-
Glasgow Coma Scale-Motor Score
- GFAP:
-
Glial fibrillary acidic protein
- ICU:
-
Intensive care unit
- IHCA:
-
In-hospital cardiac arrest
- IQR:
-
Interquartile range
- NFL:
-
Neurofilament light
- NSE:
-
Neuron-specific enolase
- OHCA:
-
Out-of-hospital cardiac arrest
- PCI:
-
Percutaneous cardiac intervention
- PEA:
-
Pulseless electric activity
- ROSC:
-
Return of spontaneous circulation
- TTM:
-
Targeted temperature management
- WLST:
-
Withdrawal of life-sustaining therapy
References
Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–35.
Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220–69.
Humaloja J, Ashton NJ, Skrifvars MB. Brain injury biomarkers for predicting outcome after cardiac arrest. Crit Care. 2022;26(1):81.
Humaloja J, Lähde M, Ashton NJ, Reinikainen M, Hästbacka J, Jakkula P, et al. GFAp and tau protein as predictors of neurological outcome after out-of-hospital cardiac arrest: a post hoc analysis of the COMACARE trial. Resuscitation. 2022;170:141–9.
Mattsson N, Zetterberg H, Nielsen N, Blennow K, Dankiewicz J, Friberg H, et al. Serum tau and neurological outcome in cardiac arrest. Ann Neurol. 2017;82(5):665–75.
Huesgen KW, Elmelige YO, Yang Z, Chowdhury MAB, Gul S, Maciel CB, et al. Ultra-early serum concentrations of neuronal and astroglial biomarkers predict poor neurological outcome after out-of-hospital cardiac arrest-a pilot neuroprognostic study. Resusc Plus. 2021;7: 100133.
Ebner F, Moseby-Knappe M, Mattsson-Carlgren N, Lilja G, Dragancea I, Undén J, et al. Serum GFAP and UCH-L1 for the prediction of neurological outcome in comatose cardiac arrest patients. Resuscitation. 2020;154:61–8.
Song H, Bang HJ, You Y, Park JS, Kang C, Kim HJ, et al. Novel serum biomarkers for predicting neurological outcomes in postcardiac arrest patients treated with targeted temperature management. Crit Care. 2023;27(1):113.
Klitholm M, Nørgaard Jeppesen A, Christensen S, Parkner T, Tybirk L, Kirkegaard H, et al. Neurofilament light chain and glial fibrillary acidic protein as early prognostic biomarkers after out-of-hospital cardiac arrest. Resuscitation. 2023;193:109983.
Ashton NJ, Moseby-Knappe M, Benedet AL, Grötschel L, Lantero-Rodriguez J, Karikari TK, et al. Alzheimer disease blood biomarkers in patients with out-of-hospital cardiac arrest. JAMA Neurol. 2023;80(4):388–96.
Hoiland RL, Rikhraj KJK, Thiara S, Fordyce C, Kramer AH, Skrifvars MB, et al. Neurologic prognostication after cardiac arrest using brain biomarkers: a systematic review and meta-analysis. JAMA Neurol. 2022;79(4):390–8.
Wihersaari L, Reinikainen M, Furlan R, Mandelli A, Vaahersalo J, Kurola J, et al. Neurofilament light compared to neuron-specific enolase as a predictor of unfavourable outcome after out-of-hospital cardiac arrest. Resuscitation. 2022;174:1–8.
Gul SS, Huesgen KW, Wang KK, Mark K, Tyndall JA. Prognostic utility of neuroinjury biomarkers in post out-of-hospital cardiac arrest (OHCA) patient management. Med Hypotheses. 2017;105:34–47.
Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62(8):1303–10.
Kaneko T, Kasaoka S, Miyauchi T, Fujita M, Oda Y, Tsuruta R, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80(7):790–4.
Foerch C, Niessner M, Back T, Bauerle M, De Marchis GM, Ferbert A, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. 2012;58(1):237–45.
Liu G, Geng J. Glial fibrillary acidic protein as a prognostic marker of acute ischemic stroke. Hum Exp Toxicol. 2018;37(10):1048–53.
Missler U, Wiesmann M, Wittmann G, Magerkurth O, Hagenström H. Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem. 1999;45(1):138–41.
Benedet AL, Milà-Alomà M, Vrillon A, Ashton NJ, Pascoal TA, Lussier F, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the alzheimer disease continuum. JAMA Neurol. 2021;78(12):1471–83.
Helwig K, Seeger F, Hölschermann H, Lischke V, Gerriets T, Niessner M, et al. Elevated serum glial fibrillary acidic protein (GFAP) is associated with poor functional outcome after cardiopulmonary resuscitation. Neurocrit Care. 2017;27(1):68–74.
Larsson IM, Wallin E, Kristofferzon ML, Niessner M, Zetterberg H, Rubertsson S. Post-cardiac arrest serum levels of glial fibrillary acidic protein for predicting neurological outcome. Resuscitation. 2014;85(12):1654–61.
Levin H, Lybeck A, Frigyesi A, Arctaedius I, Thorgeirsdóttir B, Annborn M, et al. Plasma neurofilament light is a predictor of neurological outcome 12 h after cardiac arrest. Crit Care. 2023;27(1):74.
Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11): e012799.
Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85(12):1779–89.
Lissner Östlund E, Levin H, Nielsen N, Frigyesi A, Lybeck A. Neuron-specific enolase and long-term neurological outcome after OHCA—a validation study. Resuscitation. 2021;168:206–13.
Stammet P, Collignon O, Hassager C, Wise MP, Hovdenes J, Åneman A, et al. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33°C and 36°C. J Am Coll Cardiol. 2015;65(19):2104–14.
Streitberger KJ, Leithner C, Wattenberg M, Tonner PH, Hasslacher J, Joannidis M, et al. Neuron-specific enolase predicts poor outcome after cardiac arrest and targeted temperature management: a multicenter study on 1053 patients. Crit Care Med. 2017;45(7):1145–51.
Bogoslovsky T, Wilson D, Chen Y, Hanlon D, Gill J, Jeromin A, et al. Increases of plasma levels of glial fibrillary acidic protein, tau, and amyloid β up to 90 days after traumatic brain injury. J Neurotrauma. 2017;34(1):66–73.
Randall J, Mörtberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84(3):351–6.
Acknowledgements
We would like to thank the staff at the ICUs in Skane for contributing to this study. We are also grateful to the participating patients and their families.
Funding
Open access funding provided by Lund University. Regional research support, Region Skane; Government funding of clinical research within the Swedish National Health Services (ALF); Biobanking and BioMolecular resources Research Infrastructure (BBMRI, Sweden); Hans‐Gabriel and Alice Trolle‐Wacht‐ meister Foundation for Medical Research; Anna‐Lisa & Sven‐Eric Lundgrens Foundation for medical research; European Union Interreg programme IVA. IA: Tegger Stiftelsen. MM-K: Swedish National Health Service and Regional Research Support Region Skåne. Segerfalk Foundation and the Bundy Academy at Lund University. NN: Swedish Research Council, Regional research support, Region Skane; Government funding of clinical research within the Swedish National Health Services (ALF); HZ: is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union's Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme—Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003). AF: Regional research support, Region Skåne #2022-1284; Governmental funding of clinical research within the Swedish National Health Service (ALF) #2022:YF0009 and #2022-0075; Crafoord Foundation grant number #2021-0833; Lions Skåne research grants; Skåne University Hospital grants; Swedish Heart and Lund Foundation (HLF) #2022-0352 and #2022-0458. HF: Hans‐Gabriel and Alice Trolle‐Wachtmeister Foundation for Medical Research, Regional research support Region Skåne, Governmental funding of clinical research within the Swedish National Health Service (ALF), Swedish Heart–Lung foundation. AL: Region Research Support Region Skåne, Skåne University Hospital grants, Maggie Stevens Foundation, and fees for supervising students from the Swedish National Health Services ALF via Lund University. NMC: Grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (2022-Projekt0107), Familjen Rönnströms Stiftelse, Skåne University Hospital grants, Kockska Stiftelsen, Swedish Brain Foundation (FO2023-0163), WASP and DDLS Joint call for research projects (WASP/DDLS22-066), Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Swedish Alzheimer Foundation (AF-980832), Regional research support, Region Skåne, Swedish Research Council (2021-02219). NMC is a Wallenberg Molecular Medicine Fellow, supported by grants from the Knut and Alice Wallenberg Foundation (2017-0383), The Medical Faculty at Lund University, Region Skåne, and EU Joint Programme Neurodegenerative Diseases (2019-03-401).
The funding organizations had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conceptualisation: IA, HL, AL, NMC, HF. Data Curation: IA, HL, BT. Formal Analysis: HL, IA, NMC. Funding Acquisition: AL, AF, HF. Investigation: IA, HL, AL, NMC. Methodology: IA, HL, AL, NMC. Project Administration: AL, HF, HL. Resources: AL, HF, AF, KB, HZ, NJA, MA, NN. Supervision: AL, NMC, MMK, TC, HF. Visualisation: HL. Writing—original draft: IA, HL, AL, NMC. Writing—review & editing: All authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Regional Ethical Review Board in Lund, Sweden, approved the study protocol (registration no. 2014/47 and 2022-02681-01). For patients who regained consciousness, written informed consent was obtained.
Consent for publication
Not applicable.
Competing interests
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material including additional tables and figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Arctaedius, I., Levin, H., Thorgeirsdóttir, B. et al. Plasma glial fibrillary acidic protein and tau: predictors of neurological outcome after cardiac arrest. Crit Care 28, 116 (2024). https://doi.org/10.1186/s13054-024-04889-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-04889-0