Abstract
Background
Prone positioning (PP) homogenizes ventilation distribution and may limit ventilator-induced lung injury (VILI) in patients with moderate to severe acute respiratory distress syndrome (ARDS). The static and dynamic components of ventilation that may cause VILI have been aggregated in mechanical power, considered a unifying driver of VILI. PP may affect mechanical power components differently due to changes in respiratory mechanics; however, the effects of PP on lung mechanical power components are unclear. This study aimed to compare the following parameters during supine positioning (SP) and PP: lung total elastic power and its components (elastic static power and elastic dynamic power) and these variables normalized to end-expiratory lung volume (EELV).
Methods
This prospective physiologic study included 55 patients with moderate to severe ARDS. Lung total elastic power and its static and dynamic components were compared during SP and PP using an esophageal pressure-guided ventilation strategy. In SP, the esophageal pressure-guided ventilation strategy was further compared with an oxygenation-guided ventilation strategy defined as baseline SP. The primary endpoint was the effect of PP on lung total elastic power non-normalized and normalized to EELV. Secondary endpoints were the effects of PP and ventilation strategies on lung elastic static and dynamic power components non-normalized and normalized to EELV, respiratory mechanics, gas exchange, and hemodynamic parameters.
Results
Lung total elastic power (median [interquartile range]) was lower during PP compared with SP (6.7 [4.9–10.6] versus 11.0 [6.6–14.8] J/min; P < 0.001) non-normalized and normalized to EELV (3.2 [2.1–5.0] versus 5.3 [3.3–7.5] J/min/L; P < 0.001). Comparing PP with SP, transpulmonary pressures and EELV did not significantly differ despite lower positive end-expiratory pressure and plateau airway pressure, thereby reducing non-normalized and normalized lung elastic static power in PP. PP improved gas exchange, cardiac output, and increased oxygen delivery compared with SP.
Conclusions
In patients with moderate to severe ARDS, PP reduced lung total elastic and elastic static power compared with SP regardless of EELV normalization because comparable transpulmonary pressures and EELV were achieved at lower airway pressures. This resulted in improved gas exchange, hemodynamics, and oxygen delivery.
Trial registration: German Clinical Trials Register (DRKS00017449). Registered June 27, 2019. https://drks.de/search/en/trial/DRKS00017449
Similar content being viewed by others
Background
The improved survival associated with prone positioning (PP) in patients with moderate to severe acute respiratory distress syndrome (ARDS) in the PROSEVA trial [1] has been attributed to a reduction in overdistension and cyclical airway opening and closing [2,3,4]. Combining PP and protective ventilation with positive end-expiratory pressure (PEEP) to improve ventilation distribution [5] may therefore limit ventilator-induced lung injury (VILI) [2, 3, 6]. PP reduces the pleural pressure gradient and decreases the dependence on PEEP to homogenize lung ventilation [7] by increasing transpulmonary pressures (PTP) and end-expiratory lung volume (EELV) [8]. This may result in reduced mechanical power (MP), defined as the mechanical energy delivered by the ventilator to the respiratory system [9, 10], and MP components normalized to EELV [11]. MP integrates static (respiratory system peak, plateau, and driving pressures [ΔPRS], PEEP, and tidal volume) and dynamic (airflow amplitude, inspiratory time fraction, and respiratory rate) components and has been considered a unifying driver of VILI [9, 10].
PP and supine positioning (SP) may affect static and dynamic MP components differently due to changes in pleural pressures and lung and chest wall mechanics [7, 12,13,14]; however, to date, no study has evaluated whether these changes have an impact on the transmission of lung MP components and their normalization to EELV, which reflects the energy transfer per aerated lung volume.
To clarify this issue, the current study compared the following parameters during PP and SP: lung total elastic power and its components (elastic static power and elastic dynamic power) and these variables normalized to EELV when using an esophageal pressure (Peso)-guided ventilation strategy [5]. We hypothesized that PP combined with protective ventilation reduces lung total elastic power and its static and dynamic components, as well as the energy transfer per aerated lung volume in patients with moderate to severe ARDS. The primary endpoint was the effect of PP on lung total elastic power non-normalized and normalized to EELV. Secondary endpoints were the effects of PP and different ventilation strategies in SP on elastic static and dynamic power components non-normalized and normalized to EELV, respiratory mechanics, EELV, gas exchange, and hemodynamic parameters.
Methods
This prospective, physiologic study was conducted as part of a multipurpose study [8], with independent research questions and study protocol, approved by the local ethical committee (Medizinische Ethikkomission II, University Medical Center Mannheim, Medical Faculty Mannheim of the University of Heidelberg, Mannheim, Germany; registration number 2018-609N-MA-Amend3) and after study registration at the German Clinical Trials Register (DRKS00017449, https://drks.de/search/en/trial/DRKS00017449).
Patients
Consecutive patients with moderate to severe ARDS (defined by the ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen [PaO2/FiO2] ≤ 150 mmHg according to the Berlin definition [15]) were enrolled between July 2019 and June 2023. Informed consent was obtained from the legal representative of each patient before enrollment. Exclusion criteria were age < 18 years, pregnancy, end-stage chronic organ failure, inherited cardiac malformations, severe head injury, and severe hemodynamic instability.
Clinical management
All patients were sedated with sufentanil (20–30 μg/h) and midazolam (10–20 mg/h) to achieve a score of − 5 on the Richmond Agitation-Sedation Scale, and complete neuromuscular blockade was maintained throughout the study period with cisatracurium. An esophageal balloon catheter (NutriVent nasogastric catheter; Sidam, Mirandola, Italy) was advanced into the stomach, inflated, and withdrawn into the esophagus until the appearance of cardiac artifacts on the pressure tracing [16]. The balloon was inflated with the lowest volume to obtain the largest swings in Peso during tidal ventilation. Peso measurements were considered reliable if the ratio of change in Peso to change in airway pressure was 0.8–1.2 during an end-expiratory occlusion test [17, 18].
A thermodilution catheter (5F Pulsiocath, Pulsion Medical Systems, Munich, Germany) was inserted via the femoral artery in all patients included in the study to allow hemodynamic measurements with a pulse contour cardiac output monitor (PiCCOplus; Pulsion Medical Systems, Munich, Germany). Norepinephrine was administered if the mean arterial pressure (MAP) was < 65 mmHg despite preload optimization. Dobutamine was administered if the cardiac index measured by transpulmonary thermodilution was < 2.0 L/min/m2 despite sufficient cardiac pre- and afterload.
Study protocol
Patients were passively ventilated in a horizontal position with 0° body inclination throughout the study period with an Engström Carescape R860 ventilator in a volume-controlled ventilation mode using a tidal volume (VT) of 6 mL/kg of predicted body weight and a respiratory rate (RR) and fraction of inspired oxygen (FiO2) according to recent guidelines [19]. VT and RR were modified only if ΔPRS was greater than 14 cmH2O and pHa was < 7.25.
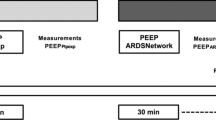
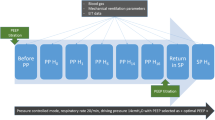
The study protocol included three steps (Fig. 1A): (1) baseline measurement in SP using a ventilation strategy with PEEP based on the lower PEEP/FiO2 table (baseline) [19, 20]; (2) Peso-guided ventilation strategy with PEEP targeting an end-expiratory PTP of 0 to 2 cmH2O in SP [5]; (3) Peso-guided ventilation strategy with PEEP targeting an end-expiratory PTP of 0 to 2 cmH2O in PP. To standardize lung volume history and allow for comparisons between ventilation strategies and positioning, a dynamic recruitment maneuver was performed before each ventilation strategy, as detailed in Additional file 1: Fig. S1. Therefore, a recruitment maneuver was performed before baseline ventilation with PEEP based on the lower PEEP/FiO2 table, esophageal pressure-guided PEEP in SP, and esophageal pressure-guided PEEP in PP [21].
A Schematic workflow of the study. B Mechanical power components during volume-controlled ventilation with constant inspiratory flow. Area A (white) describes the resistive power component. Areas B and C describe the elastic dynamic and elastic static power components, respectively; together they are the total elastic power (teal). PpeakRS, airway peak pressure; PplatRS, airway plateau pressure
In SP, patients were ventilated in a volume-controlled mode after the recruitment maneuver starting with a PEEP of 25 cmH2O. PEEP was decreased stepwise by 2 cmH2O every 2 min, and end-expiratory Peso was measured during a 2-s expiratory hold. The lowest PEEP to achieve an end-expiratory PTP of 0 to 2 cmH2O was used for the Peso-guided ventilation strategy (Additional file 1: Fig. S1). After completing the measurements for the Peso-guided ventilation strategy in SP, patients were placed in PP with unchanged ventilator settings. A recruitment maneuver was then performed, and PEEP was titrated to end-expiratory Peso targeting an end-expiratory PTP of 0 to 2 cmH2O. Physiologic measurements were obtained after a 30-min equilibration period with each ventilation strategy.
Measurements
Expiratory and inspiratory airway pressures and Peso were measured during a 2-s inspiratory and 2-s expiratory hold at zero flow to compute end-inspiratory and end-expiratory PTP (airway pressure − Peso), respectively. ΔPRS was calculated as airway plateau pressure (PplatRS) − PEEP, and transpulmonary driving pressure (ΔPTP) as end-inspiratory PTP − end-expiratory PTP. Static respiratory system and lung elastance were calculated as ΔPRS/VT and ΔPTP/VT, respectively. Chest wall elastance was calculated as end-inspiratory − end-expiratory Peso/VT. The elastance ratio of the lung to the respiratory system (EL/ERS) was used to calculate lung MP components.
Lung MP was calculated as
Lung total elastic power was calculated as [22, 23]
The elastic static (related to PEEP) and dynamic (related to ΔPRS) components of lung elastic power (Fig. 1B) were calculated as
and
, respectively [23]. Resistive power (related to the resistance of the airway) [23] was calculated as
Lung total elastic power and elastic static and dynamic power components were normalized to EELV to describe the energy transfer relative to the aerated lung volume (Additional file 1) [10, 11]. Lung stress was calculated using the elastance-derived method as PplatRS × (EL/ERS) [18]. EELV was measured with a modified nitrogen wash-out (20% FiO2 increase)/wash-in (20% FiO2 decrease) technique [24, 25]. At the end of the equilibration period, the alveolar dead space fraction was calculated, and arterial blood gas was analyzed. Hemodynamic parameters were obtained using transpulmonary thermodilution, and oxygen delivery was calculated. Vascular pressure transducers were zeroed to ambient pressure after changes in positioning and aligned with the phlebostatic axis corresponding to the right atrium and aortic root. For this purpose, pressure transducers were placed in the midaxillary line of the 4th intercostal space in both SP and PP to obtain comparable measurements of hemodynamic variables.
Statistical analysis
The effect of PP on total elastic power transmitted to the lung has not been studied in patients with ARDS. Based on the results of the previous study by our group [8], an estimated sample size of 55 patients was required to measure a change in lung total elastic power (primary endpoint of the study) between SP and PP with a power of 0.8 and a significance level of 0.05. The effect size was calculated based on the change in lung total elastic power when using a Peso-guided ventilation strategy during SP and PP. Fifty-five patients underwent the current study protocol, which was part of a multipurpose study. Of these, forty patients had also been enrolled in a prior study [8], but with a different research question and study protocol. The normality of the data was tested by the Shapiro–Wilk test and subsequently analyzed using the Wilcoxon matched-pairs test for non-normally distributed data. The results are expressed as medians (interquartile range) with a level of significance set at P < 0.05. Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA).
Results
Fifty-five patients with moderate to severe ARDS (PaO2/FiO2 ≤ 150 mmHg) were included in the analysis. Demographic and clinical characteristics of the patients are shown in Table 1. During the study period, 48 patients required only norepinephrine, whereas one patient also required dobutamine.
Effects of prone positioning
PP resulted in lower PEEP and PplatRS compared with SP, but end-inspiratory and end-expiratory PTP as well as EELV did not differ by positioning (Table 2).
Lung total elastic power was lower during PP compared with SP (6.7 [4.9–10.6] versus 11.0 [6.6–14.8] J/min; P < 0.001) (Fig. 2A). PP also resulted in lower lung total elastic power normalized to EELV compared with SP (3.2 [2.1–5.0] versus 5.3 [3.3–7.5] J/min/L; P < 0.001) (Fig. 2B).
Effects of supine and prone positioning on A lung total elastic power and B lung total elastic power normalized to end-expiratory lung volume (EELV). A ventilation strategy with positive end-expiratory pressure (PEEP) based on the PEEP/FiO2 table during supine positioning was used as the baseline. A ventilation strategy with esophageal pressure-guided PEEP was used for the comparison between supine and prone positioning. Boxplots show the interquartile range and median with whiskers according to Tukey's method. Outliers are shown as circles. Brackets denote statistically significant differences between positioning and ventilation strategies; P values are shown above the brackets
PP reduced lung elastic static power compared with SP (4.2 [3.2–7.4] versus 8.5 [4.8–10.7] J/min; P < 0.001) (Fig. 3A) and lung elastic static power normalized to EELV (2.1 [1.4–3.8] versus 4.2 [2.7–6.0] J/min/L; P < 0.001) (Fig. 3B). PP did not affect lung elastic dynamic power non-normalized and normalized to EELV (Fig. 4A, B). Compared with SP, PP reduced EL/ERS and lung stress and increased chest wall elastance when using Peso-guided ventilation (Table 2).
Effects of supine and prone positioning on A lung elastic static power and B lung elastic static power normalized to end-expiratory lung volume (EELV). A ventilation strategy with positive end-expiratory pressure (PEEP) based on the PEEP/FiO2 table during supine positioning was used as the baseline. A ventilation strategy with esophageal pressure-guided PEEP was used for the comparison between supine and prone positioning. Boxplots show the interquartile range and median with whiskers according to Tukey's method. Outliers are shown as circles. Brackets denote statistically significant differences between positioning and ventilation strategies; P values are shown above the brackets
Effects of supine and prone positioning on A lung elastic dynamic power and B lung elastic dynamic power normalized to end-expiratory lung volume (EELV). A ventilation strategy with positive end-expiratory pressure (PEEP) based on the PEEP/FiO2 table during supine positioning was used as the baseline. A ventilation strategy with esophageal pressure-guided PEEP was used for the comparison between supine and prone positioning. Boxplots show the interquartile range and median with whiskers according to Tukey's method. Outliers are shown as circles. Brackets denote statistically significant differences between positioning and ventilation strategies; P values are shown above the brackets
PaO2/FiO2 increased and the shunt fraction and the alveolar dead space fraction decreased during PP compared with SP with Peso-guided ventilation (Table 3). PP resulted in higher MAP and cardiac output and increased oxygen delivery compared with SP. Further details regarding the effect of PP on respiratory parameters, gas exchange, and hemodynamic parameters are shown in Tables 2, 3, and Additional file 1: Tables S2 and S3.
Effects of ventilation strategies during supine positioning
Peso-guided ventilation in SP resulted in higher PEEP and PplatRS compared with an oxygenation-guided ventilation strategy using the PEEP/FiO2 table (baseline). This increased end-inspiratory and end-expiratory PTP as well as EELV (Table 2).
Peso-guided ventilation during SP compared with baseline increased lung total elastic power (11.0 [6.6–14.8] versus 8.1 [5.4–11.0] J/min; P < 0.001) (Fig. 2A), but lung total elastic power normalized to EELV did not differ according to the ventilation strategy during SP (5.3 [3.3–7.5] versus 5.1 [3.6–6.9] J/min/L; P = 0.538) (Fig. 2B). Lung elastic static power was higher with Peso-guided ventilation during SP compared with baseline (8.5 [4.8–10.7] versus 5.5 [3.1–7.3] J/min; P < 0.001) (Fig. 3A). Similarly, Peso-guided ventilation increased lung elastic static power normalized to EELV compared with baseline (4.2 [2.7–6.0] versus 3.3 [2.3–5.5] J/min/L; P = 0.006) (Fig. 3B). Peso-guided ventilation during SP compared with baseline decreased ΔPTP, lung elastic dynamic power (2.5 [1.8–3.5] versus 2.9 [1.9–3.7] J/min; P = 0.029) (Fig. 4A) and lung elastic dynamic power normalized to EELV (1.2 [0.7–1.9] versus 1.7 [1.3–2.7] J/min/L; P < 0.001) (Fig. 4B), but lung stress was higher (Table 2).
PaO2/FiO2 increased and the shunt fraction decreased with Peso-guided ventilation in SP compared with baseline (Table 3). Peso-guided ventilation resulted in lower MAP and cardiac output during SP compared with baseline but did not affect oxygen delivery (Table 3).
Discussion
In this prospective, physiologic study on the effect of PP combined with protective ventilation on lung total elastic power in fifty-five patients with moderate to severe ARDS, we found that: (A) PP reduced lung total elastic power and lung total elastic power normalized to EELV compared with SP; (B) lung elastic static power and lung elastic static power normalized to EELV were lower in PP compared with SP using Peso-guided ventilation because comparable PTP and EELV were achieved at lower airway pressures; (C) PP did not reduce lung elastic dynamic power with Peso-guided ventilation compared with SP regardless of EELV normalization; (D) PP improved gas exchange and hemodynamics, thus mitigating the adverse effects of higher airway pressures associated with Peso-guided ventilation while optimizing oxygen delivery.
To the best of our knowledge, this is the first study prospectively investigating the physiologic effects of PP on lung-transmitted static and dynamic MP components (excluding MP transmission to the chest wall). Normalization of static and dynamic MP components to EELV, which surrogates energy transfer per aerated lung volume, may further enhance the relevance of the present data.
Effects of prone positioning
Total MP, which combines static and dynamic parameters of ventilation, has been investigated to quantify the invasiveness of ventilation and may be related to the risk of VILI [26, 27]. Thus, the present study compared elastic power and its components (static and dynamic), to estimate lung stress and strain, according to different positioning and ventilation strategies [22].
However, the role of MP, its static and dynamic components, and the relevance in comparison with simpler bedside indices, e.g., 4 × ΔPRS + RR, or any other predictor of VILI remains unclear [23, 28]. Despite the debate about the importance of each MP component, high MP per se, which combines different ventilator variables, increases the risk of VILI in patients with ARDS. Moreover, reducing only one variable may be insufficient to significantly modify MP [26]. MP, compared to single-ventilator variables or simpler indices, may thus provide a holistic picture on the invasiveness of different ventilation strategies and the effect of positioning. Normalizing MP transfer to the size of the aerated lung may be the essential step for the clinical use of MP and definition of safety thresholds [11, 27]. This has been shown to correlate power transfer with lung stress and strain [29] and further improve the prediction of mortality in patients with ARDS [30]. However, estimating energy transfer per aerated lung volume in clinical practice may be hindered by the requirement to measure EELV. Although normalization to body weight or compliance has been suggested, the optimal method remains unclear [27].
Another method to measure global lung stress is to isolate the fraction of airway pressure applied to the lung (PTP) using Peso measurements [5]. This can provide relevant information regarding the invasiveness of ventilation in situations with altered chest wall mechanics such as PP [8, 14, 31]. In this situation, Peso-guided ventilation with PEEP titrated to maintain a positive end-expiratory PTP may be clinically useful to balance lung recruitment and overdistension in patients with ARDS [32].
Thus, utilizing the Peso measurement to quantify lung MP and normalizing to EELV may add important information regarding the invasiveness of ventilation in patients with ARDS managed with PP, because PP modifies chest wall elastance and increases EELV and lung homogeneity [27, 33]. We modified PEEP in PP to account for the reduced vertical pleural pressures and the accompanying regional changes in lung mechanics in each individual patient [7, 12,13,14, 34] and to avoid the influence of inadequate (excessive or insufficient) PEEP on total elastic power transmitted to the lung [6]. In our study, VT and RR were comparable between ventilation strategies and positioning; thus, changes in lung total elastic power and its components were due to changes in respiratory mechanics addressed by individualized ventilation strategies.
A recent study by Morais et al. evaluated respiratory mechanics in SP and PP over a range of PEEP levels in patients with ARDS and found a variety of responses in global and regional mechanics induced by PP, suggesting the need to individualize PEEP according to the positioning [35]. On the contrary, a study by Mezidi et al. found no significant differences in PEEP titrated according to end-expiratory PTP when patients were turned from SP to PP [36]. Of note and in contrast to our study with 0° body inclination for both SP and PP, the study compared SP with 30° to PP with 0° to 15° body inclination [36]. Body inclination has been shown to affect respiratory mechanics and EELV in mechanically ventilated patients with ARDS due to changes in chest wall elastance and PTP [37, 38].
The effect of PEEP in patients with ARDS is critically dependent on lung recruitability [39]; however, the large vertical pleural pressure gradient present in supine patients with ARDS [17] may not allow for significant recruitment without concomitant overdistension due to differences in regional PTP [40]. In our study, PP resulted in a significant reduction of lung total elastic power and lung total elastic power normalized to EELV compared with SP. Although the role of static and dynamic MP components in the pathogenesis of VILI is debated [23, 28], excessive MP, regardless of the constituents, causes similar lung injury [26, 27]. PP decreases pleural pressure gradients and homogenizes ventilation [7], reducing the risk of VILI [4, 41] by limiting regional lung strain due to overdistension and tidal recruitment [3].
In moderate to severe ARDS patients with recruitable lung parenchyma, PP increases end-expiratory PTP and EELV [8, 39]. As demonstrated in the present physiologic study, PP may therefore be a part of a lung-protective ventilation strategy aimed at reducing lung total elastic power transmission per aerated lung volume. This may reduce damaging ventilation above the proposed parenchymal stress threshold by decreasing lung strain while increasing the size of the aerated lung [10, 42]. Our results expand the mechanistic understanding of the effects of PP to improve lung protection by reducing energy transfer per aerated lung volume, which has been discussed as a major factor for improved survival in the PROSEVA trial [2,3,4].
On the other hand, in our study, PP did not reduce lung elastic dynamic power non-normalized and normalized to EELV with Peso-guided ventilation. The dynamic component of lung MP is exponentially affected by VT [9] and may lead to increased inspiratory lung strain, as indicated by the resulting ΔPTP [43]. High MP due to excessive VT causing damaging lung stress and strain is clinically indicated by altered respiratory mechanics with sharply increased PplatRS and ΔPTP when EELV is kept constant [26]. Although the decrease in ΔPTP and lung elastance with comparable EELV during PP compared to SP was non-significant, this trend may signify the opening of new lung units and/or improved mechanical properties of previously ventilated lung units [44]. Consequently, VT is evenly distributed between dependent and non-dependent lung regions during PP and overdistension is limited [7], as reflected by reduced end-inspiratory lung stress and total elastic power normalized to EELV. Prolonged periods of PP may further reduce total elastic power transfer per aerated lung volume, as EELV has been shown to increase over time in PP [36].
The key message of our study for clinical practice is that PP allows for a reduction in lung elastic power transmission per aerated lung volume because comparable PTP and EELV can be achieved at lower airway pressures. This may have important implications for the ventilator management during PP.
Effects of ventilation strategies during supine positioning
In our study, compared with baseline, Peso-guided ventilation in SP resulted in higher PEEP, PplatRS, and EELV, thereby improving PaO2/FiO2 due to a reduced pulmonary shunt. However, this ventilation strategy increased lung total elastic power and lung stress. This is consistent with the results of the EPVent-2 trial, where Peso-guided ventilation resulted in PEEP levels similar to ours and did not reduce ΔPTP compared with a ventilation strategy using the higher PEEP/FiO2 table [45]. Although there was no significant difference in lung total elastic power normalized to EELV when using Peso-guided ventilation in SP compared with baseline, higher PEEP and PplatRS may have resulted in overdistension of non-dependent lung regions, despite improving dependent lung aeration by maintaining positive end-expiratory PTP and increasing EELV [18]. Our study demonstrates that PP compared to SP can offset the need for higher airway pressures to maintain positive end-expiratory PTP and reduce pulmonary shunt. This results in a reduction in total elastic and elastic static power transmission per aerated lung volume compared to SP.
Theoretically, the overall effect of a reduction in end-expiratory PTP on lung MP is less pronounced than the effect of changes in VT, ΔPRS, and inspiratory airflow [9]. This suggests that reducing elastic static power may have a minor impact on lung protection; however, a U-shaped relationship between end-expiratory PTP and the risk of VILI has been discussed, with both insufficient and excessive end-expiratory PTP causing VILI due to atelectrauma and overdistension, respectively [22, 26, 32, 46]. As shown experimentally, the application of inadequate lung elastic static power can impair lung structural architecture and elastance, and increase extravascular lung water and inflammation [26, 46]. Furthermore, the combination of elastic static and dynamic power, but not elastic static or dynamic power alone, correlated with alveolar collapse and regional overdistension as hallmarks of VILI [22]. This highlights the importance of not reaching a critical lung stress and strain threshold [10].
In SP, Peso-guided ventilation with higher PEEP and PplatRS resulted in a reduction of cardiac output in comparison with baseline. The results of our study are consistent with the findings in an animal model, where higher elastic static power caused severe hemodynamic impairment [26], which could be translated to the clinical setting. Adverse hemodynamic effects of mechanical ventilation are common in patients with ARDS [47], but may be limited by PP [48]. PP with Peso-guided ventilation restored cardiac output and increased MAP and oxygen delivery in comparison with SP. Possible mechanisms for this effect of PP in our study may include an increased gradient for venous return by increasing intra-abdominal pressure and reduced lung overdistension with decreased pulmonary vascular resistance and right ventricular afterload, thereby improving right ventricular function [47,48,49].
Clinical Implications
PP improves survival in patients with moderate to severe ARDS, possibly by improving lung protection and reducing VILI [2,3,4]. This might be due to a reduction in the pleural pressure gradient with changes in PTP and EELV that affect the transmission of static and dynamic MP components. The short-term physiologic data from our study suggest that PP may allow for a reduction in lung elastic power without a loss of EELV, thereby minimizing energy transfer per aerated lung volume and improving gas exchange, hemodynamics, and oxygen delivery in patients with moderate to severe ARDS. Furthermore, quantifying lung MP and normalizing it to EELV may be an essential step to describe the energy transfer relative to the aerated lung volume at the bedside to better monitor mechanically ventilated patients with ARDS.
Limitations
Our study has several limitations. We studied the short-term physiologic effects of PP using Peso-guided ventilation with similar per protocol VT and RR; thus, we cannot exclude that the effects of PP on lung MP components differ according to ventilation strategy. In line with the mechanistic understanding of VILI, maintaining a positive end-expiratory PTP has been associated with lower mortality in a post hoc re-analysis of the EPVent-2 trial [5]; however, the optimal ventilation strategy during PP is unclear.
Individual lung recruitability was not assessed before the study, and the effect of PEEP on lung total elastic power, lung elastic static, and dynamic power components, as well as respiratory and hemodynamic parameters, may depend on recruitability [50]. During protective ventilation, a U-shaped relationship between PEEP and VILI has been suggested by experimental studies [22, 26, 46], but the best method to individualize PEEP is unknown [19].
Our study focused on lung total elastic power including its elastic static and dynamic components to approximate lung stress and strain [22]. We excluded the resistive component of MP [9] because the biological impact of this component in comparison with the elastic power components is unclear [10, 11, 51]. Additionally, estimating energy transfer per aerated lung volume by normalizing MP in clinical practice may be hindered by the requirement to measure EELV, and the optimal method to normalize MP is unclear [27].
Another limitation is the lack of an imaging technology, e.g., electrical impedance tomography, to quantify regional lung aeration, as the physiologic effects of PP may be heterogeneous [35]. Although lung total elastic power, power components normalized to EELV, and lung stress were minimized, we cannot formally exclude regional hyperinflation in PP.
Conclusions
In patients with moderate to severe ARDS, compared with SP, PP reduced lung total elastic and elastic static power, minimizing total elastic and elastic static power transmission per aerated lung volume, because comparable values for PTP and EELV were achieved at lower airway pressures. This resulted in improved gas exchange, hemodynamics, and oxygen delivery. Due to the changes in chest wall mechanics and lung aeration during pronation, quantifying lung MP and normalizing it to EELV may be an essential step to describe the energy transfer relative to the aerated lung volume and further understand the lung-protective effect of PP.
Availability of data and materials
The datasets analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- ΔP RS :
-
Respiratory system driving pressure
- ΔP TP :
-
Transpulmonary driving pressure
- ARDS:
-
Acute respiratory distress syndrome
- EELV:
-
End-expiratory lung volume
- E L/E RS :
-
Elastance ratio of the lung to the respiratory system
- FiO2 :
-
Fraction of inspired oxygen
- ICU:
-
Intensive care unit
- MAP:
-
Mean arterial pressure
- MP:
-
Mechanical power
- PaO2/FiO2 :
-
Ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen
- PEEP:
-
Positive end-expiratory pressure
- P eso :
-
Esophageal pressure
- pHa:
-
Negative logarithm of the molar concentration of dissolved hydronium ions in arterial blood
- P peakR S :
-
Airway peak pressure
- P platRS :
-
Airway plateau pressure
- PP:
-
Prone positioning
- P TP :
-
Transpulmonary pressure
- RR:
-
Respiratory rate
- SP:
-
Supine positioning
- SAPS II:
-
Simplified Acute Physiology Score
- SOFA:
-
Sequential Organ Failure Assessment
- VILI:
-
Ventilator-induced lung injury
- V T :
-
Tidal volume
References
Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–68.
Guerin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, Munshi L, Papazian L, Pesenti A, Vieillard-Baron A, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46(12):2385–96.
Cornejo RA, Diaz JC, Tobar EA, Bruhn AR, Ramos CA, Gonzalez RA, Repetto CA, Romero CM, Galvez LR, Llanos O, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188(4):440–8.
Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med. 2000;28(2):295–303.
Sarge T, Baedorf-Kassis E, Banner-Goodspeed V, Novack V, Loring SH, Gong MN, Cook D, Talmor D, Beitler JR. EPVent-2 study group: effect of esophageal pressure-guided positive end-expiratory pressure on survival from acute respiratory distress syndrome: a risk-based and mechanistic reanalysis of the EPVent-2 trial. Am J Respir Crit Care Med. 2021;204(10):1153–63.
Beitler JR, Guerin C, Ayzac L, Mancebo J, Bates DM, Malhotra A, Talmor D. PEEP titration during prone positioning for acute respiratory distress syndrome. Crit Care (London, England). 2015;19:436.
Katira BH, Osada K, Engelberts D, Bastia L, Damiani LF, Li X, Chan H, Yoshida T, Amato MBP, Ferguson ND, et al. Positive end-expiratory pressure, pleural pressure, and regional compliance during pronation: an experimental study. Am J Respir Crit Care Med. 2021;203(10):1266–74.
Boesing C, Graf PT, Schmitt F, Thiel M, Pelosi P, Rocco PRM, Luecke T, Krebs J. Effects of different positive end-expiratory pressure titration strategies during prone positioning in patients with acute respiratory distress syndrome: a prospective interventional study. Crit Care (London, England). 2022;26(1):82.
Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42(10):1567–75.
Marini JJ, Rocco PRM, Gattinoni L. Static and dynamic contributors to ventilator-induced lung injury in clinical practice. Pressure, energy, and power. Am J Respir Crit Care Med. 2020, 201(7):767–774.
Silva PL, Ball L, Rocco PRM, Pelosi P. Power to mechanical power to minimize ventilator-induced lung injury? Intensive Care Med Exp. 2019;7(Suppl 1):38.
Scaramuzzo G, Ball L, Pino F, Ricci L, Larsson A, Guerin C, Pelosi P, Perchiazzi G. Influence of positive end-expiratory pressure titration on the effects of pronation in acute respiratory distress syndrome: a comprehensive experimental study. Front Physiol. 2020;11:179.
Kumaresan A, Gerber R, Mueller A, Loring SH, Talmor D. Effects of prone positioning on transpulmonary pressures and end-expiratory volumes in patients without lung disease. Anesthesiology. 2018;128(6):1187–92.
Riad Z, Mezidi M, Subtil F, Louis B, Guerin C. Short-term effects of the prone positioning maneuver on lung and chest wall mechanics in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197(10):1355–8.
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–82.
Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42(9):1360–73.
Grieco DL, Chen L, Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med. 2017;5(14):285.
Yoshida T, Amato MBP, Grieco DL, Chen L, Lima CAS, Roldan R, Morais CCA, Gomes S, Costa ELV, Cardoso PFG, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med. 2018;197(8):1018–26.
Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, Arabi YM, Baroncelli F, Beitler JR, Bellani G, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med 2023.
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. National Heart Lung Blood Institute, ARDS Clinical Trials Network: higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–36.
Nishida T, Suchodolski K, Schettino GP, Sedeek K, Takeuch M, Kacmarek RM. Peak volume history and peak pressure-volume curve pressures independently affect the shape of the pressure-volume curve of the respiratory system. Crit Care Med. 2004;32(6):1358–64.
Rocco PRM, Silva PL, Samary CS, Hayat Syed MK, Marini JJ. Elastic power but not driving power is the key promoter of ventilator-induced lung injury in experimental acute respiratory distress syndrome. Crit Care (London, England). 2020;24(1):284.
Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, Mercat A, Meade M, Morais CCA, Goligher E, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204(3):303–11.
Chiumello D, Cressoni M, Chierichetti M, Tallarini F, Botticelli M, Berto V, Mietto C, Gattinoni L. Nitrogen washout/washin, helium dilution and computed tomography in the assessment of end expiratory lung volume. Crit Care (London, England). 2008;12(6):R150.
Olegard C, Sondergaard S, Houltz E, Lundin S, Stenqvist O. Estimation of functional residual capacity at the bedside using standard monitoring equipment: a modified nitrogen washout/washin technique requiring a small change of the inspired oxygen fraction. Anesth Analg. 2005;101(1):206–12.
Vassalli F, Pasticci I, Romitti F, Duscio E, Assmann DJ, Grunhagen H, Vasques F, Bonifazi M, Busana M, Macri MM, et al. Does iso-mechanical power lead to iso-lung damage?: An experimental study in a porcine model. Anesthesiology. 2020;132(5):1126–37.
Gattinoni L, Collino F, Camporota L. Mechanical power: meaning, uses and limitations. Intensive Care Med. 2023;49(4):465–7.
Tonna JE, Peltan I, Brown SM, Herrick JS, Keenan HT. University of Utah Mechanical Power Study Group: mechanical power and driving pressure as predictors of mortality among patients with ARDS. Intensive Care Med. 2020;46(10):1941–3.
Pistillo N, Castelluccio P, Suzuki I, Castiblanco L. Mechanical power correlates with stress, strain, and atelectrauma only when normalized to aerated lung size in patients with acute respiratory distress syndrome. Crit Care Explor. 2023;5(10):e0982.
Coppola S, Caccioppola A, Froio S, Formenti P, De Giorgis V, Galanti V, Consonni D, Chiumello D. Effect of mechanical power on intensive care mortality in ARDS patients. Crit Care (London, England). 2020;24(1):246.
Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, Gattinoni L, Laffey JG, Marini JJ, Myatra SN, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care (London, England). 2021;25(1):250.
Madahar P, Talmor D, Beitler JR. Transpulmonary pressure-guided ventilation to attenuate atelectrauma and hyperinflation in acute lung injury. Am J Respir Crit Care Med. 2021;203(8):934–7.
Schaefer MS, Loring SH, Talmor D, Baedorf-Kassis EN. Comparison of mechanical power estimations in mechanically ventilated patients with ARDS: a secondary data analysis from the EPVent study. Intensive Care Med. 2021;47(1):130–2.
Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med. 2000;161(5):1660–5.
Morais CCA, Alcala G, De Santis Santiago RR, Valsecchi C, Diaz E, Wanderley H, Fakhr BS, Di Fenza R, Gianni S, Foote S, et al. Pronation reveals a heterogeneous response of global and regional respiratory mechanics in patients with acute hypoxemic respiratory failure. Crit Care Explor. 2023;5(10):e0983.
Mezidi M, Parrilla FJ, Yonis H, Riad Z, Bohm SH, Waldmann AD, Richard JC, Lissonde F, Tapponnier R, Baboi L, et al. Effects of positive end-expiratory pressure strategy in supine and prone position on lung and chest wall mechanics in acute respiratory distress syndrome. Ann Intensive Care. 2018;8(1):86.
Mezidi M, Guerin C. Effect of body position and inclination in supine and prone position on respiratory mechanics in acute respiratory distress syndrome. Intensive Care Med. 2019;45(2):292–4.
Marrazzo F, Spina S, Forlini C, Guarnieri M, Giudici R, Bassi G, Bastia L, Bottiroli M, Fumagalli R, Langer T. Effects of trunk inclination on respiratory mechanics in patients with COVID-19-associated acute respiratory distress syndrome: let’s always report the angle! Am J Respir Crit Care Med. 2022;205(5):582–4.
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775–86.
Cressoni M, Chiumello D, Algieri I, Brioni M, Chiurazzi C, Colombo A, Colombo A, Crimella F, Guanziroli M, Tomic I, et al. Opening pressures and atelectrauma in acute respiratory distress syndrome. Intensive Care Med. 2017;43(5):603–11.
Valenza F, Guglielmi M, Maffioletti M, Tedesco C, Maccagni P, Fossali T, Aletti G, Porro GA, Irace M, Carlesso E, et al. Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med. 2005;33(2):361–7.
Marini JJ, Thornton LT, Rocco PRM, Gattinoni L, Crooke PS. Practical assessment of risk of VILI from ventilating power: a conceptual model. Crit Care (London, England). 2023;27(1):157.
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55.
Chiumello D, Marino A, Brioni M, Cigada I, Menga F, Colombo A, Crimella F, Algieri I, Cressoni M, Carlesso E, et al. Lung recruitment assessed by respiratory mechanics and computed tomography in patients with acute respiratory distress syndrome. What is the relationship? Am J Respir Crit Care Med. 2016;193(11):1254–63.
Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D. EPVent-2 study group: effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321(9):846–57.
Collino F, Rapetti F, Vasques F, Maiolo G, Tonetti T, Romitti F, Niewenhuys J, Behnemann T, Camporota L, Hahn G, et al. Positive end-expiratory pressure and mechanical power. Anesthesiology. 2019;130(1):119–30.
Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S, Marini JJ. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med. 2016;42(5):739–49.
Lai C, Monnet X, Teboul JL. Hemodynamic implications of prone positioning in patients with ARDS. Crit Care (London, England). 2023;27(1):98.
Vieillard-Baron A, Naeije R, Haddad F, Bogaard HJ, Bull TM, Fletcher N, Lahm T, Magder S, Orde S, Schmidt G, et al. Diagnostic workup, etiologies and management of acute right ventricle failure: a state-of-the-art paper. Intensive Care Med. 2018;44(6):774–90.
Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, Sklar MC, Rauseo M, Ferguson ND, Fan E, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome: a clinical trial. Am J Respir Crit Care Med 2020, 201(2):178–87.
Huhle R, Serpa Neto A, Schultz MJ. Gama de Abreu M: Is mechanical power the final word on ventilator-induced lung injury?-no. Ann Transl Med. 2018;6(19):394.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by departmental funds.
Author information
Authors and Affiliations
Contributions
CB, JK, PRMR, and TL participated in the study design. CB and JK performed the study. CB and LS processed the data and performed the statistical analysis. CB, JK, AMC, MO, GB, MT, PRMR, TL, and LS wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This prospective, physiologic study was conducted as part of a multipurpose study [8], with independent research questions and study protocol, approved by the local ethical committee (Medizinische Ethikkomission II, University Medical Center Mannheim, Medical Faculty Mannheim of the University of Heidelberg, Mannheim; registration number 2018-609N-MA-Amend3) and after study registration at the German Clinical Trials Register (DRKS00017449, https://drks.de/search/en/trial/DRKS00017449). Informed consent was obtained from the legal representative of each patient before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Study details and equations for physiologic variables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Boesing, C., Krebs, J., Conrad, A.M. et al. Effects of prone positioning on lung mechanical power components in patients with acute respiratory distress syndrome: a physiologic study. Crit Care 28, 82 (2024). https://doi.org/10.1186/s13054-024-04867-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-024-04867-6