Abstract
Background
Current practice guidelines for optimal infusion rates during early intravenous hydration in patients with acute pancreatitis (AP) remain inconsistent. This systematic review and meta-analysis aimed to compare treatment outcomes between aggressive and non-aggressive intravenous hydration in severe and non-severe AP.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We systematically searched PubMed, Embase and Cochrane Library for randomized controlled trials (RCTs) on November 23, 2022, and hand-searched the reference lists of included RCTs, relevant review articles and clinical guidelines. We included RCTs that compared clinical outcomes from aggressive and non-aggressive intravenous hydration in AP. Meta-analysis was performed using a random-effects model for participants with severe AP and non-severe AP. Our primary outcome was all-cause mortality, and several secondary outcomes included fluid-related complications, clinical improvement and APACHE II scores within 48 h.
Results
We included a total of 9 RCTs with 953 participants. The meta-analysis indicated that, compared to non-aggressive intravenous hydration, aggressive intravenous hydration significantly increased mortality risk in severe AP (pooled RR: 2.45, 95% CI: 1.37, 4.40), while the result in non-severe AP was inconclusive (pooled RR: 2.26, 95% CI: 0.54, 9.44). However, aggressive intravenous hydration significantly increased fluid-related complication risk in both severe (pooled RR: 2.22, 95% CI 1.36, 3.63) and non-severe AP (pooled RR: 3.25, 95% CI: 1.53, 6.93). The meta-analysis indicated worse APACHE II scores (pooled mean difference: 3.31, 95% CI: 1.79, 4.84) in severe AP, and no increased likelihood of clinical improvement (pooled RR:1.20, 95% CI: 0.63, 2.29) in non-severe AP. Sensitivity analyses including only RCTs with goal-directed fluid therapy after initial fluid resuscitation therapy yielded consistent results.
Conclusions
Aggressive intravenous hydration increased the mortality risk in severe AP, and fluid-related complication risk in both severe and non-severe AP. More conservative intravenous fluid resuscitation protocols for AP are suggested.
Graphical Abstract

Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is one of the most frequent and critical gastrointestinal diseases resulting in hospital admissions worldwide [1,2,3]. The global incidence and mortality of acute pancreatitis is estimated at 34 cases per 100,000 person-years and 1.6 deaths per 100,000 person-years, respectively, and the incidence has been reported to be rising in recent years [4,5,6]. Appropriate initial management decisions for AP can significantly affect the disease course and hospitalization duration [5, 7,8,9,10].
According to a number of international guidelines, early fluid resuscitation with predominantly isotonic crystalloid (i.e., normal saline or Ringer’s lactate solution) is widely indicated for AP management to prevent hypovolemia and organ hypoperfusion, without waiting for hemodynamic worsening [7, 8, 11, 12]. However, the guidelines for the design of fluid resuscitation protocols remain inconsistent when it comes to the infusion rate [12,13,14,15,16,17,18,19]. For example, the American College of Gastroenterology (ACG) guidelines suggest that aggressive intravenous hydration (250–500 ml/hour) should be given to all patients with AP in the first 12–24 h unless cardiovascular and/or renal comorbidities exist [11]. For severe AP, the Italian Association for the Study of the Pancreas (AISP) suggests early aggressive hydration at 2 ml/kg/h, with an initial bolus of 20 ml/kg within 30–45 min in the first 24 h [20]. However, the guidelines of the American Gastroenterological Association (AGA) and experts from ACG’s Acute Pancreatitis Task Force suggest a goal-directed fluid therapy, but are unable to make specific recommendations on the optimal initial rate of fluid resuscitation in AP, due to the paucity of evidence [7, 21].
Three previous systematic review and meta-analysis studies, potentially with methodological flaws, yielded inconsistent findings on the effects of aggressive intravenous hydration in AP [22,23,24]. Gad MM et al. concluded that aggressive intravenous fluid therapy did not reduce mortality in AP, based on 9 included studies (3 randomized controlled trials (RCTs) and 6 cohort studies) [23], and Liao J et al. [22] concluded that aggressive hydration increases in-hospital mortality in AP by 1.66 times, based on 12 included studies (4 RCTs and 8 cohort studies). However, the certainty of evidence (CoE) from these reviews was compromised since the inclusion of observational studies with RCTs in meta-analyses frequently increases heterogeneity among the included studies and is therefore discouraged [25]. Another systematic review and meta-analysis from Di Martino M et al. [24] reported that high-rate fluid infusion increased mortality about threefold in AP, compared to moderate-rate fluid infusion, based on 4 RCTs. However, these previous meta-analyses did not separately analyze the severity of AP, so firm conclusions regarding specific AP populations cannot be drawn. Recently, interim findings from the WATERFALL trial indicated about threefold increased risks of fluid overload, and potentially threefold increased risks of mortality, in patients with non-severe AP receiving aggressive intravenous fluid resuscitation, compared to those receiving non-aggressive fluid resuscitation [26]. Since the heterogeneity, in terms of disease severity, of the population studied in previous RCTs has probably contributed to inconsistent findings, we conducted this systematic review and meta-analysis of RCTs, separately reporting and contrasting the benefits and harms of aggressive and non-aggressive intravenous hydration protocols for severe and non-severe AP.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Additional file 1: Appendix Table S1) [27], and the pre-defined study protocol has been published on the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) with the registration number INPLASY2022110068.
Search strategy
We searched PubMed, Embase and the Cochrane Library from database inception to November 23, 2022, with no limitation on language, to identify relevant RCTs. The search strategy was developed by an experienced evidence-based medicine researcher (SCS) in collaboration with a senior librarian (CCT) (Additional file 1: Appendix Table S2). Important keywords with MeSH terms included “acute pancreatitis”, “normal saline” and “Lactated Ringer’s solution.” To make our search more comprehensive, we also manually reviewed the reference lists of the included studies, previous review articles and published guidelines regarding AP.
Inclusion criteria
After removing duplicate records from the different databases, two independent reviewers (XWL and CHW) selected the included studies based on the following PICOS criteria: (1) Participants: Adults with AP. The diagnosis of AP should be based on two of the following criteria developed by the Atlanta international symposium and revised Atlanta classification: a.) Abdominal pain consistent with AP (e.g., acute onset of persistent, severe, epigastric pain often radiating to the back); b.) Serum lipase or amylase activity at least three times greater than the upper limit of normal; or c.) Classic image findings from computed tomography, magnetic resonance imaging or abdominal ultrasonography [1, 28]. We defined the severity of AP based on the Atlanta international symposium and revised Atlanta classification [1, 28]. For example, mild AP is defined by the absence of organ failure and local or systemic complications, and moderately severe AP is defined by the presence of transient organ failure or local or systemic complications. We classified mild and moderately severe AP into the non-severe AP group, because severe AP, characterized by persistent organ failure, may pose a higher mortality risk [2]; (2) Interventions: aggressive intravenous fluid resuscitation, defined as a.) Fluid administration (predominantly normal saline or lactated Ringer’s solution) at a rate greater than 10 ml/kg/hour as the initial management [12]; b.) Fluid bolus 20 ml/kg for 2 h, then 2–3 ml/kg/hour in the first 24 h [20]; c.) Isotonic crystalloid > 500 ml/hour for the first 12–24 h [11]. If the RCTs did not report the fluid infusion rate in the study protocols, the crystalloid fluid administration should be greater than 4000 ml in the first 24 h [29]; (3) Comparisons: non-aggressive intravenous fluid resuscitation, defined as a.) Fluid administration at a rate lower than 10 ml/kg/hour; b.) Fluid bolus 10 ml/kg for 2 h; then, 1.5 ml/kg/hour in the first 24 h or c.) Isotonic crystalloid < 500 ml/hour for the first 12–24 h. If the RCTs did not report the fluid infusion rate in the study protocols, the crystalloid fluid administration should be less than 4000 ml in the first 24 h [29]; (4) Primary outcome: all-cause mortality. Other secondary outcomes, such as the rate of clinical improvement (based on the objective parameters, including systemic inflammatory response syndrome (SIRS) subsides and time period therefore, decrease in hematocrit (Hct), blood urea nitrogen (BUN) and creatinine from baseline, and subjective measurements, including decrease in epigastric pain degree, assessed by visual analogue scale (VAS) and tolerance of oral nutrition within 48 h) in non-severe AP and the changes of Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, Sequential Organ Failure Assessment (SOFA) scores and Multiple Organ Dysfunction (MOD) scores for severe AP [30,31,32], fluid-related complications, such as abdominal compartment syndrome, pulmonary/peripheral edema and any sign of volume overload (e.g., rapid weight gain, incident ascites or jugular vein engorgement), as defined by previous guidelines [20, 33], sepsis, acute respiratory failure, acute kidney injury, pancreatic necrosis, SIRS subsiding within 48 h [34, 35], SIRS persisting > 48 h [34, 35], persistent organ failure (any organ failure > 48 h), defined by the revised Atlanta classification [1] and total hospitalization days were also evaluated if they were reported in the included studies. Specifically, decrease in epigastric pain may be related to better prognosis of AP and better subjective perception of quality of life for patients [7, 36]. In addition, we included APACHE II score changes as the clinical prognosis parameter, based on the suggestions of the ACG guidelines [37], and the prediction performance of poor outcome in severe AP has been validated [37, 38]. We also evaluated the Hct and BUN changes within 48 h [39, 40], because these parameters have been considered as surrogate markers for successful hydration for AP [11]; (5) Designs: RCTs. In cases of disagreement over study selection, the senior author (SCS) made the final decision.
Data extraction and risk-of-bias assessment
The data extraction and risk-of-bias assessment were performed by two independent reviewers (XWL and CHW). Data extracted included the study (e.g., first author with publication year and study period), participants (e.g., mean age and sex proportion, SIRS on admission and BUN levels) and intervention/comparison (e.g., fluid infusion protocols). We extracted data for the event number and mean with standard deviation (SD) in the intervention and comparison groups for categorical and continuous outcomes, respectively. In RCTs not reporting the SD for changes from baseline in continuous variables, we imputed a change-from-baseline SD using a correlation coefficient approach [41]. We used the Cochrane Collaboration's ROB tool 2.0, addressing the critical domains of randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result and overall bias, to evaluate the methodological quality of the included RCTs [42]. Any disagreements between the two reviewers were resolved by the senior author (SCS).
Data synthesis and statistical analysis
We used Review Manager Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to conduct a random-effects meta-analysis due to the expected clinical heterogeneity among the included RCTs [43]. Considering the heterogeneity with regard to disease severity among trial participants with AP in the RCTs, we separately calculated the pooled risk ratio (RR) and mean difference (MD) with a 95% confidence interval (CI) for categorical and continuous outcomes, respectively, for severe and non-severe AP. Where multiple scales were employed to measure the same continuous outcome, we used the standardized mean difference (SMD) to express the results. The I2 statistic was used to determine the extent of statistical heterogeneity among the included RCTs, and a value > 50% was considered as significant heterogeneity [44]. Funnel plots were to be constructed to visually examine for the presence of publication bias if there were at least 10 RCTs included in the meta-analysis [45].
Subgroup analysis
We assessed the heterogeneity of intravenous hydration effects on the primary and secondary outcomes of study interest based on the trial-level subgroups, including study countries (e.g., Asian or non-Asian), and mean or median age (e.g., < 50 or > 50 years).
Sensitivity analyses
Recent practice guidelines from AGA and the expert consensus of the ACG Acute Pancreatitis Task Force have highlighted the importance of goal-directed fluid therapy for AP, defined as the titration of intravenous fluids to specific clinical and biochemical perfusion targets (e.g., heart rate, mean arterial pressure, central venous pressure, urine output, BUN and Hct levels) [7, 21]. We therefore replicated all analyses by including only RCTs with goal-directed fluid therapy as a sensitivity analysis to determine the robustness of our results.
Certainty of evidence of the study outcomes
Two independent reviewers (JWD and CHW) evaluated the CoE for each study outcome based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria [46]. Any discrepancy between the review authors was resolved by discussion with the senior author (SCS).
Results
Characteristics of included studies
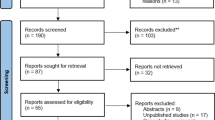
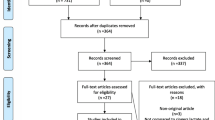
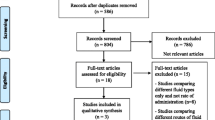
We retrieved a total of 239 records from the different databases, plus 7 records from additional reviews found on the reference lists of the included studies, previous review articles and published guidelines regarding AP. The study selection flowchart is presented in Additional file 1: Appendix Fig. S1, and the reasons for exclusion of records after full-text review are presented in Additional file 1: Appendix Table S3. We finally included 9 RCTs (severe AP: 2 RCTs; non-severe AP: 7 RCTs), covering a total of 953 trial participants in this systematic review and meta-analysis [26, 47,48,49,50,51,52,53,54]. We found both of the included RCTs focusing on severe AP were from China [47, 48], while 3, 1, 1, 1 and 1 of the included studies focusing on non-severe AP were from China, Thailand, Mexico, USA and multiple countries, respectively [26, 49,50,51,52,53,54]. Other important study characteristics of the included RCTs are shown in Table 1 and Additional file 1: Appendix Table S4.
Risk of bias of included studies
We judged only 1 study with 249 participants to have a low risk of bias in all domains [26], and 8 studies as having non-low risk of bias (either “some concerns” or “high risk of bias” in at least one domain). The greatest sources of bias were unclear randomization process and the possibility of selective reporting of results (Additional file 1: Appendix Table S5).
Primary outcome: all-cause mortality
A meta-analysis of 9 RCTs with a total of 953 participants with AP revealed an increased risk of mortality in the aggressive intravenous hydration group, compared to the non-aggressive intravenous hydration group (pooled RR: 2.42, 95% CI: 1.41, 4.17; I2: 0%) (Fig. 1). However, the significantly increased risk of mortality in the aggressive intravenous hydration group was only observed in severe AP (2 RCTs; 191 participants; pooled RR: 2.45, 95% CI: 1.37, 4.40; I2: 0%), while in non-severe AP the results remained similar but did not reach statistical significance (7 RCTs, of which only 3 contributed mortality events; 762 participants; pooled RR: 2.26, 95% CI: 0.54, 9.44; I2: 0%).
Secondary outcomes: fluid-related complications, clinical improvement, sepsis, acute respiratory failure, acute kidney injury, pancreatic necrosis, SIRS subsiding within 48 h, SIRS persisting > 48 h, persistent organ failure, BUN changes within 48 h, Hct changes within 48 h, and total hospitalization days
A meta-analysis of 5 RCTs (of which only 2 contributed outcome events) with a total of 517 participants with AP revealed an increased risk of fluid-related complications in the aggressive intravenous hydration group, compared to the non-aggressive intravenous hydration group (pooled RR: 2.49, 95% CI: 1.65, 3.75; I2: 0%) (Fig. 2). In addition, an increased risk of fluid-related complications in the aggressive intravenous hydration group was found both in severe AP (1 RCTs; 76 participants; RR: 2.22, 95% CI: 1.36, 3.63; I2: not applicable), and in non-severe AP (4 RCTs; 441 participants; pooled RR: 3.25, 95% CI: 1.53, 6.93; I2: not applicable).
A meta-analysis of 2 RCTs with a total of 104 participants with non-severe AP revealed no additional clinical improvement in the aggressive intravenous hydration group, compared to the non-aggressive intravenous hydration group (pooled RR: 1.20, 95% CI: 0.63, 2.29; I2: 72%) (Fig. 3a). None of the included RCTs reported the changes of SOFA or MOD scores. For severe AP, results from the meta-analyses indicated significantly greater changes in APACHE II scores for the aggressive vs. the non-aggressive intravenous hydration group (2 RCTs, 191 participants; pooled MD: 3.31, 95% CI: 1.79, 4.84; I2: 0%) (Fig. 3b). Another meta-analysis of 3 RCTs with a total of 440 participants with AP revealed an increased risk of sepsis in the aggressive intravenous hydration group, compared to the non-aggressive intravenous hydration group (pooled RR: 1.44, 95% CI: 1.15, 1.80; I2: 0%) (Additional file 1: Appendix Fig. S2). However, an increased risk of sepsis in the aggressive intravenous hydration group was only found in severe AP (2 RCTs; 191 participants; RR: 1.44, 95% CI: 1.15, 1.80; I2: 0%), but not in non-severe AP (1 RCTs; 249 participants; pooled RR: 1.73, 95% CI: 0.42, 7.10; I2: not applicable).
A meta-analysis of 3 RCTs with a total of 442 participants with AP revealed an increased risk of acute respiratory failure in the aggressive intravenous hydration group, compared to the non-aggressive intravenous hydration group (pooled RR: 1.49, 95% CI: 1.18, 1.89; I2: 0%) (Additional file 1: Appendix Fig. S3). However, an increased risk of acute respiratory failure in the aggressive intravenous hydration group was only found with severe AP (1 RCT; 76 participants; RR: 1.45, 95% CI: 1.14, 1.85; I2: not applicable), but not with non-severe AP (2 RCTs; 366 participants; pooled RR: 2.46, 95% CI: 0.85, 7.15; I2: 0%).
No RCTs focusing on severe AP reported acute kidney injury, pancreatic necrosis, SIRS subsiding within 48 h, SIRS persisting > 48 h or persistent organ failure. For those with non-severe AP, results from the meta-analyses did not indicate significant differences between the aggressive and non-aggressive intravenous hydration groups with regard to acute kidney injury (3 RCTs, 441 participants; pooled RR: 0.83, 95% CI: 0.32, 2.16; I2: 0%), pancreatic necrosis (2 RCTs, 337 participants; pooled RR: 1.82, 95% CI: 0.92, 3.59; I2: 0%), SIRS subsiding within 48 h (4 RCTs, 441 participants; pooled RR: 1.09, 95% CI: 0.70, 1.70; I2: 0%), SIRS persisting > 48 h (3 RCTs, 348 participants; pooled RR: 1.04, 95% CI: 0.50, 2.16; I2: 30%) or persistent organ failure (5 RCTs, of which only 4 contributed outcome events, 558 participants; pooled RR: 1.34, 95% CI: 0.65, 2.79; I2: 22%) (Additional file 1: Appendix Fig. S4–S8).
A meta-analysis of 2 RCTs with a total of 191 participants with severe AP revealed no significant differences in Hct changes within 48 h in the aggressive intravenous hydration group, compared to the non-aggressive intravenous hydration group (pooled MD: − 4.41, 95% CI: − 15.97, 7.16; I2: 100%). No RCTs focusing on severe AP reported BUN changes within 48 h or total hospitalization days. For non-severe AP, we did not find significant differences between the aggressive and non-aggressive intravenous hydration protocols as regards BUN changes within 48 h (2 RCTs, 104 participants; pooled MD: − 1.84, 95% CI: − 3.76, 0.08; I2: 0%), Hct changes within 48 h (2 RCTs, 104 participants; pooled MD: − 1.25, 95% CI: − 3.90, 1.41; I2: 71%) and total hospitalization days (6 RCTs, 673 participants; pooled MD: − 0.43, 95% CI: − 2.03, 1.17; I2: 98%) (Additional file 1: Appendix Figs. S9–S11).
Subgroup analyses
These results from the subgroup analyses of primary (Additional file 1: Appendix Table S6) and secondary outcomes (Additional file 1: Appendix Table S7–S19), comparing aggressive and non-aggressive intravenous hydration protocols for severe and non-severe AP, were generally similar to those from the main analysis. For example, compared with non-aggressive intravenous hydration protocols, the increased mortality risk associated with aggressive intravenous hydration protocols was also observed in participants with severe AP from Asian countries (2 RCTs, 191 participants; pooled RR: 2.45, 95% CI: 1.37, 4.40; I2: 0%), while inconclusive results were observed for non-severe AP.
Sensitivity analyses
In the sensitivity analyses, we included 7 RCTs (severe AP: 2 RCTs; non-severe AP: 5 RCTs) examining intravenous hydration protocols based on goal-directed fluid therapy after the initial fluid resuscitation therapy. The results also indicated an increased risk of mortality in the severe AP group receiving aggressive intravenous hydration (2 RCTs, 191 participants; pooled RR: 2.45, 95% CI: 1.37, 4.40; I2: 0%) (Additional file 1: Appendix Fig. S12), and increased risks of fluid-related complications in both the severe (1 RCT, 76 participants; pooled RR: 2.22, 95% CI: 1.36, 3.63; I2: not applicable) and non-severe AP group (3 RCTs, of which only 1 contributed outcome events, 353 participants; pooled RR: 3.25, 95% CI: 1.53, 6.93; I2: not applicable) receiving aggressive intravenous hydration, compared to the non-aggressive intravenous hydration group (Additional file 1: Appendix Fig. S13). Other secondary outcomes from sensitivity analyses were consistent with those from the main analyses (Additional file 1: Appendix Figs. S12–S25 and Tables S20–33).
GRADE assessment
Since there was a serious risk of bias and imprecise results due to the small sample sizes in the included RCTs, we judged the CoE by the GRADE criteria for our primary and secondary outcomes to be low to very low (Additional file 1: Appendix Table S34).
Discussion
In this systematic review and meta-analysis of 9 RCTs, the mortality risk from selecting aggressive intravenous hydration protocols over non-aggressive intravenous hydration protocols for fluid resuscitation therapy significantly increased 2.45-fold in severe AP, while in non-severe AP the results remained similar but did not reach statistical significance. This finding may be explained by our analyses of secondary outcomes, which indicated that aggressive intravenous hydration protocols did not decrease APACHE II scores in severe AP, or improve the clinical conditions in non-severe AP. In addition, aggressive intravenous hydration protocols increased the fluid-related complication risk in severe and non-severe AP by 2.22–3.25 times. Our findings suggested that aggressive intravenous hydration protocols should not be recommended for fluid therapy in severe and non-severe AP and may serve as an important reference not only for clinical practitioners but also for future practice guideline makers.
Determining an appropriate fluid therapy strategy for AP, especially as regards infusion rate, is critical but remains controversial. Previous animal studies have suggested that aggressive fluid therapy could improve splenic blood flow, correct pancreatic hypoperfusion and ultimately reduce pancreatic damage and mortality [55, 56], but may cause higher central venous pressure, leading to side effects of interstitial edema without significant changes in mean arterial pressure [57]. Conflicting treatment effects from aggressive intravenous fluid therapy have been found in previous RCTs and observational studies in patients with AP [23, 24, 29, 43, 44]. Nonetheless, ACG guidelines recommend aggressive intravenous hydration (defined as 250–500 ml per hour of isotonic crystalloid solution), to be administered early to most patients with AP [11], but the AGA guidelines and the ACG Acute Pancreatitis Task Force consensus make no recommendations on the hydration infusion rate [7, 21]. Our findings did not support the practice guidelines of the ACG or AISP and extended the knowledge gaps in the ACG/Acute Pancreatitis Task Force consensus. Our meta-analysis indicated a greater than twofold increased risk of mortality and fluid overload associated with aggressive intravenous hydration protocols in severe AP cases, and a similarly increased risk of fluid overload in non-severe AP cases.
AP induces interstitial edema and increases inter-capillary distance and therefore, leads to focal ischemia [14, 45]. In severe AP, in addition to interstitial edema, the production of free radicals and vasoconstriction of small arterioles with inflammatory cells adhering to the endothelial cells all contribute to a cytokine cascade [55, 56, 58], leading to multi-organ dysfunction syndrome, which is associated with higher mortality [13, 57, 59, 60]. Fluid overload itself may worsen the natural course of severe AP, with the accumulating fluid leading to abdominal compartment syndrome which then further compromises the kidneys, lungs and peritoneal viscera, which also potentially increases the risk of multi-organ failure and mortality [59, 61]. The aforementioned mechanisms could explain the poorer results in several of our secondary outcomes, such as APACHE II changes, acute respiratory failure and sepsis, that may derive from aggressive vs. non-aggressive intravenous hydration protocols in severe AP. Overall, aggressive intravenous hydration is not suggested for patients with severe AP because it not only increases the risk of mortality, but also the risk of fluid overload.
In contrast to patients with severe AP, this meta-analysis did not find a significantly increased mortality risk or clinical improvement rate from aggressive intravenous hydration in patients with non-severe AP. Mild AP is mostly self-limiting, causes only local inflammation and usually resolves within one week [10], and therefore is unlikely to increase mortality, affect the clinical progression of pancreatitis or cause multi-organ failure due to fluid-related complications. Our analyses of several secondary outcomes such as clinical improvement, sepsis, acute respiratory failure, acute kidney injury, pancreatic necrosis, BUN changes within 48 h, Hct changes within 48 h, SIRS subsiding within 48 h, SIRS persisting > 48 h, and persistent organ failure further supported this viewpoint. Our results were also consistent with previous large cohort studies [40, 62]. Specifically, the clinical improvement outcome included the subsiding of epigastric pain, which reflected overall clinical improvement and integrated the patient’s subjective symptoms, and our finding may imply that patients with AP receiving aggressive intravenous hydration, compared to non-aggressive intravenous hydration, may not achieve better quality of life in terms of pain relief [63]. Taken together, our findings suggest that aggressive intravenous hydration is not recommended for patients with non-severe AP, because it may increase the fluid overload risk while not actually improving clinical conditions.
Early goal-directed therapy has recently been suggested to titrate the intravenous fluid to specific clinical and biochemical targets of perfusion (e.g., heart rate, mean arterial pressure, central venous pressure, urine output, BUN, and Hct). The treatment benefits of goal-directed therapy in AP, compared to non-targeted therapy, remain inconsistent according to the findings of previous RCTs, but the AGA guidelines included a conditional recommendation suggesting the use of goal-directed fluid therapy versus other methods [7]. In this systematic review and meta-analysis, we replicated the analyses by only including RCTs using early goal-directed therapy in intravenous hydration protocols, and the results remained consistent to those from the main analyses. We found that aggressive intravenous hydration protocols were still associated with a higher risk of mortality and fluid-related complications in AP, even with the goal-directed fluid strategy. Our findings provided further robust evidence supporting the use of conservative intravenous hydration protocols for AP [7].
Strength and limitations
To the best of our knowledge, this is the first and most comprehensive systematic review and meta-analysis of RCTs that examine and compare outcomes between aggressive and non-aggressive intravenous hydration protocols for fluid therapy to treat severe and non-severe AP. In contrast to previous reviews which included both RCTs and cohort studies [22,23,24, 64], we included only RCTs in order to achieve more homogeneity between studies, and in order to provide the highest quality of evidence to address the specific research questions of clinicians, researchers, and health policy makers [65]. More importantly, we critically examined every potential record to ensure eligibility of the trials for our review. For example, we excluded one RCT by Wu et al. which was frequently included in previous meta-analyses on this topic, because the comparison of this RCT did not receive the non-aggressive intravenous hydration protocol [22, 23, 66]. Furthermore, we performed more pre-specified subgroup analyses to examine the heterogeneity of treatment effects, and the results from these subgroup analyses were generally consistent with our main analysis. Finally, we replicated all analyses by including only RCTs with goal-directed fluid therapy for AP, as suggested by the AGA and ACG Acute Pancreatitis Task Force since 2018 [2], and these findings were also consistent with our main analysis. Overall, our findings highlighted the clinical importance of a more conservative intravenous hydration therapy.
Several limitations should be noted before final interpretation of our findings. First, due to the limited number of included trial participants, the statistical power to detect significant differences in the secondary outcomes and the results from subgroup analyses may be suboptimal. However, our review did include four Asian RCTs and one multi-country RCT that had never been included in previous reviews, thus providing greater precision around estimates of treatment effects. Second, no individual-level data were obtained to examine the heterogeneity of treatment effects with different etiologies of AP [67, 68]. However, our included RCTs had participants with various etiologies of AP, so our findings may be applicable to the overall AP population. Third, the included RCTs did not report the total volumes of resuscitation fluid for the trial participants during hospitalization, so the actual volume differences between the aggressive and non-aggressive intravenous hydration groups remain unclear. Fourth, we could not investigate publication bias since we only included 9 RCTs in this meta-analysis. Finally, we judged the CoE of all study outcomes as low to very low, largely due to methodological concerns and small sample sizes of the included RCTs. We suggest regularly updating systematic reviews to include newly published RCTs on this critical topic.
Conclusion
This systematic review and meta-analysis of currently existing RCTs indicates that aggressive intravenous hydration protocols increased the mortality risk in severe AP and the fluid-related complication risk in both severe and non-severe AP. Our findings highlighted the clinical importance of a more conservative approach to fluid therapy for AP in order to mitigate excessive risk of avoidable side effects and mortality.
Availability of data and materials
We present the study data, in the form of tables or appendix files, in this manuscript.
Abbreviations
- ACG:
-
American college of gastroenterology
- AGA:
-
American gastroenterological association
- AISP:
-
Association for the study of the pancreas
- AP:
-
Acute pancreatitis
- APACHE II:
-
Acute physiology and chronic health evaluation II
- Hct:
-
Hematocrit
- BUN:
-
Blood urea nitrogen
- CI:
-
Confidence interval
- CoE:
-
Certainty of evidence
- GRADE:
-
Grading of recommendations assessment, development and evaluation
- INPLASY:
-
International platform of registered systematic review and meta-analysis protocols
- MD:
-
Mean difference
- RCTs:
-
Randomized controlled trials
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- RR:
-
Risk ratio
- SD:
-
Standard deviation
- SIRS:
-
Systemic inflammatory response syndrome
- SMD:
-
Standardized mean difference
- SOFA:
-
Sequential organ failure assessment
- MOD:
-
Multiple organ dysfunction
References
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11.
Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325:382–90.
Waller A, Long B, Koyfman A, et al. Acute pancreatitis: updates for emergency clinicians. J Emerg Med. 2018;55:769–79.
Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55.
Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162:122–34.
Sternby H, Bolado F, Canaval-Zuleta HJ, et al. Determinants of severity in acute pancreatitis: a nation-wide multicenter prospective cohort study. Ann Surg. 2019;270:348–55.
Crockett SD, Wani S, Gardner TB, et al. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154:1096–101.
Leppäniemi A, Tolonen M, Tarasconi A, et al. WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27.
Gardner TB. Acute pancreatitis. Ann Intern Med. 2021;174:Itc17-32.
Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet. 2020;396:726–34.
Tenner S, Baillie J, DeWitt J, et al. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(1400–15):1416.
IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1–15.
Bassi D, Kollias N, Fernandez-del Castillo C, et al. Impairment of pancreatic microcirculation correlates with the severity of acute experimental pancreatitis. J Am Coll Surg. 1994;179:257–63.
Kerner T, Vollmar B, Menger MD, et al. Determinants of pancreatic microcirculation in acute pancreatitis in rats. J Surg Res. 1996;62:165–71.
Vege SS, DiMagno MJ, Forsmark CE, et al. Initial medical treatment of acute pancreatitis: American gastroenterological association institute technical review. Gastroenterology. 2018;154:1103–39.
Jaber S, Garnier M, Asehnoune K, et al. Guidelines for the management of patients with severe acute pancreatitis, 2021. Anaesth Crit Care Pain Med. 2022;41: 101060.
Giakoumakis MI, Gkionis IG, Marinis AI, et al. Management of acute pancreatitis: conservative treatment and step-up invasive approaches—evidence-based guidance for clinicians. GastroHep. 2022;2022:2527696.
Eckerwall G, Olin H, Andersson B, et al. Fluid resuscitation and nutritional support during severe acute pancreatitis in the past: what have we learned and how can we do better? Clin Nutr. 2006;25:497–504.
Crosignani A, Spina S, Marrazzo F, et al. Intravenous fluid therapy in patients with severe acute pancreatitis admitted to the intensive care unit: a narrative review. Ann Intensive Care. 2022;12:98.
Pezzilli R, Zerbi A, Campra D, et al. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis. 2015;47:532–43.
Vivian E, Cler L, Conwell D, et al. Acute pancreatitis task force on quality: development of quality indicators for acute pancreatitis management. Am J Gastroenterol. 2019;114:1322–42.
Liao J, Zhan Y, Wu H, et al. Effect of aggressive versus conservative hydration for early phase of acute pancreatitis in adult patients: a meta-analysis of 3,127 cases. Pancreatology. 2022;22:226–34.
Gad MM, Simons-Linares CR. Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? A meta-analysis of randomized control trials and cohort studies. World J Gastroenterol. 2020;26:1098–106.
Di Martino M, Van Laarhoven S, Ielpo B, et al. Systematic review and meta-analysis of fluid therapy protocols in acute pancreatitis: type, rate and route. HPB (Oxford). 2021;23:1629–38.
Bun RS, Scheer J, Guillo S, et al. Meta-analyses frequently pooled different study types together: a meta-epidemiological study. J Clin Epidemiol. 2020;118:18–28.
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387:989–1000.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the international symposium on acute pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–90.
de-Madaria E, Soler-Sala G, Sánchez-Payá J, et al. Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am J Gastroenterol. 2011;106:1843–50.
Sharma A, Bhatt DL, Calvo G, et al. Heart failure event definitions in drug trials in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4:294–6.
Lambden S, Laterre PF, Levy MM, et al. The SOFA score—development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23:374.
Buckley TA, Gomersall CD, Ramsay SJ. Validation of the multiple organ dysfunction (MOD) score in critically ill medical and surgical patients. Intensive Care Med. 2003;29:2216–22.
Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147-239.
Buter A, Imrie CW, Carter CR, et al. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298–302.
Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:1247–51.
Wu BU, Banks PA. Clinical management of patients with acute pancreatitis. Gastroenterology. 2013;144:1272–81.
Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–400.
Khan AA, Parekh D, Cho Y, et al. Improved prediction of outcome in patients with severe acute pancreatitis by the APACHE II score at 48 hours after hospital admission compared with the APACHE II score at admission. Acute physiology and chronic health evaluation. Arch Surg. 2002;137:1136–40.
Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–82.
Wu BU, Johannes RS, Sun X, et al. Early changes in blood urea nitrogen predict mortality in acute pancreatitis. Gastroenterology. 2009;137:129–35.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Choosakul S, Harinwan K, Chirapongsathorn S, et al. Comparison of normal saline versus Lactated Ringer’s solution for fluid resuscitation in patients with mild acute pancreatitis. Randomized Control Trial Pancreatol. 2018;18:507–12.
Klar E, Messmer K, Warshaw AL, et al. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990;77:1205–10.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Mao EQ, Tang YQ, Fei J, et al. Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl). 2009;122:169–73.
Mao EQ, Fei J, Peng YB, et al. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl). 2010;123:1639–44.
Buxbaum JL, Quezada M, Da B, et al. Early aggressive hydration hastens clinical improvement in mild acute pancreatitis. Am J Gastroenterol. 2017;112:797–803.
Cuéllar-Monterrubio JE, Monreal-Robles R, González-Moreno EI, et al. Nonaggressive versus aggressive intravenous fluid therapy in acute pancreatitis with more than 24 hours from disease onset: a randomized controlled trial. Pancreas. 2020;49:579–83.
Angsubhakorn A, Tipchaichatta K, Chirapongsathorn S. Comparison of aggressive versus standard intravenous hydration for clinical improvement among patients with mild acute pancreatitis: a randomized controlled trial. Pancreatology. 2021;21:1224–30.
Liu J. Preliminary impact of the initial fluid resuscitation with different volume on the prognosis of acute pancreatitis not match the standard of severe AP. Master degree. Luzhou: Southwest Medical University 2021.
Wang SY. The application value of early aggressive hydration among patients with mild acute pancreatitis. Guangdong Med J. 2020;41:844–8.
Li H. The value of early aggressive hydration in patients with mild acute pancreatitis. Chin J Emerg Med. 2019;28:794–7.
Kusterer K, Enghofer M, Zendler S, et al. Microcirculatory changes in sodium taurocholate-induced pancreatitis in rats. Am J Physiol. 1991;260:G346–51.
Aho HJ, Nevalainen TJ, Aho AJ. Experimental pancreatitis in the rat. Development of pancreatic necrosis, ischemia and edema after intraductal sodium taurocholate injection. Eur Surg Res. 1983;15:28–36.
Knol JA, Inman MG, Strodel WE, et al. Pancreatic response to crystalloid resuscitation in experimental pancreatitis. J Surg Res. 1987;43:387–92.
Kusterer K, Poschmann T, Friedemann A, et al. Arterial constriction, ischemia-reperfusion, and leukocyte adherence in acute pancreatitis. Am J Physiol. 1993;265:G165–71.
van Brunschot S, Schut AJ, Bouwense SA, et al. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas. 2014;43:665–74.
De Waele JJ, Leppäniemi AK. Intra-abdominal hypertension in acute pancreatitis. World J Surg. 2009;33:1128–33.
Mofidi R, Duff MD, Wigmore SJ, et al. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93:738–44.
Wu BU, Bakker OJ, Papachristou GI, et al. Blood urea nitrogen in the early assessment of acute pancreatitis: an international validation study. Arch Intern Med. 2011;171:669–76.
Szatmary P, Grammatikopoulos T, Cai W, et al. Acute pancreatitis: diagnosis and treatment. Drugs. 2022;82:1251–76.
Wu F, She D, Ao Q, et al. Aggressive intravenous hydration protocol of Lactated Ringer’s solution benefits patients with mild acute pancreatitis: a meta-analysis of 5 randomized controlled trials. Front Med (Lausanne). 2022;9: 966824.
Patel JJ, Hill A, Lee ZY, et al. Critical appraisal of a systematic review: a concise review. Crit Care Med. 2022;50:1371–9.
Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710-717.e1.
Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27:286–90.
Toouli J, Brooke-Smith M, Bassi C, et al. Guidelines for the management of acute pancreatitis. J Gastroenterol Hepatol. 2002;17(Suppl):S15-39.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
X-WL, C-HW, J-WD, S-HT, P-HW and S-CS contributed to critical analysis and interpretation of the data. X-WL and S-CS contributed to drafting of the manuscript. R-NC and EC-CL contributed to study supervision and administrative, technical, or material support. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the submission of the manuscript.
Competing interests
There are no possible competing interests related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary tables and figures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, XW., Wang, CH., Dai, JW. et al. Comparison of clinical outcomes between aggressive and non-aggressive intravenous hydration for acute pancreatitis: a systematic review and meta-analysis. Crit Care 27, 122 (2023). https://doi.org/10.1186/s13054-023-04401-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04401-0