Abstract
Background
The end-tidal alveolar dead space fraction (AVDSf = [PaCO2−PETCO2]/PaCO2) is a metric used to estimate alveolar dead space. Higher AVDSf on the first day of mechanical ventilation is associated with mortality and fewer ventilator-free days. It is not clear if AVDSf is associated with length of ventilation in survivors, how AVDSf performs for risk stratification beyond the first day of ventilation, or whether AVDSf adds predictive value to oxygenation (oxygenation index [OI]) or severity of illness (Pediatric Risk of Mortality [PRISM III]) markers.
Methods
Retrospective single-center observational cohort study of children and young adults receiving invasive mechanical ventilation. In those with arterial or capillary blood gases, AVDSf was calculated at the time of every blood gas for the first week of mechanical ventilation.
Results
There were 2335 children and young adults (median age 5.8 years [IQR 1.2, 13.2]) enrolled with 8004 analyzed AVDSf values. Higher AVDSf was associated with mortality and longer length of ventilation in survivors throughout the first week of ventilation after controlling for OI and PRISM III. Higher OI was not associated with increased mortality until ≥ 48 h of ventilation after controlling for AVDSf and PRISM III. When using standardized variables, AVDSf effect estimates were generally higher than OI for mortality, whereas OI effect estimates were generally higher than AVDSf for the length of ventilation in survivors. An AVDSf > 0.3 was associated with a higher mortality than an AVDSf < 0.2 within each pediatric acute respiratory distress syndrome severity category.
The maximum AVDSf within 12 h of intensive care unit admission demonstrated good risk stratification for mortality (AUC 0.768 [95% CI 0.732, 0.803]). AVDSf did not improve mortality risk stratification when added to PRISM III but did improve mortality risk stratification when added to the gas exchange components of PRISM III (minimum 12-h PaO2 and maximum 12-h PCO2) (p < 0.00001).
Conclusions
AVDSf is associated with mortality and length of ventilation in survivors throughout the first week of invasive mechanical ventilation. Some analyses suggest AVDSf may better stratify mortality risk than OI, whereas OI may better stratify risk for prolonged ventilation in survivors than AVDSf.
Similar content being viewed by others
Background
Alveolar dead space, alveoli that receive ventilation without perfusion, is common in critically ill patients receiving mechanical ventilation [1,2,3]. Primary etiologies for increased alveolar dead space include alveolar overdistension, pulmonary vascular dysfunction or thrombosis, and low cardiac output. An estimate of alveolar dead space is the end-tidal alveolar dead space fraction (AVDSf = [PaCO2−PETCO2]/PaCO2) [4]. Several studies have demonstrated the prognostic value of elevated AVDSf in the first 24 h of mechanical ventilation in children with acute hypoxemic respiratory failure and in general cohorts of critically ill mechanically ventilated children [1, 5, 6]. An association between dead space metrics and mortality is also consistently found in adult critically ill patients [7,8,9,10].
In children with parenchymal lung disease, oxygenation and alveolar dead space abnormalities often occur together with each providing discrete data on ventilation and perfusion in the diseased lung [11]. Although there is conflicting evidence, there is some research to suggest that AVDSf may have a stronger association with mortality than oxygenation markers early in pediatric acute hypoxemic respiratory failure [5, 6, 11]. While markers of dead space have been considered for both adult and pediatric definitions of acute respiratory distress syndrome (ARDS), they have not been included in these definitions for several reasons [12, 13]. These may relate to the infrequent use of capnography in adults and neonates and imprecise surrogate markers for dead space (such as corrected minute ventilation or ventilatory ratio) without capnography. Furthermore, there are limited data on the prognostic value of AVDSf beyond the initial 24 h of invasive mechanical ventilation and whether AVDSf adds predictive value over readily available oxygenation markers. Notably, there are no data on whether AVDSf improves the predictive ability of current pediatric critical care severity of illness scores.
We sought to determine, in a large general cohort of critically ill mechanically ventilated children and young adults with a broad range of primary diagnoses, the association between AVDSf and (1) mortality and (2) length of ventilation in survivors over the first week of invasive mechanical ventilation and evaluate whether AVDSf provides additive predictive value over oxygenation markers and severity of illness scores. We hypothesized that AVDSf is a strong prognostic marker for mortality and prolonged ventilation in survivors independent of the severity of oxygenation abnormality or severity of illness.
Methods
This is a retrospective observational cohort study of children and young adults who received invasive mechanical ventilation for any reason in the Children’s Hospital Los Angeles (CHLA) Pediatric Intensive Care Unit (PICU) and were monitored with time-based capnography between August 2012 and September 2021. Children with no arterial or capillary blood gas values within the first 7 days of invasive mechanical ventilation were excluded. Children excluded for this reason generally had central venous access and were monitored with venous blood gases. Venous PCO2 is not well accepted as an accurate estimate of PaCO2, particularly in patients with hemodynamic instability; therefore, these patients were excluded [14]. Time-based capnography is part of standard monitoring for children on conventional ventilation in the CHLA PICU. There were no age-based exclusion criteria; however, neonates and young adults older than 21 years of age are rarely admitted to the CHLA PICU. Children with cyanotic heart disease are admitted to a separate cardiothoracic ICU at CHLA. The CHLA institutional review board approved this study with a waiver of informed consent (CHLA 22-00050).
Data extraction
Continuous data from the bedside monitor at the time of each arterial or capillary blood gas, which were obtained for clinical purposes, were averaged during the 1 min before and after the blood gas. Monitor data included PETCO2 and SpO2. For a subgroup of patients, continuous data on ventilator parameters including mean airway pressure (MAP) and FiO2 were also available. Demographic, diagnostic, severity of illness (Pediatric Risk of Mortality [PRISM III]), and blood gas data were obtained from the electronic medical record and an administrative database [15].
Variable definition
Alveolar dead space was quantified with the end-tidal alveolar dead space fraction (AVDSf = [PaCO2−PETCO2]/PaCO2) at the time of each blood gas during the first week of invasive mechanical ventilation [4]. An arterial blood gas (PaCO2) was used preferentially, but a capillary PCO2 could be substituted for AVDSf calculation when an arterial blood gas was not available. If the calculation of AVDSf resulted in a negative value, a zero was imputed for analysis. We have previously demonstrated that AVDSf is a reasonable surrogate for alveolar dead space calculated from volumetric capnography [4]. However, AVDSf, similar to most commonly used methods of dead space estimation, is influenced by the degree of intrapulmonary shunt [16, 17]. Oxygenation abnormality was quantified at the time of each blood gas with oxygenation index (OI = [MAP*FiO2*100]/PaO2) and, when MAP was unavailable, PF ratio (PaO2/FiO2) (if FiO2 was available). Ventilator data were obtained through a continuous data feed to the monitor, and when this data feed was not set up or disconnected, some variables were not available. When a capillary blood gas was used, PaO2 was estimated from SpO2, using the reported formula by Sauthier et al. to calculate OI and PF ratio [18].
Time windows were created for blood gases from the time of initiation of invasive ventilation (intubation [0–6 h], 12 h [6–18 h], 24 h [18–36 h], 48 h [36–60 h], 72 h [60–84 h], 96 h [84–120 h] and 144 h [120–168 h]) with the closest blood gas to the time point of interest, which was within the time window, identified for the analysis. Pediatric acute respiratory distress syndrome (PARDS) severity was determined using OI based on Pediatric Acute Lung Injury Consensus Conference (PALICC) criteria [19]. When OI was unavailable (missing MAP), ARDS severity was determined with Berlin criteria (using PF ratio) [12]. The first AVDSf within each PARDS severity category for each patient (if the patient had at least one AVDSf within a given PARDS severity category) was identified for analysis of mortality by AVDSf. AVDSf was categorized for analyses using the 75th and 90th percentile in children with a nonzero AVDSf (< 75th percentile: AVDSf < 0.2, 75th to 90th percentile: AVDSf ≥ 0.2 and < 0.3, ≥ 90th percentile: AVDSf ≥ 0.3) [1]. The natural log of PRISM III probability of death (POD) was used in modeling.
Outcomes
Our primary outcomes were PICU mortality and length of invasive mechanical ventilation in survivors. Multiple courses of invasive mechanical ventilation were summed to determine the total length of invasive mechanical ventilation if the time interval between courses was < 24 h.
Statistical analyses
We first performed descriptive analyses to determine the association between AVDSf categories and demographics, ventilator settings, blood gas data, and outcomes. AVDSf between survivors and non-survivors at every time point was compared with a Mann–Whitney U test. Differences in mortality by AVDSf category within each PARDS severity category were compared with chi-square tests. A Bonferroni correction was used to adjust for multiple comparisons. In children with OI available, multivariable logistic regression models were constructed to determine the association between AVDSf and mortality at each time interval adjusting for OI and severity of illness (PRISM III). OI and PRISM III were a priori chosen as likely confounders based on previous research and biological plausibility. Similarly, cox regression models at each time point of interest were constructed for the association between AVDSf and length of ventilation in survivors (modeled as time to liberation from invasive mechanical ventilation). Age, categorized by quartiles, and primary diagnosis were evaluated as potential effect modifiers in multivariable modeling using interaction terms. An interaction with a p value < 0.2 was considered significant. Nested models (1) AVDSf and PRISM III or (2) OI and PRISM III were compared to the full model with all three variables (AVDSf, PRISM III, and OI) using likelihood ratio tests and change in AIC (Akaike information criterion) and BIC (Bayesian information criterion) at each time point. To quantify the association between each variable (AVDSf and OI) and outcome, each variable was standardized to their standard deviation and these values were also used in modeling. The area under the receiver operating curve (AUC) estimates for logistic regression models using (1) maximum 12-h AVDSf, (2) PRISM III, and (3) minimum 12-h PaO2 and maximum 12-h PCO2 (the gas exchange components of PRISM III) for the outcome of mortality were determined. The AUC estimates for the latter two models were compared to models when a maximum of 12-h AVDSf was added as a covariate using the STATA roccomp command. All analyses were performed with STATA 17 (StataCorp LLC).
Results
There were 14,299 children and young adults admitted during the study time period with 4163 receiving invasive mechanical ventilation. Of these children and young adults, 2335 had one or more arterial or capillary blood gas and corresponding capnography data within the first 7 days of invasive mechanical ventilation. There were 1920 children and young adults with simultaneous MAP and FiO2 data to calculate at least 1 OI in the first 7 days of invasive mechanical ventilation. The analysis included 8004 AVDSf values, 2741 (34.2%) using a capillary PCO2, with a median of 3 (IQR 1, 5) AVDSf values used per patient.
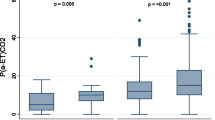
When categorizing AVDSf, 1834 (78.5%) of values were < 0.2, 274 (11.7%) were between 0.2 and 0.3, and 227 (9.7%) were ≥ 0.3. In general, AVDSf was more likely to be elevated in children with primary respiratory or cardiovascular diagnoses, and higher AVDSf was associated with pH, PCO2, FiO2, positive end-expiratory pressure (PEEP), MAP, OI, and severity of illness (PRISM III)(all p < 0.0001) (Table 1). At every time point of interest during the first week of invasive mechanical ventilation, AVDSf was higher in non-survivors than survivors (all p = 0.0001) (Fig. 1). When stratifying by PARDS severity (no PARDS, mild PARDS, moderate PARDS, or severe PARDS), the risk of mortality increased in a near stepwise fashion as a function of AVDSf (all p < 0.01) and an AVDSf ≥ 0.3 was associated with a significantly higher mortality than an AVDSf < 0.2 within each PARDS severity category (Fig. 2, Additional file 1: Table S1).
Mortality by AVDSf category within each PARDS severity category. For mortality comparisons within each PARDS category, a p value of 0.0167 was considered significant using a Bonferroni correction. Significant differences between categories are represented by lower case letters. The first AVDSf within each PARDS category for each patient was used in the analysis. Some patients are represented multiple times across PARDS categories. There are no patients represented multiple times within a PARDS category
Mortality models
In multivariable models for all time points of interest, higher AVDSf was associated with mortality after controlling for OI and PRISM III (all p < 0.05 except 48-h [p = 0.087]) (Table 2). OI was not independently associated with increased mortality in multivariable modeling before the 48-h time window. At the time of intubation, adding OI to a model with AVDSf and PRISM III for the outcome of mortality was significant, but a higher OI was associated with lower mortality (OR 0.97 [0.95, 0.99], p = 0.043). Model performance improved at most time points when adding AVDSf to a model with OI and PRISM III (Additional file 1: Table S2). However, model performance did not improve when adding OI to a model with AVDSf and PRISM III before 48 h for the outcome of mortality (Additional file 1: Table S2). Using standardized variables, the effect size of AVDSf was higher than OI prior to 48 h and then generally similar to OI over subsequent time points for mortality models (Additional file 1: Table S3).
Age and primary diagnosis modified the relationship between AVDSf and mortality in all models (Additional file 1: Tables S4 and S5). However, there was no clear trend for any subgroup where the association between higher AVDSf and mortality was not present. The magnitude of the association and the statistical significance of the associations did differ by subgroup, with older children (> 1.2 years of age) and those with the cardiovascular disease having a stronger association between AVDSf and mortality.
In the subgroup of children with AVDSf values within 12 h of PICU admission (n = 1602), the maximum 12-h AVDSf demonstrated good risk stratification for mortality (AUC 0.768 [95% CI 0.732, 0.803]). PRISM III had an AUC of 0.925 (95% CI 0.908, 0.943) for mortality with no significant change when maximum 12-h AVDSf was added to the model (AUC 0.926 [95% CI 0.909, 0.943], p = 0.40). When evaluating minimum 12-h PaO2 and maximum 12-h PCO2, the primary gas exchange components of PRISM III, the AUC for mortality was 0.670 (95% CI 0.627, 0.712) and the addition of AVDSf to the model significantly improved the AUC to 0.775 (95% CI 0.740, 0.810)(p < 0.00001).
Length of ventilation models
In multivariable models for each time point of interest, higher AVDSf was associated with a longer length of mechanical ventilation in survivors after controlling for OI and PRISM III (all p < 0.05 except at intubation [p = 0.076], 72 h [p = 0.28], and 144 h [p = 0.18]) (Table 3). Higher OI was independently associated with longer length of mechanical ventilation in survivors at all time points (all p < 0.05). Model performance improved at most time points for the length of ventilation in survivors when adding AVDSf to a model with OI and PRISM III (Additional file 1: Table S6). Using standardized variables, OI had a larger effect size than AVDSf on the length of mechanical ventilation in survivors at almost all time points (Additional file 1: Table S7).
Age and primary diagnosis modified the relationship between AVDSf and length of ventilation in survivors in most models (Additional file 1: Tables S8, S9). However, there was no clear trend for any subgroup where the association between higher AVDSf and longer length of ventilation was not present. The magnitude and statistical significance of the associations did differ by subgroup, with older children (> 1.2 years of age) in general having a stronger association between AVDSf and length of ventilation in survivors.
In sensitivity analyses, limited to children with arterial blood gases, multivariable modeling results were similar (Additional file 1: Tables S10, S11). In these models, OI was not significantly associated with increased mortality until 96 h and AVDSf was not significantly associated with longer length of ventilation in survivors at intubation.
Discussion
Our analyses confirm and expand on previous research demonstrating the strong association between higher AVDSf and mortality and prolonged length of mechanical ventilation in critically ill children. We have demonstrated that AVDSf is an important prognostic marker throughout the first week of invasive mechanical ventilation, beyond the previously reported range of the first 24 h. AVDSf provides additional risk stratification information independent of the severity of oxygenation abnormality or severity of illness at most time points in the first week of mechanical ventilation. In fact, early in the course of mechanical ventilation, higher OI is not associated with mortality after controlling for AVDSf. Previous reports of the association between AVDSf and length of ventilation have been limited to analyses of ventilator-free days which is a composite outcome of both length of ventilation in survivors and mortality. We have now demonstrated the independent association between AVDSf and each outcome (mortality and length of mechanical ventilation in survivors). Lastly, when considering a common pediatric severity of illness score (PRISM III), AVDSf improves risk discrimination for mortality when added to the current gas exchange components of PRISM III (maximum PaCO2 and minimum PaO2).
During the first hours of mechanical ventilation, patient physiology may change rapidly. In children with PARDS, ventilator management is aimed to achieve lung recruitment and decrease intrapulmonary shunt, thereby improving oxygenation. This likely contributes to the noted stronger association of AVDSf with mortality, in comparison with OI, early in mechanical ventilation that then equalizes over time. Poor oxygenation attributable to atelectasis or pulmonary edema may be more easily improved with positive pressure ventilation and reflect lower disease severity, than true alveolar consolidation. Previous studies have also demonstrated that OI may not perform as well as AVDSf for mortality risk stratification early in ARDS [5]. Nevertheless, no current diagnostic methods for ARDS or severity of illness scoring, in adults and children, include dead space metrics and most rely entirely on oxygenation metrics to assess the severity of pulmonary disease. Identification of patients at the highest risk for mortality early in the course of critical illness is crucial for weighing the risk–benefit ratio of potentially harmful interventions, such as neuromuscular blockade, and for timely enrollment in clinical trials to study new interventions. We have demonstrated in comprehensive analyses that across PARDS severity categories, ages, and primary diagnoses, AVDSf provides additional risk stratification information to oxygenation markers. Our results add to the accumulating evidence suggesting that dead space markers should be further considered for diagnostic severity scoring in ARDS and potentially for other severity of illness scores in both children and adults.
However, it is important to note that this analysis represents a subgroup of critically ill children, those that require conventional mechanical ventilation and are monitored with either arterial or capillary blood gases. This limits the generalizability of our results to critically ill children who do not meet these criteria. Capnography cannot be performed reliably when patients are not on respiratory support or in those supported with either noninvasive or non-conventional ventilation. Currently, there are also no validated methods for dead space calculation that do not require blood gas monitoring. While arterial blood gas monitoring practices vary between institutions, most critically ill children, even those with PARDS, are not monitored with arterial blood gases [19, 20]. In critically ill adults, the ventilatory ratio has been suggested as a method to estimate dead space as this method does not require capnography data [7]. However, the ventilatory ratio continues to require arterial blood gases monitoring and has not performed well in comparison to AVDSf in children [5]. Capillary PCO2 is likely an acceptable estimate of PaCO2, and the exclusion of capillary blood gases did not significantly alter our results in sensitivity analyses [21]. However, frequent monitoring of capillary blood gases may not be acceptable to patients and families. Other noninvasive methods of dead space assessment, including volumetric capnography curve analysis, have been proposed, but these have not been validated in children [22]. Additionally, most methods for dead space estimation such as AVDSf, physiologic dead space from the Bohr–Enghoff equation, or ventilatory ratio rely on the assumption that arterial PCO2 equals alveolar PCO2 and therefore may be influenced by intrapulmonary shunt [23, 24]. The development of methods to monitor dead space that overcomes these issues should be pursued to determine if dead space metrics may add prognostic information in all critically ill children.
It is often assumed that the strongest predictors of mortality are also most strongly associated with the length of ventilation. This forms the rationale for the common critical care outcome of ventilator-free days. However, as our data have demonstrated, this may not always be the case. AVDSf had a larger estimated effect size on mortality in the first 24 h of mechanical ventilation and continued to be significantly associated with mortality throughout the first week of ventilation. When considering that most children with PARDS die from multisystem organ failure, the potential importance of vascular dysfunction is highlighted [19]. Dead space reflects pulmonary perfusion abnormality and is more likely to reflect vascular dysfunction than oxygenation markers. On the other hand, at most time points, OI had a significantly larger estimated effect on the length of mechanical ventilation in survivors than AVDSf. The reasons for prolonged mechanical ventilation are numerous but mechanical ventilation liberation practices and spontaneous breathing trial eligibility are often reliant on oxygenation metrics (MAP or PEEP, FiO2, SpO2) [25, 26]. Therefore, it may not be surprising that OI performed better than AVDSf for risk discrimination of length of ventilation in survivors. It is crucial that these relationships are further studied and considered when choosing the appropriate patients and outcomes for clinical trials based on the expected mechanism of the effect of the intervention.
AVDSf did not add significantly to the performance of PRISM III for mortality prediction demonstrating that the prognostic data reflected by elevated AVDSf are captured in the variables considered in PRISM III. However, PRISM III is a cumbersome score that uses numerous data points and is rarely used clinically. AVDSf did significantly outperform PaCO2 and PaO2, the gas exchange metrics of PRISM III. Risk prediction, in various formats, in critically ill adults and children is the focus of a large existing, and growing, body of research. However, although evidence is accumulating on the prognostic importance of the easily calculated marker of dead space, almost no studies have considered dead space in predictive modeling. This is an area ripe for further research and consideration of dead space may improve risk stratification in diagnostic criteria, such as those for PARDS, and for the severity of illness scores.
The large cohort and closely matched physiologic values using continuous monitoring data are clear unique strengths of this study. The primary limitation of this study is obtaining data from a single center. It is possible that some site-specific practices, criteria for arterial line catheter placement, use of inhaled nitric oxide, or ventilator liberation for example, may lead to less applicability at other centers. However, the study results are consistent with numerous other studies demonstrating the prognostic relevance of dead space markers seemingly making this possibility less likely. We did not have the continuous ventilator data for OI calculation (MAP or FiO2 missing) for some patients. In patients with FiO2, we used Berlin Criteria for PARDS severity stratification but excluded them from multivariable modeling. It is possible PARDS severity was misclassified in some of these patients or that the relationship between AVDSf and outcome is different in the patients excluded from modeling. Our findings may require validation in multicenter observational studies.
Conclusions
In conclusion, AVDSf is an important marker of the severity of illness associated with both mortality and length of ventilation in survivors, initially and over the first week of invasive mechanical ventilation for critically ill children. Some analyses suggest AVDSf performs better than OI for risk stratification of mortality, whereas OI performs better for risk stratification of the length of ventilation in survivors. AVDSf may be useful for additional mortality risk stratification within PARDS severity categories.
Availability of data and materials
The data used for this study analysis are available from the corresponding author given there is a reasonable request for the data.
Abbreviations
- AIC:
-
Akaike information criterion
- AVDSf:
-
End-tidal alveolar dead space fraction
- BIC:
-
Bayesian information criterion
- FiO2 :
-
Fractional inspired oxygen
- MAP:
-
Mean airway pressure
- OI:
-
Oxygenation index
- PARDS:
-
Pediatric acute respiratory distress syndrome
- PCO2 :
-
Partial pressure of carbon dioxide
- PEEP:
-
Positive end-expiratory pressure
- PRISM:
-
Pediatric risk of mortality
- SpO2 :
-
Oxygen saturation (pulse oximetry)
References
Bhalla AK, Belani S, Leung D, Newth CJ, Khemani RG. Higher dead space is associated with increased mortality in critically ill children. Crit Care Med. 2015;43(11):2439–45.
Oh SB, Aguilan A, Tan HL, Ma YJ, Sultana R, Lee JH, et al. The association between alveolar dead space fraction and mortality in pediatric acute respiratory distress syndrome: a prospective cohort study. Front Pediatr. 2022;10: 814484.
Beda A, Winkler T, Wellman TJ, De Prost N, Tucci M, Vidal Melo MF. Physiological mechanism and spatial distribution of increased alveolar dead-space in early ARDS: an experimental study. Acta Anaesthesiol Scand. 2021;65(1):100–8.
Bhalla AK, Rubin S, Newth CJ, Ross P, Morzov R, Soto-Campos G, et al. Monitoring dead space in mechanically ventilated children: volumetric capnography versus time-based capnography. Respir Care. 2015;60(11):1548–55.
Bhalla AK, Dong J, Klein MJ, Khemani RG, Newth CJ. The association between ventilatory ratio and mortality in children and young adults. Respir Care. 2021;66(2):205–12.
Yehya N, Bhalla AK, Thomas NJ, Khemani RG. Alveolar dead space fraction discriminates mortality in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med. 2016;17(2):101–9.
Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199(3):333–41.
Kallet RH, Zhuo H, Liu KD, Calfee CS, Matthay MA. The association between physiologic dead-space fraction and mortality in subjects with ARDS enrolled in a prospective multi-center clinical trial. Respir Care. 2014;59(11):1611–8.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–6.
Siegel ER, Zhuo H, Sinha P, Papolos AI, Ni SA, Vessel K, et al. Ventilatory ratio is a valuable prognostic indicator in an observational cohort of patients with ARDS. Respir Care. 2022;67(9):1075–81.
Ghuman AK, Newth CJ, Khemani RG. The association between the end tidal alveolar dead space fraction and mortality in pediatric acute hypoxemic respiratory failure. Pediatr Crit Care Med. 2012;13(1):11–5.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Pediatric Acute Lung Injury C. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5):S23–40.
Chong WH, Saha BK, Medarov BI. Comparing central venous blood gas to arterial blood gas and determining its utility in critically ill patients: narrative review. Anesth Analg. 2021;133(2):374–8.
Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(5):743–52.
Bonifazi M, Romitti F, Busana M, Palumbo MM, Steinberg I, Gattarello S, et al. End-tidal to arterial PCO(2) ratio: a bedside meter of the overall gas exchanger performance. Intensive Care Med Exp. 2021;9(1):21.
Suarez-Sipmann F, Bohm SH, Tusman G. Volumetric capnography: the time has come. Curr Opin Crit Care. 2014;20(3):333–9.
Sauthier M, Tuli G, Jouvet PA, Brownstein JS, Randolph AG. Estimated Pao(2): a continuous and noninvasive method to estimate Pao(2) and oxygenation index. Crit Care Explor. 2021;3(10): e0546.
Khemani RG, Smith L, Lopez-Fernandez YM, Kwok J, Morzov R, Klein MJ, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2019;7(2):115–28.
Mahendra M, McQuillen P, Dudley RA, Steurer MA. Variation in arterial and central venous catheter use in pediatric intensive care units. J Intensive Care Med. 2021;36(11):1250–7.
Harrison AM, Lynch JM, Dean JM, Witte MK. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med. 1997;25(11):1904–8.
Tusman G, Sipmann FS, Borges JB, Hedenstierna G, Bohm SH. Validation of Bohr dead space measured by volumetric capnography. Intensive Care Med. 2011;37(5):870–4.
Bourgoin P, Baudin F, Brossier D, Emeriaud G, Wysocki M, Jouvet P. Assessment of Bohr and Enghoff dead space equations in mechanically ventilated children. Respir Care. 2017;62(4):468–74.
Maj R, Palermo P, Gattarello S, Brusatori S, D’Albo R, Zinnato C, et al. Ventilatory ratio, dead space, and venous admixture in acute respiratory distress syndrome. Br J Anaesth. 2022. https://doi.org/10.1016/j.bja.2022.10.035.
Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 2009;10(1):1–11.
Abu-Sultaneh S, Iyer NP, Fernández A, Gaies M, González-Dambrauskas S, Hotz JC, et al. Executive summary: international clinical practice guidelines for pediatric ventilator liberation, a PALISI network document. Am J Respir Crit Care Med. 2022;207:17–28.
Acknowledgements
Not applicable.
Funding
Dr. Bhalla receives funding from a K23 Career Development Award through the National Institutes of Health (K23 HL153756).
Author information
Authors and Affiliations
Contributions
AKB designed the study, performed the data analysis, and wrote the manuscript. AC aided with data collection and review of the manuscript. RGK aided with the study design, and review of the data analysis and manuscript. C.J.L.N. aided with study design, data collection, data analysis and review of the manuscript. All authors read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Children’s Hospital Los Angeles institutional review board approved this study with a waiver of informed consent (CHLA 22–00050).
Consent for publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplement Tables 1–11.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bhalla, A.K., Chau, A., Khemani, R.G. et al. The end-tidal alveolar dead space fraction for risk stratification during the first week of invasive mechanical ventilation: an observational cohort study. Crit Care 27, 54 (2023). https://doi.org/10.1186/s13054-023-04339-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04339-3