Abstract
Heat stroke (HS) is a life-threatening systemic disease characterized by an elevated core body temperature of more than 40 ℃ and subsequent multiple organ dysfunction syndrome. With the growing frequency of global heatwaves, the incidence rate of HS has increased significantly, which has caused a huge burden on people's lives and health. Liver injury is a well-documented complication of HS and usually constitutes the direct cause of patient death. In recent years, a lot of research has been carried out on the pathogenesis and treatment strategies of HS-induced liver injury. In this review, we summarized the important pathogenesis of HS-induced liver injury that has been confirmed so far. In addition to the comprehensive effect of systemic factors such as heat cytotoxicity, coagulopathy, and systemic inflammatory response syndrome, excessive hepatocyte cell pyroptosis, dysfunction of Kupffer cells, abnormal expression of heat shock protein expression, and other factors are also involved in the pathogenesis of HS-induced liver injury. Furthermore, we have also established the current therapeutic strategies for HS-induced liver injury. Our study is of great significance in promoting the understanding of the pathogenesis and treatment of HS-induced liver injury.
Similar content being viewed by others
Introduction
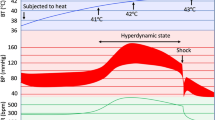
Heatstroke (HS) was a severe heat illness characterized by an elevated core body temperature exceeding 40 °C and often accompanied by central nervous system dysfunction and multiple organ injury such as liver, intestine, and lung [1, 2]. With the continuous warming of the global climate and the frequent occurrence of extreme weather in summer, the incidence and mortality of HS have shown an obvious upward trend globally [3]. Accordingly, HS has attracted more and more researchers' attention in recent years. However, although the exploration of the pathogenesis and treatment strategy of HS has been ongoing, the pathogenesis of HS has not been fully elucidated so far, and effective treatments are lacking.
According to the presence or absence of labor-related factors, the HS can be divided into classic heat stroke (CHS) and external heat stroke (EHS) [1]. CHS mainly occurs in the elderly, children, and people with underlying diseases [4], which is caused by the dysfunction of the thermoregulation in a high-temperature environment, while EHS mainly occurs in military officers, soldiers and young adults who perform high-intensity physical work in a high-temperature and high-humidity environment.
Clinically, HS is identified as a syndrome of extremely high fever, bleeding and coagulation disorders, circulatory failure, systemic inflammatory reaction, and multiple organ dysfunction [5]. In addition to the direct injury caused by heat exposure, the key pathophysiological changes induced by systemic inflammatory response syndrome (SIRS) [6] and multiple organ dysfunction syndrome (MODS) are also important pathological mechanisms of HS. Among the injured target organs, acute liver injury (ALI) and its more serious form acute liver failure (ALF) are well-documented complications of HS [7, 8] and served as a direct cause of HS patient death [9, 10]. A large number of hepatocytes undergo degeneration changes during the pathological process of HS [11].
The studies have shown that one of the important mechanisms of HS is the excessive opening of intestinal tight junctions, the destruction of intestinal cell structure and function, the increase in intestinal mucosal permeability, and the introduction of endotoxin into the blood [12, 13]. The blood from the gastrointestinal tract must pass through the portal vein system to the liver before entering the circulation, which enables the liver to play a vital role in metabolism, immunity, excretion, and other aspects [14]. Therefore, the liver not only plays a victim role in the course of HS, but also is a key factor in the pathogenesis of HS.
In this review, we systematically elaborated the possible mechanisms (as shown in Fig. 1) and treatment strategies (as shown in Fig. 2) of HS-induced liver injury, thus providing a theoretical basis and further research direction for the further study of HS-induced liver injury.
The related mechanism of hepatocyte injury during HS-induced liver injury. In addition to direct heat injury, HS can cause liver injury through various mechanisms, including inhibiting the expression of HSP70 of hepatocytes, promoting the production of ROS, promoting the inflammatory response and pyropsis, etc.
The main treatment strategies for HS-induced liver injury. It mainly focuses on systematic support treatment, including cooling treatment, early oxygen supply, CBP, anticoagulant therapy, and anti-inflammatory treatment. For those with irreversible liver injury, liver transplantation provides a possible choice
The related mechanisms of HS-induced liver injury.
The excessive inflammatory response
Excessive inflammation is an important cause of MODS in HS, while the translocation of endotoxin in the intestinal cavity caused by intestinal injury into the circulation is the key factor to trigger SIRS and MODS including liver injury in HS. Both animal and human studies have suggested that heat stress can cause serious damage to intestinal barrier function [15, 16]. On the one hand, heat stress can induce oxidative stress, nitrogen stress, and other negative cellular reactions in the body, and ultimately lead to intestinal epithelial cell damage and apoptosis [17, 18]. On the other hand, the maintenance of intestinal barrier function requires both active intestinal epithelial cells and the normal expression of intercellular tight junction proteins, while HS can lead to the reorganization of the intestinal cytoskeleton and downregulate the expression of tight junction proteins between intestinal cells [19, 20], thus leading to intestinal barrier dysfunction and the translocation of bacteria and endotoxin from the intestinal tract to the systemic circulation.
Cytokines are considered to be key mediators of SIRS in HS-induced systemic MODS [21] and are closely related to the severity and outcome of HS [22, 23]. Although many proinflammatory cytokines such as interleukin (IL)-1β, IL6, tumor necrosis factor-alpha(TNF-α), IL-8, etc., were found to be significantly increased in HS patients in the early years [23,24,25], the research on the pathogenesis of excessive inflammation in HS has not been elucidated.
As one of the most ubiquitous, abundant, and evolutionarily conserved transcription and growth factors in eukaryotes [26], high mobility group box 1 (HMGB1) occupies an important position in the diagnosis and treatment of HS. Consistent with multiple septic [27] or non-septic systemic inflammation [28], HMGB1 levels in HS patients were positively associated with disease severity and mortality [29]. Antithrombin III, thrombomodulin (TM), and hypothermia treatment could decrease the level of HMGB1, inhibit excessive inflammation, and improve HS-induced organ damage [30,31,32]. More precisely, Wang et al. used HMGB1 monoclonal antibody to specifically inhibit HMGB1 to explore the role of HMGB1 in HS-induced ALI [33]. They found that the intervention of the HMGB1 monoclonal antibody could significantly improve liver function and alleviate liver damage in the HS rat model. The mechanism of HMGB1 in the HS liver injury model was further explored by Yan Geng et al. [34]. As a typical damage-associated molecular patterns (DAMPs) molecule, HMGB1 can mediate HS-induced activation of the Nlrp3 inflammasome through the Toll-like receptor (TLR) 4 and receptor for advanced glycation end products (RAGE) signaling, thereby inducing the activation of IL-1β, as well as hepatocyte pyroptosis and consequent severe liver injury, while the HMGB1 inhibition, silencing of Nlrp3, or blockade of caspase-1 could significantly prevent the Nlrp3 inflammasome activation, thereby reducing liver injury in HS-induced liver injury.
Macrophages and monocytes activated by proinflammatory factors are important sources of HMGB1 [35], while HMGB1 can also leak from necrotic or damaged cells [36, 37]. Under heat stress, HMGB1 expression in liver macrophages and plasma increased significantly. The TM intervention could decrease the plasma HMGB1 levels even with delayed treatment [38]. These findings indicated that TM in HS can not only improve HS-induced liver injury by improving the systemic coagulation state but also effectively inhibit the excessive inflammatory reaction.
Coagulation disorders
The activation of coagulation and fibrin deposition secondary to inflammation can be regarded as an important part of the host's defense against high fever, but the intensification of systemic inflammatory reaction may lead to systemic blood coagulation activation and microvascular failure, thus exacerbating organ dysfunction [39]. HS can cause obvious coagulant status disorder even more obvious than sepsis. HS-activated coagulation was indicated by the prothrombin time, activated partial thromboplastin time, and D-dimer levels, and decreased platelet count [40]. In HS patients, the whole-blood tissue factor, TAT, and soluble TM levels dramatically increased, while the levels of the major physiologic anticoagulants were significantly decreased, including antithrombin, protein C, and protein S [39]. TM can bind thrombin to convert protein C to activated protein C(APC) [41, 42].
TM is an endothelial anticoagulant cofactor, which exerts an important role in the regulation of intravascular coagulation [43]. In addition to regulating the blood coagulation state of the whole body, the complex formed by TM and thrombin can promote the activation of protein C, which has the effect of inhibiting monocytes and macrophages [44, 45]. The positive role of APC in HS in reducing systemic inflammation, hypercoagulability, and organ damage has been confirmed [46]. To sum up, TM can effectively improve liver injury in HS, which may involve many mechanisms. TM supplementation should be considered an important treatment strategy for patients with HS.
Endothelial cells play an important role in maintaining vascular homeostasis and normal physiological function [47]. Endothelial cell injury is one of the most important pathological features of HS. Intense heat stress could induce obvious endothelial cell damage and apoptosis [48, 49]. In addition to causing the release of cytokines, which further amplifies the inflammatory response [50], the damaged endothelial cells will lead to the exposure of collagen fibers under the endothelium, activate coagulation factor XII, and then activate fibrinogen to form fibrin thrombus, leading to endogenous coagulation dysfunction [51]. At the same time, endothelial cells can release tissue factors and activate coagulation factor VII, leading to exogenous coagulation dysfunction. Furthermore, studies showed that the reduction of endothelial cell apoptosis was closely related to the improvement of coagulation disturbances in HS, which can effectively reduce HS-induced liver injury [52, 53]. These findings suggested that endothelial cells could be an important target for improving HS-induced liver injury.
Abnormal hepatocyte death
The most common pathological change of HS-induced liver injury is the massive degeneration of hepatocytes including abnormal cell death [11], while pyroptosis exerts an important role in abnormal cell death in HS-induced liver injury [34]. Pyroptosis is a caspase-1-dependent programmed cell death characterized by cell swelling, rapid plasma membrane rupture, and release of proinflammatory intracellular substances [54, 55]. NOD-like receptor family pyrin domain containing3 (NLRP3) is an intracellular pattern recognition receptor, which was involved in cell pyroptosis by assembling into an inflammasome [56]. NLRP3-dependent pyroptosis is an important cause of abnormal cell death in HS-induced liver injury [34]. HS-induced liver injury is accompanied by the activated inflammasome, which can effectively induce the activation of IL-1 β and hepatocyte pyroptosis, thus leading to severe liver injury.
Excessive ROS will be produced during heat stress [57], which has been proven to be a key stimulator of NLRP3 inflammasome and a potential target for negative regulation of cell pyroptosis [58]. Ming Zhang et al. further found that the overproduction of ROS and NLRP3-dependent pyroptosis is an important pathological mechanism in HS-induced liver injury [59].
The angiotensin peptides are the important components of the renin-angiotensin system (RAS). The previous studies have confirmed the value of Ang II and Ang—[1,2,3,4,5,6,7] as potential biomarkers in inflammatory diseases [60, 61]. The level of angiotensin peptides changed during HS and appears to be associated with excessive ROS production [62]. In the HS-induced liver injury, the expression of Ang II increased, while the levels of Ang—[1,2,3,4,5,6,7] decreased, which were consistent with their receptors and converting enzymes [59]. As an analog of Ang- [1,2,3,4,5,6,7], AVE 0991 can significantly inhibit the production of ROS and reduce the protein levels of NOX4, NLRP3, caspase-1, and IL-1 in HS-induced liver injury. These findings indicated that RAS exerted an important role in HS-induced liver injury, while the mechanism is closely related to the excessive production of ROS and the consequent induction of cell pyroptosis.
Another mechanism of cell death in HS is the Z-DNA binding protein 1 (ZBP1)-mediated programmed cell death. As a Z-nuclear acid sensor, ZBP1 can activate receptor-interacting protein kinase 3(RIPK3)-mixed lineage kinase domain-like(MLKL) pathway-dependent programmed cell necrosis [63, 64]. Recently, the research of Fangfang Yuan et al. suggested that the expression of ZBP1 in HS was significantly increased and could induce RIPK-dependent cell death. The deletion of ZBP1 and RIPK3 can significantly reduce cell apoptosis in HS and effectively alleviate multiple organ damage including liver injury. Further, Fangfang Yuan et al. found that heat stress can enhance the activation and occupation of heat shock transcription factor1 (HSF1) binding sites in the ZBP1 promoter. Consistently, the deletion of HSF1 can inhibit the increase in ZBP1 expression and cell death induced by heat stress.
Mitophagy plays a crucial role in regulating homeostasis in the liver [65, 66]. As the most important mechanism for self-regulating mitochondrial quality control and selectively clearing the damaged mitochondria [67], the dysfunction of the mitophagy leads to the accumulation of the dysfunctional mitochondria, the excessive activation of apoptosis-inducing factors, and the excessive release of the ROS, thus leading to abnormal cell apoptosis [68]. The previous studies have suggested that mitophagy is closely related to the occurrence and development of various liver diseases, including viral hepatitis, liver ischemia/reperfusion (I/R) injury, and drug-induced liver injury [69]. In HS-induced liver injury, the important role of mitophagy has also been confirmed. P53 has been well characterized for its response to regulate the cell cycle and cell apoptosis [70, 71]. Under the stimulation of heat stress, the translocation of P53 from the nucleus to the cytoplasm is significantly increased, thereby binding to Parkin to reduce Parkin’s translocation from the cytosol to the mitochondria, which decreases mitophagy activation and exacerbates apoptosis in hepatocytes, while the inhibition of p53 with siRNA or PFT-a significantly suppressed the aberrant apoptosis in HS-induced ALI [72].
The abnormal production and release of extracellular vesicles of hepatocytes.
Extracellular vesicles (EVS) are novel mediators of intercellular communication [73]. Various biologically active substances, including proteins, lipids, and nucleic acids, can be transferred to recipient cells via EVS and modulate their biological processes and function [74]. EVS is a key event in liver pathology [74] and function in a variety of liver diseases, regulating physiological cellular events [75,76,77].
In HS-induced ALI, HS could lead to a marked increase in EVS released from hepatocytes, which promotes liver injury both in vitro and in vivo, while the EVS synthesis inhibitor GW4968 attenuated HS-induced liver injury [78]. Furthermore, the proteomic analysis suggested that the regulation of programmed cell death was the most distinctly altered pathway [78]. Necroptosis is another form of programmed cell death that was controlled by receptor-interacting protein 1 (RIP1), RIP3, and MLKL and was caspase-independent [79]. Not the same as apoptosis, necroptosis is accompanied by an increase in plasma membrane permeability, which can lead to the release of DAMPs, such as HMGB1 and mitochondrial DNA [80], that trigger powerful immune and inflammatory responses [81, 82]. Both in vitro and in vivo experiments confirmed that EVS of hepatocytes in HS can promote necroptosis and apoptosis.
Exosomes are EVS that mediate the transport of a variety of bioactive molecules that regulate the function of target cells [83, 84]. Hepatocytes are the main source of exocrine bodies in the liver. Studies have demonstrated that hepatocytes are not only victims of harmful stimuli, but also actively participate in liver injury by releasing “danger signals” [85].
The dysregulation of Kupffer cells
Liver-resident macrophages/Kupffer cells (KCs) are central to maintaining liver homeostasis [86]. In response to heat stress in the liver, KCs exert a key role in the clearance of gut-derived endotoxin by phagocytosis, while the dysfunction of KCs is the main explanation for the elevated endotoxin concentration [87]. However, at the same time, KCs are also the main source of inflammatory factors in the liver [88]. TNF-α, IL-β, and IL-6 secreted by KCs were significantly increased in HS, and inhibiting the secretion of KCs appeal-related factors can effectively alleviate HS-induced liver injury [89].
Macrophage inflammatory protein-1α (MIP-1α) is an important chemokine in the inflammatory response, which can effectively activate immune cells, and regulate the synthesis of cytokines [90]. The various immune cells, including neutrophils, macrophages, and lymphocytes, are important sources of the production of MIP-1α [91]. Accounting for 80–90% of the total resident macrophages in vivo, KCs are the main source of the MIP-1α in the liver [92]. The previous studies have suggested that MIP-1α secreted by KCs was related to the excessive inflammation that leads to MODS in ischemia–reperfusion injury. Further evidence showed that MIP-1α can promote peritoneal macrophages to secrete tumor TNF-α, IL-1β, and IL-6 [93]. Wang et al. found that there were elevated MIP-1α expression and TNF-α, IL-1β, and IL-6 in the KCs in the HS-induced liver injury, while the activation of JNK signaling is required for this pathological process. JNK plays an important role in the inflammation response, cell apoptosis, and heat stress, especially in the KCs [94, 95]. In vitro and in vivo, after inhibiting JNK phosphorylation in KCs with sp600125, the inflammatory cytokines and MIP-1 produced by KCs were significantly reduced [96]. Interestingly, the inhibition of JNK phosphorylation level and MIP-1α was accompanied by the increase in KCs phagocytosis.
The dysregulation of heat shock protein.
Different defense systems protect cells from heat injury, the most important of which is the increased expression of HSP [97, 98]. The increased expression of HSP level is related to the better prognosis of HS patients [99]. HSP is a family of highly conserved proteins that function as molecular chaperones by promoting protein folding and refolding, mediating transmembrane transport of some secreted proteins, and targeting proteins for lysosomal degradation [97]. Among them, the expression of HSP70 can effectively prevent organ damage [100]. The pre-treatment of 17‐dimethylaminoethylamino‐17‐demethoxy‐geldanamycin(17‐DMAG) could upregulate the expression of HSP70 in the liver, thereby significantly alleviating the HS-induced liver dysfunction in rats [101]. Furthermore, the symptoms of hypotension and tachycardia could also be markedly improved.
As is a highly conserved nuclear zinc-finger DNA-binding protein, the enzyme poly(ADP-ribose) polymerase-1 (PARP-1) has long been considered the key factor of DNA damage sensor and DNA repair system [102]. In recent years, it has been shown that genetic deletion or pharmacologic blockade of PARP-1 could significantly inhibit the excessive inflammation response [103,104,105]. Furthermore, the inhibition of PARP can reduce various forms of liver injury [106, 107]. In HS-induced liver injury, PARP blockage has also emerged as a promising treatment [108]. The level of IL-1β and IL-6 expression in PARP−/− mice and mice treated with PARP inhibitors was significantly lower than that in control mice after heat exposure. The PARP inhibition could significantly increase the expression of HSP70 and HSP27 at messenger RNA and protein levels. Although the upregulation of HSP protein and the inhibition of PARP showed consistency in HS-induced liver injury, the exact molecular mechanism remains unclear. José Yélamos et al. proposed two possible explanations. One possibility is that PARP-1, as a part of the complex of histone variant mH2A1.1, is related to the hsp70.1 promoter, which can inactivate the expression of hsp70.1 [109]. Another possibility is that PARP-1 destroys the DNA binding of heat shot factor-1 and the promoter of HSP genes, which has been confirmed in fibroblasts [110].
The treatment strategy for HS-induced liver
Although proper hypothermia and active treatment have certain effects, the liver injury still occurs frequently in HS patients and acts as a direct cause of death [10]. As a life-threatening condition, patients with HS could be fatal if appropriate evaluation and treatment are not initiated promptly. Many active intervention strategies have been proven to have positive effects on improving the symptoms and prognosis of HS patients. Here, we focus more on the published research on the treatment of HS-induced liver injury to make a more in-depth and systematic exposition.
Systematic treatment
As a systemic disease, the treatment of HS currently relies more on supportive treatment [111]. Medical therapy and supportive treatment are the first-line treatment strategies for HS. We summarized the main and generally accepted treatment strategies for HS at present, as follows:
Cooling Early and rapid cooling is of great significance to the prevention of irreversible tissue damage and improves the prognosis of HS patients [112, 113]. Various cooling strategies including the alcohol bath, hibernation mixture, bedside blood filtration, and cold liquid intravenous drip can rapidly reduce the patient's temperature. There is no evidence to support the superiority of any cooling technology in HS. The effects of non-invasive, evaporative, or conductive-based cooling techniques, whether alone or in combination, seem comparable [112].
Early oxygen supply Cerebral ischemia and hypoxia, and nervous system dysfunction are important characteristics of patients with HS [114]. Hyperbaric oxygen therapy (HBOT) is an effective method to treat brain injury. HBOT can improve circulation, significantly improve tissue ischemia and hypoxia, and reduce brain edema. In addition, HBOT can also exert other positive molecular biological effects, such as anti-inflammatory and antioxidant stress, and inhibit apoptosis [115]. So when the patient's vital signs are stable, hyperbaric oxygen treatment can be carried out as soon as possible.
Continuous blood purification (CBP) Simple plasma exchange (PE), continuous hemodialysis filtration (CHDF), and their combination are commonly used to treat severe HS with MODS [116,117,118]. With the help of cardiopulmonary bypass technology, CBP can achieve an ideal cooling effect compared with traditional physical cooling. It can not only reduce the catabolism of the body but also effectively eliminate the inflammatory mediators such that IL-1 and IL-2 can maintain the homeostasis of the body, which is helpful for the recovery of HS patients. CBP can significantly improve the prognosis of patients with HS, improve the survival rate of patients, and is an important means of treating HS [119].
Anticoagulant therapy In the early stage of the onset of HS patients, high fever leads to the loss of a large number of body fluids, resulting in blood concentration. At the same time, hyperthermia causes microvascular endothelial cell damage, leading to the activation of the endogenous coagulation pathway. Subsequently, fibrin is deposited in arterioles and capillaries, which, together with platelet aggregation, leads to intravascular micro thrombosis.
Anti-inflammatory treatment Systemic inflammation and coagulopathy are the two main factors that cause life-threatening organ dysfunction during HS [120]. The pathophysiological mechanism of HS is similar to sepsis, and systemic inflammatory reaction is important pathogenesis. Many drugs that have been proven to have definite anti-inflammatory effects, such as dexmedetomidine and melatonin, have positive effects on improving multiple organ damage and the prognosis of HS [121,122,123].
Liver transplantation
Although the mild or moderate liver injury is the most common in HS patients [124], only a few patients have serious liver injury that may lead to fatal consequences [8]. In these cases, the drug treatment and other active supportive treatments are ineffective, and the liver injury caused by HS progresses to ALF with organ dysfunction. At this time, liver transplantation can provide a treatment option.
A clinical observational study showed that in patients with HS-induced liver injury requiring mechanical intubation and continuous renal replacement therapy, liver transplantation can immediately restore renal function [125], In the follow-up several years after the operation, the liver function was normal without any complications after transplantation.
According to Philippe Ichai et al., the classical standard of liver transplantation does not seem to apply to HS-induced ALF [113]. And they emphasized that the decision to implement LT should not be made hastily. Even in the case of severe liver failure, the fluctuation of prothrombin time and the clinical condition of patients should also be considered. They believed that PT lasted < 10% after the onset of heat stroke, and there was no sign of elevation after 3 days of median time, which was an important reference factor for LT implementation.
Discussion
HS is a syndrome with complex pathological mechanisms including microvascular injury, thrombosis, inflammation, and cell apoptosis [126]. Among the MODS caused by HS, the liver is considered to be one of the first organs to be damaged [127]. Liver damage occurs in nearly all cases of HS and is often the site of fatal damage [128]. Although a large number of clinical and basic medical researches on HS have been carried out in recent years, most of the underlying pathological mechanisms have not been precisely understood due to the complexity of HS. In the current clinical practice of HS treatment, more systemic treatments such as plasma exchange and hypothermia are used, but specific treatments for HS-induced ALI are lacking. The liver, as the key line of defense for the collective elimination of endotoxin, may obtain surprises with specific treatment for HS-induced ALI.
At present, the research on HS is mainly limited by two aspects. One is the complex pathological mechanism of HS. Secondly, although the incidence rate of HS is increasing year by year [129], the number of patients with HS, especially those with severe multiple organ dysfunction and even death, is still relatively less. Therefore, at present, there is no large cohort of clinical studies to provide accurate evidence-based medical evidence for the pathogenesis and treatment of HS. For example, although it is generally recognized that early and rapid cooling treatment is of positive significance for patients with HS. However, so far, no evidence has been found for a specific end-point temperature at which cooling can be safely stopped. The previous studies have shown that the conduction-based cooling method, that is, immersion in ice water has a positive effect on labor heat stroke, and Wang et al. proposed the use of 38.6° C as a safe rectal temperature cooling limit for the cold water bath, Wang et al.'s research is based on the subjects whose core temperature reaches 39.5 through temperature and exercise, not HS patients [130].
In brief, the mechanism of HS-induced liver injury is not only related to systemic factors such as systemic inflammatory reaction and coagulation dysfunction but also closely related to pathological mechanisms such as abnormal death of liver cells and abnormal function of KCs. Therefore, for the treatment of HS-induced liver injury, we should not only implement systematic and supportive treatment but also target the liver for precise treatment. We need to conduct more and more accurate research to reveal the mechanism of HS-induced and find effective treatment strategies.
Future perspective
In the past decades, a large number of studies on the pathogenesis and treatment strategies of HS have indeed revealed many possible pathogenesis and treatment targets; however, most of the studies are at the animal level. Besides liver transplantation, there are few liver-targeted therapies for HS patients. Therefore, in our view, the future focus of research on HS-induced liver injury is the clinical verification of relevant research, especially the data integration of liver-targeted therapy and its efficacy. Further studies, including clinical randomized controlled trials, are expected.
Availability of data and materials
Not applicable.
Abbreviations
- ALI:
-
Acute liver injury
- ALF:
-
Acute liver failure
- APC:
-
Activated protein C
- CHS:
-
Classic heat stroke
- CHDF:
-
Continuous hemodialysis filtration
- DAMPs:
-
Damage-associated molecular patterns
- EVS:
-
Extracellular vesicles
- EHS:
-
External heat stroke
- HSP:
-
Heat shock protein
- HSF1:
-
Heat shock transcription factor 1
- HS:
-
Heat stroke
- HMGB1:
-
High mobility group box 1
- HBOT:
-
Hyperbaric oxygen therapy
- I/R:
-
Ischemia/reperfusion
- IL:
-
Interleukin
- KCs:
-
Kupffer cells
- MIP-1α:
-
Macrophage inflammatory protein-1α
- MLKL:
-
Mixed lineage kinase domain-like
- MODS:
-
Multiple organ system failure
- NLRP3:
-
NOD-like receptor family pyrin domain containing3
- PARP-1:
-
Poly(ADP-ribose) polymerase-1
- RAGE:
-
Receptor for advanced glycation end products
- RIP1:
-
Receptor-interacting protein 1
- RIPK3:
-
Receptor-interacting protein kinase 3
- RAS:
-
Renin-angiotensin system
- PE:
-
Simple plasma exchange
- SIRS:
-
Systemic inflammatory response syndrome
- TM:
-
Thrombomodulin
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor
- ZBP1:
-
Z-DNA binding protein 1
- 17‐DMAG:
-
17‐Dimethylaminoethylamino‐17‐demethoxy‐geldanamycin
References
Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O’Connor FG, et al. Classic and exertional heatstroke. Nat Rev Dis Prim. 2022;8(1):8.
Tsai HY, Hsu YJ, Lu CY, Tsai MC, Hung WC, Chen PC, et al. Pharmacological activation of aldehyde dehydrogenase 2 protects against heatstroke-induced acute lung injury by modulating oxidative stress and endothelial dysfunction. Front Immunol. 2021;12: 740562.
Epstein Y, Yanovich R. Heatstroke. N Engl J Med. 2019;380(25):2449–59.
Davis BC, Tillman H, Chung RT, Stravitz RT, Reddy R, Fontana RJ, et al. Heat stroke leading to acute liver injury & failure: a case series from the acute liver failure study group. Liver Int Off J Int Assoc Study Liver. 2017;37(4):509–13.
Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341(8):586–92.
Bouchama A, Ollivier V, Roberts G, Al Mohanna F, de Prost D, Eldali A, et al. Experimental heatstroke in baboon: analysis of the systemic inflammatory response. Shock (Augusta, Ga). 2005;24(4):332–5.
Hassanein T, Razack A, Gavaler JS, Van Thiel DH. Heatstroke: its clinical and pathological presentation, with particular attention to the liver. Am J Gastroenterol. 1992;87(10):1382–9.
Bianchi L, Ohnacker H, Beck K, Zimmerli-Ning M. Liver damage in heatstroke and its regression. A biopsy study. Human Pathol. 1972;3(2):237–48.
Kew M, Bersohn I, Seftel H, Kent G. Liver damage in heatstroke. Am J Med. 1970;49(2):192–202.
Weigand K, Riediger C, Stremmel W, Flechtenmacher C, Encke J. Are heat stroke and physical exhaustion underestimated causes of acute hepatic failure? World J Gastroenterol. 2007;13(2):306–9.
Kew MC, Minick OT, Bahu RM, Stein RJ, Kent G. Ultrastructural changes in the liver in heatstroke. Am J Pathol. 1978;90(3):609–18.
Camus G, Deby-Dupont G, Duchateau J, Deby C, Pincemail J, Lamy M. Are similar inflammatory factors involved in strenuous exercise and sepsis? Intensive Care Med. 1994;20(8):602–10.
Garcin JM, Bronstein JA, Cremades S, Courbin P, Cointet F. Acute liver failure is frequent during heat stroke. World J Gastroenterol. 2008;14(1):158–9.
Frise CJ, Williamson C. Gastrointestinal and liver disease in pregnancy. Clin Med (London, England). 2013;13(3):269–74.
Sato A, Ikawa Y, Inoue N, Kuroda M, Shimizu M, Yachie A. Massive intestinal liquid retention in a case of severe heat stroke. J Paediatr Child Health. 2019;55(2):248–9.
Gathiram P, Wells MT, Raidoo D, Brock-Utne JG, Gaffin SL. Changes in lipopolysaccharide concentrations in hepatic portal and systemic arterial plasma during intestinal ischemia in monkeys. Circ Shock. 1989;27(2):103–9.
Yu J, Liu F, Yin P, Zhao H, Luan W, Hou X, et al. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Stress (Amsterdam, Netherlands). 2013;16(1):99–113.
Oliver SR, Phillips NA, Novosad VL, Bakos MP, Talbert EE, Clanton TL. Hyperthermia induces injury to the intestinal mucosa in the mouse: evidence for an oxidative stress mechanism. Am J Physiol Regul Integr Comp Physiol. 2012;302(7):R845–53.
Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G204–12.
Yang WR, Li BB, Hu Y, Zhang L, Wang XZ. Oxidative stress mediates heat-induced changes of tight junction proteins in porcine sertoli cells via inhibiting CaMKKβ-AMPK pathway. Theriogenology. 2020;142:104–13.
Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–88.
Lu KC, Wang JY, Lin SH, Chu P, Lin YF. Role of circulating cytokines and chemokines in exertional heatstroke. Crit Care Med. 2004;32(2):399–403.
Bouchama A, Al-Sedairy S, Siddiqui S, Shail E, Rezeig M. Elevated pyrogenic cytokines in heatstroke. Chest. 1993;104(5):1498–502.
Bouchama A, Parhar RS, El-Yazigi A, Sheth K, Al-Sedairy S. Endotoxemia and release of tumor necrosis factor and interleukin 1 alpha in acute heatstroke. J Appl Physiol (Bethesda, Md: 1985). 1991;70(6):2640–4.
Chang DM. The role of cytokines in heat stroke. Immunol Invest. 1993;22(8):553–61.
Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255(3):320–31.
Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33(3):564–73.
Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med (Cambridge, Mass). 2006;12(4–6):105–14.
Tong HS, Tang YQ, Chen Y, Qiu JM, Wen Q, Su L. Early elevated HMGB1 level predicting the outcome in exertional heatstroke. J Trauma. 2011;71(4):808–14.
Todani M, Fujita M, Tsuruta R, Nakahara T, Yagi T, Oshima C, et al. Moderate hypothermia suppressed excessive generation of superoxide anion radical and inflammatory reactions in blood and liver in heatstroke: laboratory study in rats. Free Radic Res. 2010;44(4):462–72.
Hagiwara S, Iwasaka H, Shingu C, Matsumoto S, Uchida T, Noguchi T. High-dose antithrombin III prevents heat stroke by attenuating systemic inflammation in rats. Inflamm Res. 2010;59(7):511–8.
Hagiwara S, Iwasaka H, Goto K, Ochi Y, Mizunaga S, Saikawa T, et al. Recombinant thrombomodulin prevents heatstroke by inhibition of high-mobility group box 1 protein in sera of rats. Shock (Augusta, Ga). 2010;34(4):402–6.
Tong H, Tang Y, Chen Y, Yuan F, Liu Z, Peng N, et al. HMGB1 activity inhibition alleviating liver injury in heatstroke. J Trauma Acute Care Surg. 2013;74(3):801–7.
Geng Y, Ma Q, Liu YN, Peng N, Yuan FF, Li XG, et al. Heatstroke induces liver injury via IL-1β and HMGB1-induced pyroptosis. J Hepatol. 2015;63(3):622–33.
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science (New York, NY). 1999;285(5425):248–51.
Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–5.
Müller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, et al. New EMBO members; review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20(16):4337–40.
Kawasaki T, Okamoto K, Kawasaki C, Sata T. Thrombomodulin improved liver injury, coagulopathy, and mortality in an experimental heatstroke model in mice. Anesth Analg. 2014;118(5):956–63.
Huisse MG, Pease S, Hurtado-Nedelec M, Arnaud B, Malaquin C, Wolff M, et al. Leukocyte activation: the link between inflammation and coagulation during heatstroke. A study of patients during the 2003 heat wave in Paris. Crit Care Med. 2008;36(8):2288–95.
Bouchama A, Roberts G, Al Mohanna F, El-Sayed R, Lach B, Chollet-Martin S, et al. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J Appl Physiol (Bethesda, Md: 1985). 2005;98(2):697–705.
Suzuki K, Kusumoto H, Deyashiki Y, Nishioka J, Maruyama I, Zushi M, et al. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987;6(7):1891–7.
Esmon CT, Esmon NL, Harris KW. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982;257(14):7944–7.
Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–30.
Grey ST, Tsuchida A, Hau H, Orthner CL, Salem HH, Hancock WW. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J Immunol (Baltimore, Md: 1950). 1994;153(8):3664–72.
Esmon CT. The regulation of natural anticoagulant pathways. Science (New York, NY). 1987;235(4794):1348–52.
Chen CM, Hou CC, Cheng KC, Tian RL, Chang CP, Lin MT. Activated protein C therapy in a rat heat stroke model. Crit Care Med. 2006;34(7):1960–6.
Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91(1):1–77.
Brinton MR, Tagge CA, Stewart RJ, Cheung AK, Shiu YT, Christensen DA. Thermal sensitivity of endothelial cells on synthetic vascular graft material. Int J Hyperth Off J Eur Soc Hyperth Oncol North Am Hyperth Group. 2012;28(2):163–74.
Gu ZT, Wang H, Li L, Liu YS, Deng XB, Huo SF, et al. Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci Rep. 2014;4:4469.
Tong H, Wan P, Zhang X, Duan P, Tang Y, Chen Y, et al. Vascular endothelial cell injury partly induced by mesenteric lymph in heat stroke. Inflammation. 2014;37(1):27–34.
Liu J, Zhu G, Xu S, Liu S, Lu Q, Tang Z. Analysis of miRNA expression profiling in human umbilical vein endothelial cells affected by heat stress. Int J Mol Med. 2017;40(6):1719–30.
Xu Q, Liu J, Guo X, Tang Y, Zhou G, Liu Y, et al. Xuebijing injection reduces organ injuries and improves survival by attenuating inflammatory responses and endothelial injury in heatstroke mice. BMC Complement Altern Med. 2015;15:4.
Chen F, Li H, Zhu G, Chen X, Tang Z. Sodium tanshinone IIA sulfonate improves inflammation, aortic endothelial cell apoptosis, disseminated intravascular coagulation and multiple organ damage in a rat heat stroke model. Mol Med Rep. 2017;16(1):87–94.
Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243(1):206–14.
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108(49):19725–30.
Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology (Baltimore, MD). 2014;59(3):898–910.
Liu Y, Wang Z, Xie W, Gu Z, Xu Q, Su L. Oxidative stress regulates mitogen-activated protein kinases and c-Jun activation involved in heat stress and lipopolysaccharide-induced intestinal epithelial cell apoptosis. Mol Med Rep. 2017;16(3):2579–87.
Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5.
Zhang M, Zhu X, Tong H, Lou A, Li Y, Li Y, et al. AVE 0991 attenuates pyroptosis and liver damage after heatstroke by inhibiting the ROS-NLRP3 inflammatory signalling pathway. Biomed Res Int. 2019;2019:1806234.
Jiang T, Tan L, Gao Q, Lu H, Zhu XC, Zhou JS, et al. Plasma angiotensin-(1–7) is a potential biomarker for Alzheimer’s disease. Curr Neurovasc Res. 2016;13(2):96–9.
Huang F, Guo J, Zou Z, Liu J, Cao B, Zhang S, et al. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun. 2014;5:3595.
Pan Z, Shao Y, Dong W, Liu C, Chen Y, Jin H, et al. Xuebijing attenuates hypotension through the upregulation of angiotensin II type 1 receptor-associated protein 1 in rats suffering from heat stroke. Int J Mol Med. 2014;34(6):1699–705.
Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH 3rd, Ingram JP, et al. RIPK3 activates parallel pathways of MLKL-driven necroptosis and FADD-mediated apoptosis to protect against influenza A virus. Cell Host Microbe. 2016;20(1):13–24.
Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, Eren RO, et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. 2020;580(7803):391–5.
Ke PY. Mitophagy in the pathogenesis of liver diseases. Cells. 2020;9(4):831.
Williams JA, Ding WX. Targeting Pink1-Parkin-mediated mitophagy for treating liver injury. Pharmacol Res. 2015;102:264–9.
Youle RJ. Mitochondria—Striking a balance between host and endosymbiont. Science (New York, NY). 2019;365(6454):eaaw9855.
Samaiya PK, Krishnamurthy S, Kumar A. Mitochondrial dysfunction in perinatal asphyxia: role in pathogenesis and potential therapeutic interventions. Mol Cell Biochem. 2021;476(12):4421–34.
Ma X, McKeen T, Zhang J, Ding WX. Role and mechanisms of mitophagy in liver diseases. Cells. 2020;9(4):837.
Engeland K. Cell cycle regulation: p53–p21-RB signaling. Cell Death Differ. 2022;29(5):946–60.
Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med. 2016;6(3): a026104.
Huang W, Xie W, Zhong H, Cai S, Huang Q, Liu Y, et al. Cytosolic p53 inhibits parkin-mediated mitophagy and promotes acute liver injury induced by heat stroke. Front Immunol. 2022;13: 859231.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17.
Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol. 2016;65(1):213–21.
Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64(3):651–60.
Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28(5):1551–8.
Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900.
Li Y, Zhu X, Wang G, Tong H, Su L, Li X. Proteomic analysis of extracellular vesicles released from heat-stroked hepatocytes reveals promotion of programmed cell death pathway. Biomed Pharmacother. 2020;129:110489.
Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: mechanisms and relevance to disease. Annu Rev Pathol. 2017;12:103–30.
Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–23.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–21.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–20.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11.
Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27(1):31–9.
Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS ONE. 2014;9(12): e113651.
Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17(5):306–21.
Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12(46):7413–20.
Fukuda T, Mogami A, Tanaka H, Yoshikawa T, Hisadome M, Komatsu H. Y-40138, a multiple cytokine production modulator, protects against D-galactosamine and lipopolysaccharide-induced hepatitis. Life Sci. 2006;79(9):822–7.
Chen Y, Tong H, Zhang X, Tang L, Pan Z, Liu Z, et al. Xuebijing injection alleviates liver injury by inhibiting secretory function of Kupffer cells in heat stroke rats. J Tradit Chin Med. 2013;33(2):243–9.
Giribaldi G, Prato M, Ulliers D, Gallo V, Schwarzer E, Akide-Ndunge OB, et al. Involvement of inflammatory chemokines in survival of human monocytes fed with malarial pigment. Infect Immun. 2010;78(11):4912–21.
Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu JT, Schwacha MG, et al. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol (Baltimore, Md: 1950). 2008;181(4):2806–12.
Liu LM, Liang DY, Ye CG, Tu WJ, Zhu T. The UII/UT system mediates upregulation of proinflammatory cytokines through p38 MAPK and NF-κB pathways in LPS-stimulated Kupffer cells. PLoS ONE. 2015;10(3): e0121383.
Fahey TJ 3rd, Tracey KJ, Tekamp-Olson P, Cousens LS, Jones WG, Shires GT, et al. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol (Baltimore, Md: 1950). 1992;148(9):2764–9.
Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77(13):1502–15.
Gonda RL, Garlena RA, Stronach B. Drosophila heat shock response requires the JNK pathway and phosphorylation of mixed lineage kinase at a conserved serine-proline motif. PLoS ONE. 2012;7(7): e42369.
Chen XJ, Tang ZZ, Zhu GG, Cheng Q, Zhang WK, Li HM, et al. JNK signaling is required for the MIP-1α-associated regulation of Kupffer cells in the heat stroke response. Mol Med Rep. 2017;16(3):2389–96.
Lee WC, Wen HC, Chang CP, Chen MY, Lin MT. Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J Appl Physiol (Bethesda, Md: 1985). 2006;100(6):2073–82.
Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock (Augusta, Ga). 2006;25(3):295–9.
Wang ZZ, Wang CL, Wu TC, Pan HN, Wang SK, Jiang JD. Autoantibody response to heat shock protein 70 in patients with heatstroke. Am J Med. 2001;111(8):654–7.
Lam KK, Cheng PY, Lee YM, Liu YP, Ding C, Liu WH, et al. The role of heat shock protein 70 in the protective effect of YC-1 on heat stroke rats. Eur J Pharmacol. 2013;699(1–3):67–73.
Tsai YC, Lam KK, Peng YJ, Lee YM, Yang CY, Tsai YJ, et al. Heat shock protein 70 and AMP-activated protein kinase contribute to 17-DMAG-dependent protection against heat stroke. J Cell Mol Med. 2016;20(10):1889–97.
Ding L, Chen X, Xu X, Qian Y, Liang G, Yao F, et al. PARP1 suppresses the transcription of PD-L1 by Poly(ADP-Ribosyl)ating STAT3. Cancer Immunol Res. 2019;7(1):136–49.
Shen B, Mei M, Pu Y, Zhang H, Liu H, Tang M, et al. Necrostatin-1 attenuates renal ischemia and reperfusion injury via meditation of HIF-1α/mir-26a/TRPC6/PARP1 signaling. Mol Ther Nucleic Acids. 2019;17:701–13.
Zhang JN, Ma Y, Wei XY, Liu KY, Wang H, Han H, et al. Remifentanil protects against Lipopolysaccharide-Induced Inflammation through PARP-1/NF-κB Signaling Pathway. Mediat Inflamm. 2019;2019:3013716.
El-Hamoly T, Hajnády Z, Nagy-Pénzes M, Bakondi E, Regdon Z, Demény MA, et al. Poly(ADP-Ribose) polymerase 1 promotes inflammation and fibrosis in a mouse model of chronic pancreatitis. Int J Mol Sci. 2021;22(7):3593.
Mukhopadhyay P, Horváth B, Rajesh M, Varga ZV, Gariani K, Ryu D, et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J Hepatol. 2017;66(3):589–600.
Wang S, Shi XL, Feng M, Wang X, Zhang ZH, Zhao X, et al. Puerarin protects against CCl4-induced liver fibrosis in mice: possible role of PARP-1 inhibition. Int Immunopharmacol. 2016;38:238–45.
Mota RA, Hernández-Espinosa D, Galbis-Martinez L, Ordoñez A, Miñano A, Parrilla P, et al. Poly(ADP-ribose) polymerase-1 inhibition increases expression of heat shock proteins and attenuates heat stroke-induced liver injury. Crit Care Med. 2008;36(2):526–34.
Ouararhni K, Hadj-Slimane R, Ait-Si-Ali S, Robin P, Mietton F, Harel-Bellan A, et al. The histone variant mH2A1.1 interferes with transcription by down-regulating PARP-1 enzymatic activity. Genes Dev. 2006;20(23):3324–36.
Fossati S, Formentini L, Wang ZQ, Moroni F, Chiarugi A. Poly(ADP-ribosyl)ation regulates heat shock factor-1 activity and the heat shock response in murine fibroblasts. Biochem Cell Biol. 2006;84(5):703–12.
Azzopardi N, Chetcuti S, Sant J, Pocock J. Acute liver impairment in a young, healthy athlete: hypoxic hepatitis and rhabdomyolysis following heat stroke. Case Rep Gastroenterol. 2012;6(2):563–8.
Bouchama A, Dehbi M, Chaves-Carballo E. Cooling and hemodynamic management in heatstroke: practical recommendations. Crit Care (London, England). 2007;11(3):R54.
Ichai P, Laurent-Bellue A, Camus C, Moreau D, Boutonnet M, Saliba F, et al. Liver transplantation in patients with liver failure related to exertional heatstroke. J Hepatol. 2019;70(3):431–9.
Tsai HM, Gao CJ, Li WX, Lin MT, Niu KC. Resuscitation from experimental heatstroke by hyperbaric oxygen therapy. Crit Care Med. 2005;33(4):813–8.
Ni X, Liu Z, Xie Q, Tong H, Su L, Yu R. Cerebral injury induced by heat stroke and the therapeutic effect of hyperbaric oxygen therapy. Zhonghua wei zhong bing ji jiu yi xue. 2017;29(6):572–6.
Chen KJ, Chen TH, Sue YM, Chen TJ, Cheng CY. High-volume plasma exchange in a patient with acute liver failure due to non-exertional heat stroke in a sauna. J Clin Apheresis. 2014;29(5):281–3.
Wakino S, Hori S, Mimura T, Fujishima S, Hayashi K, Inamoto H, et al. Heat stroke with multiple organ failure treated with cold hemodialysis and cold continuous hemodiafiltration: a case report. Ther Apheresis Dial Off Peer Rev J Int Soc Apheresis Jpn Soc Apheresis JpN Soc Dial Ther. 2005;9(5):423–8.
Raj VM, Alladin A, Pfeiffer B, Katsoufis C, Defreitas M, Edwards-Richards A, et al. Therapeutic plasma exchange in the treatment of exertional heat stroke and multiorgan failure. Pediatr Nephrol (Berlin, Germany). 2013;28(6):971–4.
Lu B, Li MQ, Cheng SL. Clinical effectiveness of continuous blood purification in combination with ulinastatin in treating thermoplegia. Eur Rev Med Pharmacol Sci. 2014;18(22):3464–7.
Iba T, Connors JM, Levi M, Levy JH. Heatstroke-induced coagulopathy: biomarkers, mechanistic insights, and patient management. EClinicalMedicine. 2022;44: 101276.
Kobayashi K, Mimuro S, Sato T, Kobayashi A, Kawashima S, Makino H, et al. Dexmedetomidine preserves the endothelial glycocalyx and improves survival in a rat heatstroke model. J Anesth. 2018;32(6):880–5.
Tian YF, Lin CH, Hsu SF, Lin MT. Melatonin improves outcomes of heatstroke in mice by reducing brain inflammation and oxidative damage and multiple organ dysfunction. Mediat Inflamm. 2013;2013: 349280.
Yang HH, Hou CC, Lin MT, Chang CP. Attenuating heat-induced acute lung inflammation and injury by dextromethorphan in rats. Am J Respir Cell Mol Biol. 2012;46(3):407–13.
Glazer JL. Management of heatstroke and heat exhaustion. Am Fam Physician. 2005;71(11):2133–40.
Bi X, Deising A, Frenette C. Acute liver failure from exertional heatstroke can result in excellent long-term survival with liver transplantation. Hepatology (Baltimore, MD). 2020;71(3):1122–3.
Roberts GT, Ghebeh H, Chishti MA, Al-Mohanna F, El-Sayed R, Al-Mohanna F, et al. Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke: a study in baboon model. Arterioscler Thromb Vasc Biol. 2008;28(6):1130–6.
Ji J, Gao J, Wang C, Ouyang L, Liu Z, Liu Z. Characteristics and outcome of exertional heatstroke patients complicated by acute hepatic injury: a cohort study. J Clin Transl Hepatol. 2021;9(5):655–60.
Argaud L, Ferry T, Le QH, Marfisi A, Ciorba D, Achache P, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Internal Med. 2007;167(20):2177–83.
Gubernot DM, Anderson GB, Hunting KL. The epidemiology of occupational heat exposure in the United States: a review of the literature and assessment of research needs in a changing climate. Int J Biometeorol. 2014;58(8):1779–88.
Gagnon D, Lemire BB, Casa DJ, Kenny GP. Cold-water immersion and the treatment of hyperthermia: using 38/6°C as a safe rectal temperature cooling limit. J Athl Train. 2010;45(5):439–44.
Acknowledgements
Pictures are prepared by Figraw (www.figraw. com).
Funding
The study was supported by the Major Technological Innovation Special Project of Hubei Province of China (2019ACA167).
Author information
Authors and Affiliations
Contributions
Fuquan Wang, Yan Zhang and Jianhua Li found the relevant research basis and wrote this article. Fuquan Wang and Haifa Xia made the relevant figures. Shanglong Yao and Dingyu Zhang designed the review and revised the article. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing financial interests.
Consent for publication
All listed authors consent to the submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, F., Zhang, Y., Li, J. et al. The pathogenesis and therapeutic strategies of heat stroke-induced liver injury. Crit Care 26, 391 (2022). https://doi.org/10.1186/s13054-022-04273-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04273-w