Abstract
Background
Droplet digital PCR (ddPCR) has emerged as a promising tool of pathogen detection in bloodstream infections (BSIs) in critical care medicine. However, different ddPCR platforms have variable sensitivity and specificity for diverse microorganisms at various infection sites. There is still a lack of prospective clinical studies aimed at validating and interpreting the discrepant ddPCR results for diagnosing BSI in intensive care unit (ICU) practice.
Methods
A prospective diagnostic study of multiplex ddPCR panels was conducted in a general ICU from May 21, 2021, to December 22, 2021. Paired blood cultures (BCs) and ddPCRs (2.5 h) were obtained synchronously to detect the 12 most common BSI pathogens and three antimicrobial resistance (AMR) genes. Firstly, ddPCR performance was compared to definite BSI. Secondly, clinical validation of ddPCR was compared to composite clinical diagnosis. Sensitivity, specificity, and positive and negative predictive values were calculated. Thirdly, the positive rate of AMR genes and related analysis was presented.
Results
A total of 438 episodes of suspected BSIs occurring in 150 critical patients were enrolled. BC and ddPCR were positive for targeted bacteria in 40 (9.1%) and 180 (41.1%) cases, respectively. There were 280 concordant and 158 discordant. In comparison with BCs, the sensitivity of ddPCR ranged from 58.8 to 86.7% with an aggregate of 72.5% in different species, with corresponding specificity ranging from 73.5 to 92.2% with an aggregate of 63.1%. Furthermore, the rate of ddPCR+/BC− results was 33.6% (147/438) with 87.1% (128 of 147) cases was associated with probable (n = 108) or possible (n = 20) BSIs. When clinically diagnosed BSI was used as true positive, the final sensitivity and specificity of ddPCR increased to 84.9% and 92.5%, respectively. In addition, 40 blaKPC, 3blaNDM, and 38 mecA genes were detected, among which 90.5% were definitely positive for blaKPC. Further, 65.8% specimens were predicted to be mecA-positive in Staphylococcus sp. according to all microbiological analysis.
Conclusions
The multiplexed ddPCR is a flexible and universal platform, which can be used as an add-on complementary to conventional BC. When combined with clinical infection evidence, ddPCR shows potential advantages for rapidly diagnosing suspected BSIs and AMR genes in ICU practice.
Similar content being viewed by others
Background
Bacterial bloodstream infections (BSIs) and associated sepsis/septic shock are one of the leading causes of mortality in critically ill patients whose physical and immune barriers are disrupted [1]. This condition is further complicated by the increasing global burden of antimicrobial resistance and inappropriate use of antibiotic drugs [2]. International Guidelines for Management of Sepsis and Septic Shock 2021 recommend the administration of antimicrobials immediately, ideally within 1 h of diagnosis [3]. Therefore, rapid diagnosis and early administration of appropriate antimicrobials are crucial determinants in improving prognosis and decreasing the all-cause mortality rate in BSIs, especially in the case of drug-resistant bacterial infections [1]. Conventional blood culture (BC) is the gold standard for causative pathogen identification and antimicrobial susceptibilities test (AST) in the diagnosis of BSIs. However, this method is limited by suboptimal sensitivity ranging from ≤ 10% to about 50% in patients with suspected bacteremia, febrile neutropenia, or sepsis/septic shock [4]. This is primarily due to low levels of circulating microorganisms, slow growing microorganisms, and long turnaround times. Hence, the development of accurate BSI diagnostic tools for rapid pathogen detection and antibiotic stewardship in endemic regions such as emergency departments and intensive care units (ICUs) is a top priority.
In contrast to molecular tests performed on bacterial isolates, attempts have been made to directly detect pathogens and resistance markers in blood samples without prior incubation to improve BSI diagnosis [5, 6], including multiplex real-time polymerase chain reaction (PCR)-based MagicPlex® Sepsis Test [7], PCR combined with T2 magnetic resonance-based T2Candida [8] and T2Bacteria panel [9], metagenomics-based assays (LightCycler®SeptiFast) [10], and even next-generation sequencing [11]. However, several of these technologies are still limited and have been abandoned in the clinical environment because of medium sensitivity/specificity, high operational costs, and incapable of performing ASTs [6, 12]. Recently, digital polymerase chain reactions (dPCRs), the third-generation PCR after real-time quantitative PCR (qPCR), have been developed to offer a number of technical advantages to address these challenges [13, 14]. Mechanically speaking, a PCR Master Mix was divided into thousands of partitions using emulsified microdroplets suspended in oil (i.e., droplet digital PCR; ddPCR), followed by PCR amplification of target genes in each individual partition, thereby acting as an individual microreactor. In summary, ddPCR is less affected by PCR inhibitors and more sensitive to microbial genes, thus leading to higher sensitivity and precision. Moreover, this technology offers high reproducibility and provides absolute quantification without the need for a standard curve [15]. Various dPCR platforms have been developed in different areas [16,17,18]. In the field of critical care medicine, the functions of dPCR are attractive and have been proposed as a potential tool for pathogen identification in blood or other clinical samples, severity assessment, prognosis, treatment guidance, and profile host responses to infection [15]. Nevertheless, there is still a lack of prospective studies aimed to validate ddPCR performance in BSI diagnosis in ICU clinical practice.
In this study, we used a multiplex ddPCR panel to detect the most clinically relevant pathogens and related resistance genes in critically ill patients with suspected BSIs with a turnaround time of 2.5 h. We identified eight bacterial species involved with the most common “ESKAPE” pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Escherichia coli), as well as four fungi species (Candida parapsilosis, Candida tropicalis, Candida glabrata, and Candida albicans) and three resistance markers (blaKPC, blaNDM, and mecA). Herein, a single-center prospective study was performed in an integrated ICU to evaluate the concordance between ddPCR and conventional BC results. It is worth noting that the explanation of discrepancies results between the ddPCR and BC remains controversial. Notably, the disaccord results and clinical utility of ddPCR for diagnosing suspected BSIs were comprehensively interpreted according to all microbiological cultures and clinical evidence [9].
Methods
Study population
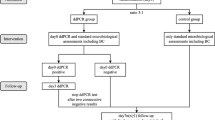
This study was a prospective pilot diagnostic study to clinically validate the multiplex ddPCR panel in diagnosing suspected BSIs in critically ill patients. This work was performed in the integrated ICU of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, from May 21, 2021, to December 22, 2021. Critically ill patients (older than 18 years) with suspected BSIs were eligible and consecutively recruited for the study (Fig. 1). Patients with mental disorders and pregnant women were excluded from the study. Contaminated or damaged samples were also eliminated. The study protocol allowed the inclusion of multiple episodes of suspected BSI occurring in one patient.
Sample collection and BSI diagnosis
Upon clinical suspicion of a BSI by training ICU physicians, two sets of blood cultures (both aerobic and anaerobic bottles, 10–20 mL per bottle) and at least 3 mL whole blood samples (EDTA blood collection tubes) were obtained from the same catheter or venipuncture for BSI diagnosis and ddPCR testing. Blood cultures were incubated for a maximum of 5 days (BD BACTEC FX; BD Biosciences), and pathogens in positive cultures were identified using matrix-assisted laser desorption ionization-time of flight mass spectrometry (bioMérieux, Marcy-l’Étoile, France) [19]. ASTs were performed using the VITEK2 compact system and interpreted according to the Clinical and Laboratory Standards Institute guidelines (M100-ED30) [20]. Conventional culturing results were analyzed in terms of pathogen detection and resistance patterns. Strains that showed resistance to imipenem or meropenem were identified as carbapenem-resistant. PCRs were performed to detect carbapenem-encoding resistance genes (blaKPC and blaNDM) [21]. Methicillin-resistant Staphylococcus sp. was speculated mecA-positive.
Plasma DNA Extraction and ddPCR testing
The multiplex ddPCR testing, which consists of five channels, allows for the detection of the eight most common bacterial pathogens, four fungal pathogens, and three antimicrobial resistance (AMR) genes directly from blood in amounts as small as 50 copies/mL (Pilot Gene Technologies. Hangzhou, China) (Fig. 2) [22]. The detection limit was determined by ddPCR detection of whole blood specimens spiked with multiple known concentrations of different microbial species. We found the detection sensitivity of ddPCR to be 50 copy units per mL (copies/mL), with the exception of blaKPC (80 copies/mL). Whole-blood samples were stored at 4 °C, and ddPCR testing was conducted on the same day or on the next day because of the timing of suspected BSI. Further, ddPCR testing procedures were performed for about 2.5 h according to the manufacturer’s protocol (Fig. 2). Samples were processed to plasma by centrifugation (1,600 r.c.f. for 15 min), and sample preparation took about 40 min. Next, the reaction mixture in the sample cup was passed through the micro-channel (Droplet Generator DG32) under the action of pressure, and tens of thousands of water-in-oil emulsion droplets were generated due to gravity and shear force in 20 min. After PCR amplification for 60 min by Thermal Cycler TC1, scanning and data analysis for droplet counts and amplitudes were performed within 30 min using a chip scanner CS5 and GenePMS software (v2.0.01.20011). The synthesized DNA fragment was used as positive control, and DNase-free water or blood samples from three healthy subjects were used as negative controls. The copies of each targeted pathogen or gene were reported by ddPCR results.
Definitions and clinical data
Culture-proven BSI is defined by positive blood cultures in a patient with systemic signs of infection and may be either secondary to a documented source or primary, according to the definitions released by National healthcare safety network (https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent) [1]. Clinical data were extracted from the patients’ medical records, including the demographic, comorbidities, organ dysfunction, surgical intervention, and clinical outcomes. Severity of disease was assessed by the Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring system. Results of ddPCRs and BCs were independently verified by two ICU physicians. Suspected infectious episodes of all routine microbiological cultures, including BC, abdominal, respiratory, skin and soft tissue, and other tissue/fluid cultures, were collected within 7 days of enrolment according to the standard microbiology laboratory procedures. A composite clinical infection standard was defined, consisting of all microbiological results plus clinical adjudication [9, 23].
Interpretation of BSI and ddPCR results
BSI results involved in the ddPCR-targeted pathogens or AMR genes were summarized for further data analysis. The ddPCR result was considered positive if 1 or more target bacteria were detected and negative if none were detected. Polymicrobial infection was defined as an episode in which more than one microorganism was detected by either ddPCR or blood culture. The BSI and ddPCR results were classified as concordant (both positive and negative) or discordant. The cases in which BCs were positive but ddPCR results were negative or different were defined as presumptive false-negative cases. To resolve discrepancies, discordant ddPCR+/BSI− results were classified as probable BSI, possible BSI, or presumptive false-positive cases similar to previous studies [9, 23]. The following definitions were used for each classification, (i) probable: ddPCR result was concordant with a microbiological test performed within seven days of sample collection from other extra-blood site; (ii) possible: without microbiological data but ddPCR result had potential for pathogenicity based on clinical presentation and laboratory findings; (iii) presumptive false-positive: ddPCR result was inconsistent with clinical presentation.
Statistical analysis
Primary outcomes were the sensitivity and specificity of ddPCR testing, which were calculated by comparing positive BC results with the ddPCR-targeted pathogens and AMR genes. The secondary outcomes were the clinical validation of the ddPCR testing for diagnosing suspected BSIs, which were compared with all microbiological cultures and the composite clinical diagnosis. Sensitivity, specificity, and positive and negative predictive values were calculated using those findings. For per-assay calculations, results for individual pathogens in each sample were considered separately. Statistical analysis was performed with IBM SPSS Statistics software (v 23.0) (IBM, Armonk, NY, USA). Continuous variables were expressed as the median and interquartile range (IQR). Categorical variables were reported as frequencies and percentages. The difference in positivity rate between BCs and ddPCRs was explored with the Chi-square test. Differences were considered to be statistically significant for if P values were ≤ 0.05.
Results
Patient clinical characteristics and BC results
In total, 438 episodes (BC and ddPCR simultaneously) of suspected BSIs occurring in 150 critically ill patients were consecutively recruited, of which 27 patients contributed two episodes and 13 patients three episodes of suspected BSI, and even more episodes involved in several patients (Table 1 and Fig. 1). Table 1 reports the underlying diseases and outcomes of the 150 patients. Among them, 40.7% were thought to be suffering septic shock and treated with vasopressors. The cumulative mortality rate at 28 day was 16.7%. Culture-proven BSI was positive for 78 microorganisms in 16.2% (71 of 438) episodes; polymicrobial BSI was detected in 8.0% (6 of 71) of cases (Fig. 3c). Pathogens included in the ddPCR panel were identified in 56.3% (40 of 71) of positive BCs, 37.5% positives for Gram-negative bacteria, 20.0% for Gram-positive bacteria, and 42.5% for fungi, including Acinetobacter baumannii (n = 2), K. pneumonia (n = 9), E. coil (n = 1), P. aeruginosa (n = 3), S. aureus (n = 3), Enterococcus faecalis (n = 1), E. faecium (n = 4), C. parapsilosis (n = 5), C. tropicalis (n = 1), C. glabrata (n = 5), and C. albicans (n = 6). Further, coagulase-negative staphylococci, Pseudomonas maltophilia, Acinetobacter junii and other species, were recovered from the remaining 43.7% BCs (Fig. 3a).
Performance of the ddPCR testing
We found that ddPCR results were positive in 41.1% (180 of 438) of episodes, with identification of 275 pathogens (Table 2). Polymicrobial infections were detected in 38.3% (69 of 180) of episodes of ddPCR testing (Fig. 3b). Among them, 159 Gram-negative bacteria detected, with the top three strains being K. pneumoniae (n = 54), A. baumannii (n = 38), and P. aeruginosa (n = 36). In addition, the 65 Gram-positive pathogens detected by ddPCR included E. faecium (n = 44), E. faecalis (n = 12), S. aureus (n = 9). Further, the most detected fungi in the remaining 51 strains were C. albicans (n = 23), C. glabrata (n = 13), C. tropicalis (n = 5), and C. parapsilosis (n = 10). The performance of the ddPCR method in diagnosing culture-proven BSIs caused by targeted bacteria is summarized in Fig. 3a and Table 2. In eight and two episodes, a second or third organism was also identified by ddPCR testing, and four organisms in one episode, indicating the difficulty of diagnosing polymicrobial infections in conventional BC method. Compared to BC results, 72.5% (29/40) of positive ddPCR tests were also positive in BC. However, this meant ddPCR failed to detect causative pathogens in 11 episodes, which were defined as presumably false-negative cases, including K. pneumoniae (n = 1), P. aeruginosa (n = 1), E. faecium (n = 1), S. aureus (n = 1), C. parapsilosis (n = 4), C. glabrata (n = 1), and C. albicans (n = 2). In four samples of the 11 episodes, ddPCR detected E. coli, K. pneumoniae, C. parapsilosis, and C. albicans pathogens, which were different in BC results as E. faecium, C. parapsilosis, C. albicans, and K. pneumoniae were detected instead, respectively.
Overall concordance between BCs and ddPCRs
Results of BC and ddPCR were concordantly positive in 29 episodes, concordantly negative in 251 episodes, and discordant in 158 episodes (Fig. 1). The level of agreement between culture-proven BSIs and ddPCR was 63.9% (280/438). For culture-proven BSIs, the aggregate ddPCR testing demonstrated a sensitivity of 72.5%, a specificity of 63.1%, a positive predictive value (PPV) of 16.5%, and a negative predictive value (NPV) of 95.8%. However, the sensitivity of individual ddPCR detection channels within the multiplex setting was highly variable at genus or gram stain level. Separately, the sensitivity of ddPCR-targeted Gram-negative bacteria was 86.7%, which was higher than 75.0% for Gram-positive bacteria and 58.8% for fungi. Correspondingly, the specificity of ddPCR testing in comparison with the BC method ranged from 73.5 to 92.2% across the detection channels (Table 3).
Evaluation of patients with discordant results between BC and ddPCR
The ddPCR results were positive in 33.6% (147 of 438) of episodes with negative BCs, which embraced 231 targeted organisms (Table 2). When we analyzed these 147 discordant results in-depth, combining composite microbiological and clinical evidence, 108 episodes (73.5%) met criteria for probable BSI, 20 episodes (13.6%) for possible BSI, and the remaining 19 cases (12.9%) were presumptive false positives (Fig. 1). Among the probable/possible BSIs, discordant results were often associated with patients diagnosed with earlier or subsequent BCs and localized infections according to pathogens summarized in Additional file 1: Table S1 [blood culture (n = 38), abdominal (n = 68), respiratory (n = 17), skin and soft tissue (n = 3), perianal (n = 11), urine (n = 2), and multiple sites (n = 11)]. The ultimately diagnostic performance of ddPCR testing is presented in Table 3. Meanwhile, the 69 polymicrobial infections detected by ddPCR have been further interpreted combined with the composite microbiological and clinical infection evidence. 37.7% episodes (26/69) were completely concordant with all microbiological testing within seven days. 55.1% episodes (38/69) were partly concordant with all microbiological testing. In addition, in comparison with all microbiological testing, 63.4% (104/164) of the polymicrobial ddPCR+ pathogens were also detected in other infection sites. 20.7% (34/164) fulfilled the criteria for possible BSI, and the remaining 15.9% (26/164) were defined as presumptive false-positive cases. If considering probable BSIs with all microbiological testing, the sensitivity and specificity of ddPCR testing were 43.1% and 67.5%, respectively, and the corresponding PPV and NPV were 77.8% and 30.9%, respectively. If considering both probable and possible BSIs were assumed to be true positive, anticipated sensitivity and specificity, as well as the PPV and NPV of ddPCR for complicated BSIs would increase to 84.9%, 92.4%, 89.2%, and 89.3%, respectively.
In summary, the sensitivity of ddPCR for suspected BSI was higher than that of conventional BCs in the 438 cases (35.8% vs 9.1%, P < 0.001). Also, we noticed in the 147 ddPCR+/BC− episodes, 44.9% (66/147) received targeted antimicrobial therapy, 27.9% (41/147) received partial targeted treatment owing to the polymicrobial infections, and 27.2% (40/147) did not receive any appropriate treatment before the BC and ddPCR tests. These preliminary data suggest that ddPCR has potentiality to rapidly identify and exclude targeted pathogens, including cases that may be missed by conventional BCs or inhibited by prior antibiotics applications.
Evaluation of the AMR genes detected by ddPCR
In our research, ddPCR showed 40 episodes positive for blaKPC, among which, K. pneumoniae and blaKPC gene were simultaneously detected in 75.0% cases, which were considered more meaningful in the clinical environment (Table 4). Compared with BC results, six cases of BC-reported K. pneumoniae showed resistance to carbapenems, and PCR results showed that these strains expressed the blaKPC gene. Considering pathogens recovered from other microbiological testing and AST, all cases were resistant to carbapenems, among which 21 strains of K. pneumoniae were stored and detected, with 90.5% (19/21) of samples expressing the blaKPC gene. As for the blaNDM gene, while positive in three cases, no causative pathogen was detected according to BC or other microbiological testing. Further, 38 episodes were positive of mecA genes in ddPCR testing. Only three samples were both positive for S. aureus and the mecA gene, but this could not be confirmed by BC (Table 4). However, 23.7% cases were predicted as mecA-positive in S. aureus combined with all microbiological and AST results. In addition, 10 (26.3%) and 25 (65.8%) cases were predicted as mecA-positive in other Staphylococcus sp. that were not included by the ddPCR panel according to the BC and other microbiological testing, respectively.
Discussion
Clinical management of BSIs in critically ill patients poses several challenges because signs and symptoms are generally nonspecific. Studies showed that about 35.0% of patients with sepsis are culture-negative, and Gupta et al. reported that mortality was significantly higher in these BC-negative patients [24]. Our study showed that the mortality rate of ddPCR+/BC− patients was 26.2%, similar to ddPCR+/BC+ patients, significantly higher than the ddPCR-/BC- group in Additional file 1: Table S2. Therefore, it is still necessary to take other appropriate diagnostic methods as add-ons complementary to conventional BC to identify the possible causative pathogens for BC-negative sepsis patients. Although numerous biomarkers have been explored to assist in the rapid diagnosis of serious infections, causative pathogen-related molecular diagnostic strategies that directly detect multiple species and resistance phenotypes in whole blood samples without the need for cultivating organisms are urgently needed to distinguish causative infection and define non-infectious inflammatory states in the context of sepsis. Based on the strengths of the ddPCR method, several studies have explored its application in infectious diseases [13]. In the EPICIII study, 41.41% gram-negative microorganisms, 43.17% gram-positive microorganisms, and 10.56% fungal microorganisms were detected among patients admitted to ICUs with proven BSI [25]. Therefore, we designed the multiplex ddPCR panel according to the global and local pathogen epidemiology.
Clinical validation has revealed that the ddPCR method is a flexible and universal platform, but this was at small-scale and not routinely used in the urgent ICU environment [26,27,28,29,30]. Nevertheless, like other molecular tests such as metagenomics-based assays, ddPCR detects microbial Cell-Free DNA (mcfDNA) in plasma, but unable to distinguish mcfDNA between live microorganisms and apoptotic microorganisms. However, mcfDNA could be continuously detected in patients with bloodstream infection or sepsis and evaluated through dynamic monitoring [31]. Also, the clinical significance of ddPCR testing in rapidly pathogens diagnosis was not clearly clarified in critically ill patients [25]. Further, ddPCR shows potential advantages over BC in terms of sensitivity and specificity when combined with clinical infection evidence among the 150 critically ill patients in our study. There were 280 (63.9%) concordant positive or negative results between the two methods. In addition, ddPCR and BC result showed that 180 (41.1%) and 40 (9.1%) of the 438 cases of BSIs yielded targeted bacterial pathogens, respectively. Among the 40 BC+ cases, 11 pathogens were missed by the ddPCR test, the overall sensitivity of ddPCR in comparison with the BC results ranged from 58.8% in fungi to 86.7% in gram-negative bacteria, with an aggregate sensitivity of 72.5%. The corresponding specificity was 92.2% and 73.5%, with an aggregate specificity of 63.1%. Importantly, discordant ddPCR+/BC− results represented 33.6% (147/438) of all reported tests, and the detailed review of clinical circumstances showed that the majority of discordant results were either probable (24.7%,108/438) or possible (4.6%,20/438) BSIs. When the clinically diagnosed BSIs criterion was used as the comparator, the overall sensitivity and specificity of ddPCR increased to 84.9% and 92.5%, respectively. The final sensitivity of ddPCR for suspected BSI was higher than that of conventional BC (35.8% vs 9.1%, P < 0.001). In addition, ddPCR also allowed for the detection of AMR genes including blaKPC, blaNDM, and mecA. We found that 90.5% (19/21) of cases were definitely positive for blaKPC, no causative pathogen was identified for blaNDM, and 65.8% cases are predicted as mecA-positive in Staphylococcus sp. according to all microbiological, AST, and PCR tests. Nonetheless, the application of ddPCR for AMR genes needs further verification.
The causative pathogens in our multiplex ddPCR panel generally covered about 60.0% of organisms recovered from BCs. Furthermore, ddPCR shows higher rate for polymicrobial infection episodes (38.3% vs 8.0%, P < 0.001); this is difficult for BC method, which regularly detects only fastest-growing microorganisms [32]. The observed sensitivity of ddPCR is different for various types of microorganisms; although it is relatively high for the majority of individual Gram-negative bacteria, it is insufficient for yeasts. In addition, six fungi were missing and defined as presumptive false-negative cases. Similar results were found in Wouters’ study for identifying 20 bacteria and six fungi, and the sensitivity of identifying Gram-positive and Gram-negative bacteria by ddPCR was 71.0% and 67.0%, respectively, but lower for fungi (60.0%) [33]. In fact, higher sensitivities for fungal detection have been previously reported by other BSI molecular assays such as the T2Candida [9]. Indeed, during our initial ddPCR development, fungal detection was deemed satisfactory in spiking experiments using laboratory-grown organisms. This was partly because of the differences in the methodology such as lysis process, and the success rate of extracting bacterial nucleic acid from blood samples by the ddPCR system was between 15 and 80% [27]. Fungi derived from clinical blood samples may be more difficult to process due to the changes in cell wall characteristics [34]. It is likely that low circulating DNA concentrations in false-negative samples were below the limit of detection of the assay. However, further evaluation and collaborative studies of flexible ddPCR panels covering different pathogens in species or genus level and related resistance genes should be designed based on epidemiological surveillance data in local ICU wards.
Previous studies showed that 10–40% of negative BCs were found to be positive using multiplex molecular assays, and there is no consensus about the interpretation of BC−/ddPCR+ results [35, 36]. We deliberately focused on the clinical interpretation of discrepant ddPCR-positive results, which, to the best of our knowledge, has not yet been addressed in the literature. Consequently, future informed decision-making will have to consider whether a positive ddPCR result represents a true BSI in the context of additional factors, including clinical, epidemiological, and other laboratory data [9, 11]. The possible reasons of BC−/ddPCR+ result might be explained by the presence of nonviable, nonproliferating, or transient or intermittent bacteremia, intracellular organisms within circulating phagocytic cells, inhibition of bacterial growth by antibiotics, or possible contamination [9]. In our study, 73.5% (108/140) BC−/ddPCR+ cases were thought to be probable BSIs because of transient or intermittent bacteremia. The same pathogen was recovered from previous and sequent blood/non-blood site cultures (mainly abdominal infection). Moreover, 14.3% (20/140) of BC−/ddPCR+ cases were considered as possible BSIs because they fit the clinical infection syndromes. Taken together, the sensitivity and specificity of ddPCR were higher than BCs, meaning that ddPCRs could be used as a tool for early detection of suspected BSIs with extra-blood site infections missed by BCs. Although the sensitivity and specificity of ddPCR were reasonable in our study, a higher sensitivity and specificity are desirable in clinical practice. This situation could be countered by dynamic monitoring or investigative ddPCRs from BCs after several hours of incubation [37, 38].
Recently, metagenomic next-generation sequencing (mNGS) has also shown promising potential as a diagnostic tool for BSIs [39]. Hu et al. reported that ddPCR was more rapid and sensitive than mNGS within the detection range of 20 common isolated pathogens and four AMR genes, while mNGS detected a broader range of pathogens than ddPCR [22]. The frequent detection of contaminants and colonizing pathogens both affects the specificity of NGS and ddPCR and complicates the interpretation of results in diagnosing BSIs. However, the clinical applications of these two methods play vital roles in different clinical scenes. Further, ddPCR showed a great potential to identify and exclude the common BSI pathogens, whereas mNGS is more appropriate in the diagnosis of rare infections and intractable diseases. In addition, the availability of a bioinformatics analysis team, a typical turnaround time of 2 days for processing and interpreting the sequencing data, and the high cost of NGS represents barriers to the application of NGS in clinical practice.
In this study, 40 blaKPC, 3 blaNDM, and 38 mecA genes were detected, meaning that AMR gene detection is more sensitive than pathogen detection in ddPCR testing. This may be due to untargeted ranges or low bacterial loads. As resistance mechanisms are not a definite proof of AST, ddPCR cannot replace it but represents a promising diagnosis method that is complementary to BC, which has imperfect sensitivity in critically ill patients [40]. Moving forward, ddPCR may also have particular value in conjunction with cultures during antimicrobial therapy and rational antimicrobial management strategies, including severity stratification. The impact of ddPCR-based interventions and optimizing treatment and patients’ outcomes requires further clinical research [15, 41].
Several limitations should be underlined. Firstly, this was a monocentric prospective study, and our results warrant further investigations. Secondly, because of the economic cost, ddPCR was simultaneously collected with only a single set of BC samples and generally identified a limited spectrum of microorganisms and AMR genes. However, the testing cost will be further reduced with the technical development and widespread application of ddPCR for diagnosing suspected BSIs. Thirdly, because several patients were repeatedly enrolled, the antimicrobial treatment impact on BC and ddPCR positivity were not performed. Finally, the correlations between quantitative ddPCR results and severity stratification inferred to pathogen load were not analyzed.
Conclusions
The multiplex ddPCR can be used as add-on complementary assay to the conventional BC method and offered some added diagnostic value for rapidly and accurately diagnosing common suspected BSIs and related AMR genes in ICU practices. Development of ddPCR assays including flexible pathogens and resistance determinants according to local epidemiology are required before it can be used as a precise bedside test.
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- ddPCR:
-
Droplet digital PCR
- BSIs:
-
Bloodstream infections
- ICU:
-
Intensive care unit
- BC:
-
Blood culture
- AMR:
-
Antimicrobial resistance
- ASTs:
-
Antimicrobial susceptibility tests
- PCR:
-
Polymerase chain reaction
- dPCRs:
-
Digital polymerase chain reactions
- qPCR:
-
Quantitative PCR
- SOFA:
-
Sequential Organ Failure Assessment
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- IQR:
-
Interquartile range
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- mNGS:
-
Metagenomic next-generation sequencing
References
Timsit JF, Ruppe E, Barbier F, Tabah A, Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–84.
Kern WV, Rieg S. Burden of bacterial bloodstream infection—a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect. 2020;26(2):151–7.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–143.
Murray PR, Masur H. Current approaches to the diagnosis of bacterial and fungal bloodstream infections in the intensive care unit. Crit Care Med. 2012;40(12):3277–82.
Kothari A, Morgan M, Haake DA. Emerging technologies for rapid identification of bloodstream pathogens. Clin Infect Dis. 2014;59(2):272–8.
Peri AM, Harris PNA, Paterson DL. Culture-independent detection systems for bloodstream infection. Clin Microbiol Infect. 2022;28(2):195–201.
Zboromyrska Y, Cilloniz C, Cobos-Trigueros N, Almela M, Hurtado JC, Vergara A, Mata C, Soriano A, Mensa J, Marco F, et al. Evaluation of the magicplex sepsis real-time test for the rapid diagnosis of bloodstream infections in adults. Front Cell Infect Microbiol. 2019;9:56.
Clancy CJ, Pappas PG, Vazquez J, Judson MA, Kontoyiannis DP, Thompson GR 3rd, Garey KW, Reboli A, Greenberg RN, Apewokin S, et al. Detecting Infections Rapidly and Easily for Candidemia Trial, part 2 (DIRECT2): a prospective, multicenter study of the T2Candida panel. Clin Infect Dis. 2018;66(11):1678–86.
Nguyen MH, Clancy CJ, Pasculle AW, Pappas PG, Alangaden G, Pankey GA, Schmitt BH, Rasool A, Weinstein MP, Widen R, et al. Performance of the T2Bacteria panel for diagnosing bloodstream infections: a diagnostic accuracy study. Ann Intern Med. 2019;170(12):845–52.
Yanagihara K, Kitagawa Y, Tomonaga M, Tsukasaki K, Kohno S, Seki M, Sugimoto H, Shimazu T, Tasaki O, Matsushima A, et al. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit Care. 2010;14(4):R159.
Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, Kawli T, Christians FC, Venkatasubrahmanyam S, Wall GD, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4(4):663–74.
Dubourg G, Lamy B, Ruimy R. Rapid phenotypic methods to improve the diagnosis of bacterial bloodstream infections: meeting the challenge to reduce the time to result. Clin Microbiol Infect. 2018;24(9):935–43.
Chen B, Jiang Y, Cao X, Liu C, Zhang N, Shi D. Droplet digital PCR as an emerging tool in detecting pathogens nucleic acids in infectious diseases. Clin Chim Acta. 2021;517:156–61.
Salipante SJ, Jerome KR. Digital PCR—an emerging technology with broad applications in microbiology. Clin Chem. 2020;66(1):117–23.
Merino I, de la Fuente A, Dominguez-Gil M, Eiros JM, Tedim AP, Bermejo-Martin JF. Digital PCR applications for the diagnosis and management of infection in critical care medicine. Crit Care. 2022;26(1):63.
Nyaruaba R, Li C, Mwaliko C, Mwau M, Odiwuor N, Muturi E, Muema C, Xiong J, Li J, Yu J, et al. Developing multiplex ddPCR assays for SARS-CoV-2 detection based on probe mix and amplitude based multiplexing. Expert Rev Mol Diagn. 2021;21(1):119–29.
Imaizumi T, Yamamoto-Shimojima K, Yamamoto T. Advantages of ddPCR in detection of PLP1 duplications. Intractable Rare Dis Res. 2019;8(3):198–202.
Oscorbin I, Kechin A, Boyarskikh U, Filipenko M. Multiplex ddPCR assay for screening copy number variations in BRCA1 gene. Breast Cancer Res Treat. 2019;178(3):545–55.
Ligozzi M, Bernini C, Bonora MG, De Fatima M, Zuliani J, Fontana R. Evaluation of the VITEK 2 system for identification and antimicrobial susceptibility testing of medically relevant gram-positive cocci. J Clin Microbiol. 2002;40(5):1681–6.
CLSI M100-ED30:2020 Performance Standards for Antimicrobial Susceptibility Testing, 30th edn.
Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–23.
Hu B, Tao Y, Shao Z, Zheng Y, Zhang R, Yang X, Liu J, Li X, Sun R. A comparison of blood pathogen detection among droplet digital PCR, metagenomic next-generation sequencing, and blood culture in critically ill patients with suspected bloodstream infections. Front Microbiol. 2021;12:641202.
Kalligeros M, Zacharioudakis IM, Tansarli GS, Tori K, Shehadeh F, Mylonakis E. In-depth analysis of T2Bacteria positive results in patients with concurrent negative blood culture: a case series. BMC Infect Dis. 2020;20(1):326.
Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB. Culture-negative severe sepsis: nationwide trends and outcomes. Chest. 2016;150(6):1251–9.
Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, Finfer S, Pelosi P, Brazzi L, Aditianingsih D, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–87.
Zheng Y, Jin J, Shao Z, Liu J, Zhang R, Sun R, Hu B. Development and clinical validation of a droplet digital PCR assay for detecting Acinetobacter baumannii and Klebsiella pneumoniae in patients with suspected bloodstream infections. Microbiologyopen. 2021;10(6): e1247.
Zhang T, Niu Z, Wu F, Chen Z, Xu J, Jiang K, Lai Z. Qualitative and quantitative detection of surgical pathogenic microorganisms Escherichia coli and Staphylococcus aureus based on ddPCR system. Sci Rep. 2021;11(1):8771.
Shin J, Shina S, Jung SH, Park C, Cho SY, Lee DG, Chung YJ. Duplex dPCR system for rapid identification of gram-negative pathogens in the blood of patients with bloodstream infection: a culture-independent approach. J Microbiol Biotechnol. 2021;31(11):1481–9.
Schell WA, Benton JL, Smith PB, Poore M, Rouse JL, Boles DJ, Johnson MD, Alexander BD, Pamula VK, Eckhardt AE, et al. Evaluation of a digital microfluidic real-time PCR platform to detect DNA of Candida albicans in blood. Eur J Clin Microbiol Infect Dis. 2012;31(9):2237–45.
Abram TJ, Cherukury H, Ou CY, Vu T, Toledano M, Li Y, Grunwald JT, Toosky MN, Tifrea DF, Slepenkin A, et al. Rapid bacterial detection and antibiotic susceptibility testing in whole blood using one-step, high throughput blood digital PCR. Lab Chip. 2020;20(3):477–89.
Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and death of circulating cell-free DNA. Cancer Biol Ther. 2019;20(8):1057–67.
Nieman AE, Savelkoul PHM, Beishuizen A, Henrich B, Lamik B, MacKenzie CR, Kindgen-Milles D, Helmers A, Diaz C, Sakka SG, et al. A prospective multicenter evaluation of direct molecular detection of blood stream infection from a clinical perspective. BMC Infect Dis. 2016;16:314.
Wouters Y, Dalloyaux D, Christenhusz A, Roelofs HMJ, Wertheim HF, Bleeker-Rovers CP, Te Morsche RH, Wanten GJA. Droplet digital polymerase chain reaction for rapid broad-spectrum detection of bloodstream infections. Microb Biotechnol. 2020;13(3):657–68.
Kumar M, Mugunthan M. Evaluation of three DNA extraction methods from fungal cultures. Med J Armed Forces India. 2018;74(4):333–6.
Wallet F, Nseir S, Baumann L, Herwegh S, Sendid B, Boulo M, Roussel-Delvallez M, Durocher AV, Courcol RJ. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin Microbiol Infect. 2010;16(6):774–9.
Peker N, Couto N, Sinha B, Rossen JW. Diagnosis of bloodstream infections from positive blood cultures and directly from blood samples: recent developments in molecular approaches. Clin Microbiol Infect. 2018;24(9):944–55.
Lin K, Zhang HC, Zhao YH, Xia J, Ai JW, Zhang WH. The direct application of plasma droplet digital PCR in the ultra-early pathogen detection and warning during sepsis: case reports. J Infect Public Health. 2022;15(4):450–4.
Badran S, Chen M, Coia JE. Multiplex droplet digital polymerase chain reaction assay for rapid molecular detection of pathogens in patients with sepsis: protocol for an assay development study. JMIR Res Protoc. 2021;10(12):e33746.
Jing C, Chen H, Liang Y, Zhong Y, Wang Q, Li L, Sun S, Guo Y, Wang R, Jiang Z, et al. Clinical evaluation of an improved metagenomic next-generation sequencing test for the diagnosis of bloodstream infections. Clin Chem. 2021;67(8):1133–43.
Suzuki S, Horinouchi T, Furusawa C. Prediction of antibiotic resistance by gene expression profiles. Nat Commun. 2014;5:5792.
Giacobbe DR, Giani T, Bassetti M, Marchese A, Viscoli C, Rossolini GM. Rapid microbiological tests for bloodstream infections due to multidrug resistant Gram-negative bacteria: therapeutic implications. Clin Microbiol Infect. 2020;26(6):713–22.
Acknowledgements
We would like to thank Pilot Gene Technologies (Hangzhou) Co., Ltd for technical support of ddPCR testing for clinical patients.
Funding
This study was supported by National Key R&D Program of China (2018YFE0102400 to Jieming Qu), Science and Technology Commission of Shanghai Municipality (19JC1413004 to Hongping Qu), Medical engineering cross research fund of Shanghai Jiaotong University (YG2019ZDA14 to Hongping Qu), Talent development project for Three-years action plan of Shanghai public health system construction (GWV-10.2-YQ50 to Xiaoli Wang) and Guangci discipline group construction of public health and disaster emergency center (XKQ-09 to Hongping Qu).
Author information
Authors and Affiliations
Contributions
HPQ, XLW, and JYS designed the study and supervised the whole project, JW, BT, and YZQ collected the clinical samples and data, analyzed, and wrote the manuscript; JX and JZ provided the technical support of ddPCR testing; RMT, JLL, and JJH analyzed the data and prepared figures; JMQ contributed to study design and manuscript revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki. The Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (RJ2021-132) reviewed and approved the study, and written informed consent was obtained from the patients prior to inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1. Detailed descriptions of the inconsistency BC−/ddPCR+ cases. Table S2. Clinical outcomes of the 150 critically ill patients according to ddPCR and BC results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, J., Tang, B., Qiu, Y. et al. Clinical validation of a multiplex droplet digital PCR for diagnosing suspected bloodstream infections in ICU practice: a promising diagnostic tool. Crit Care 26, 243 (2022). https://doi.org/10.1186/s13054-022-04116-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04116-8