Abstract
Evaluating left atrial pressure (LAP) solely from the left ventricular preload perspective is a restrained approach. Accurate assessment of LAP is particularly relevant when pulmonary congestion and/or right heart dysfunction are present since it is the pressure most closely related to pulmonary venous pressure and thus pulmonary haemodynamic load. Amalgamation of LAP measurement into assessment of the ‘transpulmonary circuit’ may have a particular role in differentiating cardiac failure phenotypes in critical care. Most of the literature in this area involves cardiology patients, and gaps of knowledge in application to the bedside of the critically ill patient remain significant. Explored in this review is an overview of left atrial physiology, invasive and non-invasive methods of LAP measurement and their potential clinical application.
Graphical abstract

Similar content being viewed by others
Background
A clinician’s interest in the left atrial pressure (LAP) usually pivots around its preload contribution to cardiac output. However, the left atrium is a key component of the ‘transpulmonary circuit’ with upstream and downstream functions as reservoir, conduit and pump [1]. Increases in LAP have important consequences for gas exchange, pulmonary haemodynamic load and right ventricular performance [2]. Raised LAP may be due to pre-existing left ventricular systolic and/or diastolic dysfunction, mitral and/or aortic valve pathology; however, acute increases in LAP can be seen in critical illnesses such as sepsis, myocardial ischemia, stress-induced cardiomyopathies and volume overload states [3,4,5]. Accurate manipulation of cardiopulmonary performance using the limited tools available demands a more in-depth understanding of LA physiology and pressure measurement.
Left atrial physiology
Although the classical anatomy is that of four pulmonary veins, two superior and two inferior, draining separately into the left atrium (LA), this is only the case in 70% of individuals [6]. Around 12–25% of the population have either the two right, or the two left pulmonary veins entering through a single ostia [6]. Flow from the pulmonary veins into the left atrium is pulsatile, and the classical pressure wave form exhibits a V wave and an A wave. The V waves are passive atrial filling waves and occur during ventricular systole. The other peak, the A wave, is the left atrial pressure wave that follows active atrial contraction [7, 8]. The relationship between the left atrial pressures and left ventricular pressures is illustrated in Fig. 1.
Blood flow from the pulmonary vein into the LA depends upon the pressure gradient, which varies throughout the cardiac cycle, i.e. the normal blood flow is both phasic and bidirectional [7]. Doppler analysis reveals four distinct waves of flow [8]. See Fig. 2. Two antegrade waves occur during the LA reservoir phase in early and mid-systole (S1 and S2, respectively), corresponding to the X descent post-A pressure wave. The V pressure wave caused by ventricular contraction reduces antegrade flow but following this during the Y descent comes the third antegrade flow during diastole, giving the pulmonary vein D wave, whose amplitude and shape mirror that of the mitral Doppler E wave. Near the end of diastole, atrial contraction occurs, resulting in a significant pressure difference between the LA and pulmonary vein creating a retrograde A wave into the pulmonary vein. This pulmonary vein Doppler A wave is related in time to the transmitral Doppler A wave and the LA pressure A wave [7, 8].
Relationship between pulmonary vein (PV) pressure, LAP and mitral inflow Doppler waves throughout the cardiac cycle. PV Doppler D wave mirrors the mitral E wave and occurs at the time of the Y descent. PV A wave is concomitant to the mitral Doppler A wave and to left atrial contraction. The corresponding reservoir, conduit and pump functions of the left atrium are shown. MV mitral valve
What are we measuring and why?
As demonstrated in Fig. 1, there is variation throughout the cardiac cycle and the pressure at a specific time point has consequences for both incoming flow from the PV (downstream) into the LA and ongoing flow from the LA into the left ventricle (LV). It is quite difficult to express LV filling pressure (LVFP) as a single value on the LV and LA pressure tracing because the pressures fluctuate and LV filling is a complex process.
Mean LAP and LVEDP are not telling us the same thing yet are often used interchangeably. The LVEDP provides information about the LV operating compliance and is the closest estimate of LV preload as a surrogate for LVEDV. Patients with similar LVEDP can have markedly different LAP, which is determined by the operating compliance of the LA [9]. This concept is perhaps most relevant to critical care as changes to compliance can occur with fluid challenges and mechanical ventilation for example. The mean LAP integrates the atrial pressure tracing throughout systole and diastole providing a measure of the hemodynamic load determined by the LA operating compliance (and indirectly left ventricular operating compliance through atrioventricular coupling). It is the mean LAP that is reflected back to the pulmonary venous circulation impacting right ventricular performance [9, 10].
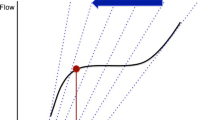
The ‘mid A wave pressure’ (mean value of the A‐wave amplitude) is recommended in consensus statements to estimate end-diastolic LAP that correlates most closely with LVEDP [11], whereas the mean LAP is obtained by temporal integration of the instantaneous PAOP over the entire cardiac cycle (Fig. 3). Mean LAP and end-diastolic LAP can differ significantly in the presence of large ‘V’ waves that occur in severe mitral regurgitation and with reduced LA compliance [12] (Fig. 3). Some suggest that the mean LAP as opposed to the end-diastolic LAP makes more sense when wanting to differentiate pre- from post-capillary pulmonary hypertension (PH) [9, 10]. Certainly, in the critically ill patient with hypoxic respiratory failure and RV dysfunction the more crucial question must be what the cumulative haemodynamic load on the pulmonary vascular system is. The answer to this lies with measurement of the mean LAP.
PAOP trace showing the ‘mid A point’ and large ‘V’ wave (patients with mitral regurgitation or reduced LA compliance). An integrated digitised mean over the entire cardiac cycle would include the ‘V’ wave and give a higher PAOP value than a PAOP measurement taken at the ‘mid A point’. PAOP pulmonary artery occlusion pressure
LAP and ‘RV–pulmonary circuit’ dysfunction
The impact of different PH haemodynamic subgroups on RV function is increasingly recognised [13]. A higher incidence of RV dysfunction and RV–pulmonary arterial uncoupling (measured by tricuspid annular planar systolic excursion (TAPSE)/systolic pulmonary artery systolic pressure (sPAP) ratio) was found in those with pre-capillary and combined pre- and post-capillary PH than in isolated post-capillary PH [14]. ePLAR (echocardiographic pulmonary-to-left atrial ratio using tricuspid regurgitant velocity and E/e′) appears to be a simple, non-invasive ratio in differentiating pre- and post-capillary PH with reasonable accuracy, albeit in non-critically ill cohorts [15] (Fig. 4). Patients with RV dysfunction coupled with a low/normal mean LAP and high pulmonary pressures may benefit from pulmonary vasodilators, e.g. nitric oxide. In contrast, those with a high mean LAP and isolated post-capillary PH may derive benefit from diuretics, and pulmonary vasodilators in this group may worsen pulmonary oedema [16]. These diverging treatment strategies emphasise the potential benefit of amalgamating LAP measurement into categorising RV–pulmonary circuit dysfunction. Further investigation of the feasibility and utility of ePLAR in critically ill patients with RV dysfunction would be of interest.
ePLAR = TRV/E/e′. Post-capillary pulmonary hypertension (PHT) is characterised by a lower ePLAR given E/e′ will be higher in these groups. Pre-capillary PHT with lower E/e′ has a higher ePLAR ratio. (A cut off value of < 0.28 m/s for post-capillary PH yielded 83% sensitivity and specificity, AUC 0.87) [12]. TRVmax tricuspid regurgitation maximum velocity, m/sec. PAP pulmonary artery pressure, mmHg
Bedside methods for assessing LAP
Invasive: pulmonary artery occlusion pressure (PAOP)
The challenges in correlating PAOP, LAP and LVEDP when using a PA catheter have been subject to intense evaluation in previously published works [17, 18] and are summarised in Table 1. Table 2 summarises non-critical care studies investigating the correlation between the PAOP and LVEDP during left heart catheterisation (LHC) showing varying results [19,20,21,22]. Data comparing PAOP and LVEDP in critical care populations are scare and conflicting, and a tabulated summary is provided in Table 3 [23,24,25]. In 1974, Lozman et al. evaluated five ventilated post-operative cardiac surgical patients without ARDS and showed that the relationship between PAOP and directly measured LAP was lost at PEEP levels above 15 cm H20 [23]. Jardin et al. demonstrated that below a PEEP of 10cmH20, PAOP correlated with invasively measured LVEDP; however, this correlation was diminished at PEEP values > 10 [24]. Teboul et al. have shown that PAOP correlated strongly with invasively measured post-A wave LVEDP in patients with ARDS with PEEPs up to 20 cm H20. They suggested this observed correlation of PAOP and LVEDP is due to surrounding diseased lung preventing alveolar vessel compression [25].
Non-invasive: echocardiography and Doppler techniques
Investigation of LAP non-invasively using Doppler has been studied for over 30 years [26]. The most recent 2016 American Society of Echocardiography and the European Association of Cardiovascular Imaging (ASE/EACVI) guidelines estimate mean LAP through Doppler assessment of diastolic blood flow between the left atrium and left ventricle (mitral E to A wave ratio), tissue Doppler imaging (TDI) of the mitral annulus, the tricuspid regurgitant flow velocity and LA volumes as shown in Fig. 5 [27]. Importantly for the critical care physician who is interested in presence of raised LAP for treatment decisions, these guidelines began to differentiate between the two major objectives—LV diastolic dysfunction and LAP (Fig. 5).
ASE/EACVI algorithms for estimating LAP in those with reduced left ventricular ejection fraction (EF) of < 50% (or normal EF with the presence of structural disease). Left panel, demonstrates where E/A ratio and E velocity, or E/A alone can differentiate normal versus elevated LAP in those with grade 1 and grade 3 diastolic dysfunction, respectively. Right panel, demonstrates a patient where 3 further criteria are required to decide if there is raised LAP: E/e′, TR Velocity and LA volume index (LAVI) showing a patient with grade 2 diastolic dysfunction and raised LAP
The Euro-Filling study enrolled 159 patients in 9 centres, comparing non-invasive LAP measurements using ASE/ESCVI guidelines with invasive measurements of LVEDP. Of those with a normal non-invasive LAP, only 65% had a normal invasive LVEDP. Of those with an elevated non-invasive LAP, 79% had elevated invasive LVEDP. Overall, the sensitivity was 75% and specificity 74% giving a PPV of 39% and NPV 93% with an AUC of 0.78 [28]. In a similar study, 90 patients undergoing invasive cardiac catheterisation underwent TTE immediately prior to the procedure. The non-invasive LAP was accurate, when compared to the pre-A invasive measurement in 75% and inaccurate in 25%. In the latter group, the non-invasive LAP was overestimated in 8/20 and underestimated in 12/20 [29]. In these and other studies, the use of a single parameter as opposed to the guidelines use of 4 parameters was found to be highly inaccurate. Even the favoured one of E/e′ delivered only a moderate correlation to invasive measurements. Once again, these studies do not include the type of patients commonly found in the critical care setting who often have cardiac pathologies other than coronary artery disease. A tabulated summary is provided in Table 2 [28,29,30].
It is of little surprise that pathophysiology unique to critical illness (mechanical ventilation, vasoactive agents, fluid shifts) can make application of these algorithms more challenging [31]. Brault et al. [32] who compared ASE/EACVI echo Doppler LAP algorithms to PAOP (measured at end expiration and averaged over five non-consecutive cardiac cycles) in 98 mechanically ventilated patients found a sensitivity and specificity of 74% for ASE/EACVI algorithms to predict elevated PAOP ≥ 18 mmHg. Agreement between echocardiography and PAOP was moderate (Cohen’s Kappa, 0.48; 95% CI, 0.39–0.70). Overall, the guidelines show better discriminatory performance in the critically ill than previous iterations as shown by Clancy et al. [33] and offer an unrivalled framework in our patient group. Table 3 provides a tabulated summary of critical care studies that have compared echo Doppler LAP to PAOP values in critically ill patients [32, 34,35,36,37,38,39,40].
The ratio of early diastolic mitral inflow to average mitral annular tissue velocity (E/e′) has been most extensively studied in the cardiology population [28, 30] and has gained some interest in the critical care literature [32, 34, 36,37,38,39]. E/e′ is less load dependent and can be used to assess for raised LAP in those with atrial fibrillation (AF), making it a favoured choice in critical care. A septal E/e′ of > 11, as well as lack of mitral E velocity beat to beat variation, are suggestive of raised LAP in AF [27].
As with any haemodynamic measurement, the use of a single parameter to evaluate LAP should be avoided, and E/e′ is no exception [19]. Although a normal E/e′ does not rule out high LAP, an E/e′ > 15 does have a high specificity in identifying a high LAP [41]. This is perhaps of greatest pragmatic benefit when decisions on further fluid resuscitation are needed at the bedside: an E/e′ > 15 in this scenario would strongly favour a patient with ‘fluid intolerance’. At the other extreme, a low lateral E/e′ of < 8 has shown good diagnostic accuracy to predict PAOP < 18 mmHg [34].
A further challenge of the algorithm to identify patients with high LAP in critical care is the inability of the LA to dilate acutely (in comparison to the right atrium) [42]. Critically ill patients can have acutely high LAP despite a normal LA size, for example, those with volume overload or sepsis and acute diastolic dysfunction [3]. In summary, a dilated LA (LA volume index (LAVI) ≥ 34mls/m2) should raise suspicion for raised LAP, but a normal LA size should not exclude raised LAP. Echocardiographic evaluation of the interatrial septal (IAS) kinetics throughout the respiratory cycle may add pertinent information. Patients with fixed bowing of the IAS to the right are more likely to have raised LAP [43]. Considering right atrial pressure is important however given it is the relative pressure difference between the atria that determines position of the interatrial septum. Additional parameters, including a reduced E wave deceleration time (< 160 ms), alterations in the pulmonary venous Doppler waveform such as a reduced contribution to left atrial filling during systole (S/D ratio < 1), reduced isovolumetric relaxation time (IVRT) of < 60 ms and a mitral ‘L’ wave of > 20 cm/sec, may be additive in identifying raised LAP. An appraisal of their merits and disadvantages is discussed by Nagueh et al. [27].
Overall, when it comes to LAP measurement there exists a lack of uniformity in methods and ‘what’ is being measured. These issues are further compounded by the heterogeneity of the populations included. We shouldn’t be too hasty however in abandoning LAP measurement at the bedside altogether. A non-invasive, rapid beside screening tool to identify patients with possible raised LAP could be ‘the rule of 8’s’: lateral E/e′ > 8 [34] and a lateral e’ ≤ 8 cm/s [32]. This tool could serve as a trigger to temporarily halt further fluid resuscitation and instigate multimodal assessment of cardiopulmonary performance as proposed in Fig. 6.
Newer non-invasive measurements of LAP: LA strain and left atrial expansion index
LA strain uses angle independent speckle tracking imaging to assess LA function and stiffness [44]. The increased participation of LA contraction to end-diastolic LV filling is increased in the presence of LV diastolic dysfunction, up to the point when LA is failing because of excessive LVEDP. Studies have demonstrated an inverse relationship between LA global strain and LV end-diastolic pressures [45]. LA strain should be measured using a non-foreshortened apical-4-chamber (A4C) view of the LA where values of LA strain for reservoir, conduit and pump functions are measured [46] (Fig. 7). Inoue et al. evaluated 322 patients referred for left or right heart catheterisation in a multicentre study [47]. Cut off values for LA reservoir strain of < 18% and LA pump strain of < 8% had an AUC of 0.76 and AUC 0.77, respectively, for detecting increased LVFP (defined as PAOP > 12 mmHg or LVEDP > 16 mmHg). LA strain didn’t perform well in predicting LVFP in those with AF. These values have been proposed to serve as substitute parameters for those with missing criteria in ASE/EACVI algorithms that would otherwise be classified as ‘indeterminate’ (Fig. 5), providing there are no exclusion criteria (AF, mitral valve disease, and left bundle branch block amongst others) [48].
LA strain using non-foreshortened A4C LA views. White dashed strain curve showing average values of 6 segments. Ventricular end-diastole is recommended as the time reference to define the zero-baseline for strain curves. As depicted by the white arrows: LA reservoir strain = difference of the strain value at mitral valve opening minus ventricular end-diastole. LA conduit strain = difference of the strain value at the onset of atrial contraction minus mitral valve opening. LA pump strain = difference of the strain value at ventricular end-diastole minus onset of atrial contraction [44]
There has been increasing interest in the utility of the relative left atrial volume change over the cardiac cycle to predict filling pressure. The hypothesis being that a smaller volume expansion of the LA between systole and diastole predicts higher LAP. The value, expressed as a percentage, is known as the left atrial expansion index (LAEI) and is calculated by the formula: (Volmax − Volmin) × 100%/Volmin, where Volmax = maximal LA volume and Volmin = minimal LA volume. Genovese et al. investigated its use in over six hundred patients with chronic cardiac disease [49]. A reasonable linear correlation was found between logarithmically transformed LAEI and PAOP (r = 0.73, p < 0.001). Whilst LA strain and LAEI have shown promise in the cardiology setting, prospective data are needed to assess their role in the critical care arena.
LAP and the ‘diastolic stress test’ of critical care
Patients may have pre-existing diastolic dysfunction or develop de novo diastolic dysfunction because of critical illness such as sepsis [4]. An important concept to appreciate is that the ‘diastolic stress test’ of critical illness can shift patients from a normal ‘resting’ LAP to a high LAP state, with corresponding increases in E/e′ ratio. This is because the mitral annular velocity (e’) of the stiff left ventricle cannot increase to match the increased mitral E velocity as occurs with increased cardiac output demand [27]. This can be particularly problematic during ventilatory weaning leading to Weaning-induced Pulmonary Oedema (WiPO). There is no validated cut off E/e′ value to predict WiPO, though higher values are associated with increased risk of weaning failure. The reader is directed to detailed review of this topic elsewhere [50, 51]. Repeated echocardiographic assessment with an approach as outlined in Fig. 6 could help identify those at risk of WiPO and help guide treatment strategies. For example, patients with elevated LAP may benefit from more aggressive diuresis, higher levels of PEEP and a planned extubation to non-invasive ventilation.
LAP and acute respiratory distress syndrome (ARDS): the ‘grey zone’ patient
A PAOP ≥ 18 mmHg was a commonly accepted criterion to define cardiogenic oedema in ARDS [52]. However, it was not a ‘hard’ value, was seldom measured and it was increasingly appreciated that raised LVFP could coexist with ARDS, hence it was removed from revised diagnostic criteria [53]. Authors of the guidelines highlight the ongoing complexities in differentiating cardiogenic from non-cardiogenic pulmonary oedema and describe scenarios [53].
An elderly patient with chronic obstructive lung disease and congestive cardiac failure, with a central venous pressure of 15 mmHg and fulfilling ARDS criteria, is described as probably having an overlap of cardiogenic and non-cardiogenic pulmonary oedema [53]. In contrast, a multi-trauma patient fulfilling ARDS criteria with a small, hyperdynamic LV without pericardial effusion is likely to have non-cardiogenic pulmonary oedema ARDS. The latter scenario highlights the benefit of incorporating echocardiography, however, it describes the extreme ends of both echocardiographic and clinical spectrums where treatment decisions are often easier.
Unfortunately, many of our patients, like the elderly patient described with pre-existing cardiorespiratory comorbidity, fall into a ‘grey zone’ and there is frequent overlap of cardiogenic and non-cardiogenic aetiologies [54]. Ray et al. studied over 500 elderly patients presenting to the emergency department with acute respiratory failure and showed that those with cardiogenic pulmonary oedema had the highest mortality at 21%. Importantly, around one third of the total cohort was deemed to have inappropriate early treatment and this was associated with a doubling of in hospital mortality [54]. Early detection of raised LAP and lung oedema to prevent inappropriate therapy therefore is a key goal.
LAP and multimodal assessment: combining lung ultrasound
Lung ultrasound (US) assessment of B lines is relatively quick and can be used to identify pulmonary oedema [55, 56]. B lines can be seen in other non-cardiogenic lung oedema states particularly relevant to our population (interstitial syndrome of ARDS, pulmonary fibrosis) [56], hence the need to contextualise echocardiographic and clinical findings [57]. B line quantification methods have shown good diagnostic accuracy against extra vascular lung water impedance techniques in critically ill patients [58, 59]. A simplified 4-sector method described by Mayr et al. only just underperformed against the more laborious 28-sector method, and a cut off value of ≥ 15 B lines resulted in a sensitivity of 91.7% and specificity of 92.1% to identify patients with increased extravascular lung water (AUC 0.978) [59]. Counting the number of B lines can be difficult in those with coalesced lines and lung US scores evaluating the percentage of B lines occupying the pleural line may be better, however, the time required for post processing and offline analysis limits its application at the bedside for most users at this time [58]. Furthermore, in the shocked patient with echocardiographic LAP parameters falling into the ‘grey zone’, the finding of a predominant A line pattern, that is highly specific for a low/normal PAOP, can increase confidence that repeating a fluid challenge is unlikely to result in pulmonary oedema [60].
Time critical decisions on haemodynamic resuscitation often centre on ‘fluid tolerance versus intolerance’, as opposed to definitive diagnosis, and we often need rapid, yet rich haemodynamic information. Tempered with an awareness of the caveats, trends in LAP coupled to the upstream hydrostatic consequence using lung US could provide this information (proposed in Fig. 6).
Conclusion
Located in a pivotal position in the journey of blood flowing from the right heart to the left ventricle, the contribution of left atrium to the circulation needs to be considered from a variety of perspectives. Seen as either a downstream station for the pulmonary blood flow, or an upstream one for filling of the left ventricle, the value of LAP to our haemodynamic armamentarium should not be underestimated. Currently utilised tools in evaluating LAP at the bedside, namely the PA catheter and Echo Doppler and 2D techniques, although having recognised technical drawbacks can be of benefit clinically if utilised correctly.
The combined strength of invasive and advanced echo techniques offers a pathway to evaluate both ends of the circuit, from LA function and pressure to pulmonary haemodynamics and RV function. Perhaps, this amalgamation can enable a more comprehensive understanding of ‘transpulmonary circuit dysfunction’ and its consequence to cardiac performance at the bedside, where it really matters.
Availability of data and materials
The data and material used in this article belong to the corresponding author and can be accessed with permission.
Abbreviations
- LAP:
-
Left atrial pressure
- LVEDP:
-
Left ventricular end-diastolic pressure
- PAOP:
-
Pulmonary artery occlusion pressure
- PVR:
-
Pulmonary vascular resistance
- RVD:
-
Right ventricular dysfunction
- RV-PA:
-
Right ventricular–pulmonary artery
- ePLAR:
-
Echocardiographic pulmonary-to-left atrial ratio
- PASP:
-
Pulmonary artery systolic pressure
- TAPSE:
-
Tricuspid annular planar systolic excursion
References
Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(15):1961–77.
Marra AM, Sherman AE, Salzano A, Guazzi M, Saggar R, Squire IB, et al. Right side of the heart pulmonary circulation unit involvement in left-sided heart failure. Chest. 2022;161(2):535–51.
Orde S, Slama M, Hilton A, Yastrebov K, McLean A. Pearls and pitfalls in comprehensive critical care echocardiography. Crit Care. 2017;21(1):1–10.
Sanfilippo F, Corredor C, Arcadipane A, Landesberg G, Vieillard-Baron A, Cecconi M, et al. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: a systematic review and meta-analysis. Br J Anaesth. 2017;119(4):583–94.
Medeiros K, O’Connor MJ, Baicu CF, Fitzgibbons TP, Shaw P, Tighe DA, et al. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation. 2014;129(16):1659–67.
Ghaye B, Szapiro D, Dacher JN, Rodriguez LM, Timmermans C, Devillers D, et al. Percutaneous ablation for atrial fibrillation: the role of cross-sectional imaging. Radiographics. 2003;23(SPEC. ISS):19–33.
Smiseth OA, Thompson CR, Lohavanichbutr K, Ling H, Abel JG, Miyagishima RT, et al. The pulmonary venous systolic flow pulse—its origin and relationship to left atrial pressure. J Am Coll Cardiol. 1999;34(3):802–9.
Fadel BM, Pibarot P, Kazzi BE, Al-Admawi M, Galzerano D, Alhumaid M, et al. Spectral Doppler interrogation of the pulmonary veins for the diagnosis of cardiac disorders: a comprehensive review. J Am Soc Echocardiogr. 2021;34(3):223–36.
Reddy YNV, El-Sabbagh A, Nishimura RA. Comparing pulmonary arterialwedge pressure and left ventricular end diastolic pressure for assessment of left-sided filling pressures. JAMA Cardiol. 2018;3(6):453–4.
Naeije R, Chin K. differentiating precapillary from postcapillary pulmonary hypertension: pulmonary artery wedge pressure versus left ventricular end-diastolic pressure. Circulation. 2019;140(9):712–4.
Manouras A, Lund LH, Gellér L, Nagy AI, Johnson J. Critical appraisal of the instantaneous end-diastolic pulmonary arterial wedge pressures. ESC Heart Fail. 2020;7(6):4247–55.
Maron BA, Kovacs G, Vaidya A, Bhatt DL, Nishimura RA, Mak S, et al. Cardiopulmonary hemodynamics in pulmonary hypertension and heart failure: JACC review topic of the week. J Am Coll Cardiol. 2020;76(22):2671–81.
Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Resp J 2019;53(1).
Caravita S, Faini A, D’Araujo SC, Dewachter C, Chomette L, Bondue A, et al. Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: role of the pre-capillary component. PLoS ONE. 2018;13(6):1–16.
Scalia GM, Scalia IG, Kierle R, Beaumont R, Cross DB, Feenstra J, et al. ePLAR—the echocardiographic pulmonary to left atrial ratio—a novel non-invasive parameter to differentiate pre-capillary and post-capillary pulmonary hypertension. Int J Cardiol. 2016;212:379–86.
Hayward CS, Macdonald PS, Keogh AM. Inhaled nitric oxide in cardiology practice. Cardiovasc Res. 1999;43:628–38.
Raper R, Sibbald WJ. Misled by the wedge? The Swan-Ganz catheter and left ventricular preload. Chest. 1986;89(3):427–34.
O’Quin, Marini JJ. Pulmonary artery occlusion pressure: clinical physiology, measurement and interpretation. Am Rev Respir Dis. 1983;128:319–26.
Sato K, Grant ADM, Negishi K, Cremer PC, Negishi T, Kumar A, et al. Reliability of updated left ventricular diastolic function recommendations in predicting elevated left ventricular filling pressure and prognosis. Vol. 189, American Heart Journal. Elsevier Inc.; 2017. 28–39 p.
Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136(1):37–43.
Hemnes AR, Opotowsky AR, Assad TR, Xu M, Doss LN, Farber-Eger E, et al. Features associated with discordance between pulmonary arterial wedge pressure and left ventricular end diastolic pressure in clinical practice: implications for pulmonary hypertension classification. Chest. 2018;154(5):1099–107.
Mascherbauer J, Zotter-Tufaro C, Duca F, Binder C, Koschutnik M, Kammerlander AA, et al. Wedge pressure rather than left ventricular end-diastolic pressure predicts outcome in heart failure with preserved ejection fraction. JACC Heart Fail. 2017;5(11):795–801.
Lozman J. Correlation of pulmonary wedge and left atrial pressures. Arch Surg. 1974;109(2):270.
Jardin F, Farcot JC, Boisante L, Curien N, Margairaz A, Bourdarias JP. Influence of positive end-expiratory pressure on left ventricular performance. N Engl J Med. 1979;301(1):44–5.
Teboul JL, Zapol WM, Brun-Buisson C, Abrouk F, Rauss ALF. A comparion of pulmonary artery occlusion pressure and left ventricular end-diastolic pressure during mechanical ventilation with PEEP in patients with Severe ARDS. Anaesthesiology. 1989;70(2):261–6.
Nishimura RA, Housmans PR, Hatle LK, Tajik AJ. Assessment of diastolic function of the heart: background and current applications of Doppler echocardiography. Part I. Physiologic and pathophysiologic features. Mayo Clin Proc. 1989;64(1):71–81.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiog. 2016;29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Lancellotti P, Galderisi M, Edvardsen T, Donal E, Goliasch G, Cardim N, et al. Echo-Doppler estimation of left ventricular filling pressure: results of themulticentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging. 2017;18(9):961–8.
Balaney B, Medvedofsky D, Mediratta A, Singh A, Ciszek B, Kruse E, et al. Invasive validation of the echocardiographic assessment of left ventricular filling pressures using the 2016 diastolic guidelines: head-to-head comparison with the 2009 Guidelines. J Am Soc Echocardiogr. 2018;31(1):79–88.
Nauta JF, Hummel YM, Meer P van der, Lam CSP, Voors AA, Joost P. van Melle. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients wi. Eur J Heart Fail. 2018;20:1303–11.
Sanfilippo F, Scolletta S, Morelli A, Vieillard-Baron A. Practical approach to diastolic dysfunction in light of the new guidelines and clinical applications in the operating room and in the intensive care. Ann Intensive Care. 2018;8(1).
Brault C, Marc J, Mercado P, Diouf M, Tribouilloy C, Zerbib Y, et al. Estimation of pulmonary artery occlusion pressure using doppler echocardiography in mechanically ventilated patients. Crit Care Med. 2020;(Dd):E943–50.
Clancy DJ, Scully T, Slama M, Huang S, McLean AS, Orde SR. Application of updated guidelines on diastolic dysfunction in patients with severe sepsis and septic shock. Ann Intensive Care. 2017;7(1):1–10.
Vignon P, AitHssain A, François B, Preux PM, Pichon N, Clavel M, et al. Echocardiographic assessment of pulmonary artery occlusion pressure in ventilated patients: a transoesophageal study. Crit Care. 2008;12(1):1–9.
Nagueh SF, Kopelen HA, Zoghbi WA. Feasibility and accuracy of Doppler echocardiographic estimation of pulmonary artery occlusive pressure in the intensive care unit. Am J Cardiol. 1995;75(17):1256–62.
Mousavi N, Czarnecki A, Ahmadie R, Tielan Fang, Kumar K, Lytwyn M, et al. The utility of tissue doppler imaging for the noninvasive determination of left ventricular filling pressures in patients with septic shock. J Intensive Care Med. 2010;25(3):163–7.
Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, et al. Optimal noninvasive assessment of left ventricular filling pressures: a comparison of tissue Doppler echocardiography and B-type natriurietic peptide in patients with pulmonary artery catheters. Circulation. 2004;109(20):2432–9.
Combes A, Arnoult F, Trouillet JL. Tissue Doppler imaging estimation of pulmonary artery occlusion pressure in ICU patients. Intensive Care Med. 2004;30(1):75–81.
Bouhemad B, Nicolas-Robin A, Benois A, Lemaire S, Goarin JP, Rouby JJ. Echocardiographic Doppler assessment of pulmonary capillary wedge pressure in surgical patients with postoperative circulatory shock and acute lung injury. Anesthesiology. 2003;98(5):1091–100.
Dabaghi SF, Rokey R, Rivera JM, Saliba WI, Majid PA. Comparison of echocardiographic assessment of cardiac hemodynamics in the intensive care unit with right-sided cardiac catheterization. Am J Cardiol. 1995;76(5):392–5.
Obokata M, Borlaug BA. The strengths and limitations of E/e’ in heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20(9):1312–4.
Patel DA, Lavie CJ, Milani R v., Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner J. 2009;9(4):191–6.
Kusumoto FM, Muhiudeen IA, Kuecherer HF, Cahalan MK, Schiller NB. Response of the interatrial septum to transatrial pressure gradients and its potential for predicting pulmonary capillary wedge pressure: An intraoperative study using transesophageal echocardiography in patients during mechanical ventilation. J Am Coll Cardiol. 1993;21(3):721–8.
Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S. Left atrial strain: a useful index in atrial fibrillation. Int J Cardiol. 2016;220:208–13.
Wakami K, Ohte N, Asada K, Fukuta H, Goto T, Mukai S, et al. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22(7):847–51. https://doi.org/10.1016/j.echo.2009.04.026
Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600.
Inoue K, Khan FH, Remme EW, Ohte N, García-Izquierdo E, Chetrit M, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. 2021;23(1):61–70.
Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23(2):e34-61.
Genovese D, Muraru D, Marra MP, Carrer A, Previtero M, Palermo C, et al. Left atrial expansion index for noninvasive estimation of pulmonary capillary wedge pressure: a cardiac catheterization validation study. J Am Soc Echocardiogr. 2021;34(12):1242–52. https://doi.org/10.1016/j.echo.2021.07.009
Sanfilippo F, Falco D di, Noto A, Santonocito C, Morelli A, Bignami E, et al. Association of weaning failure from mechanical ventilation with transthoracic echocardiography parameters : a systematic review and meta-analysis. Br J Anaesth. 2021;126(1):319–30. https://doi.org/10.1016/j.bja.2020.07.059
Vignon P. Cardiovascular failure and weaning. Ann Transl Med. 2018;6(18):354–354.
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. Report of the American-European consensus conference on ARDS: Definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Med. 1994;20(3):225–32.
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–82.
Ray P, Birolleau S, Lefort Y, Becquemin MH, Beigelman C, Isnard R, et al. Acute respiratory failure in the elderly: Etiology, emergency diagnosis and prognosis. Crit Care. 2006;10(3):1–12.
Lichtenstein D. Fluid administration limited by lung sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Med. 2012;6(2):155–62.
Lichtenstein D. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1–12.
Miglioranza MH, Gargani L, Sant’Anna RT, Rover MM, Martins VM, Mantovani A, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141–51.
Brusasco C, Santori G, Bruzzo E, Trò R, Robba C, Tavazzi G, et al. Quantitative lung ultrasonography: a putative new algorithm for automatic detection and quantification of B-lines. Crit Care. 2019;23(1):1–7.
Mayr U, Lukas M, Habenicht L, Wiessner J, Heilmaier M, Ulrich J, et al. B-lines scores derived from lung ultrasound provide accurate prediction of extravascular lung water index: an observational study in critically ill patients. J Intensive Care Med. 2022;37(1):21–31.
Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136(4):1014–20.
Acknowledgements
The authors thank Elaine Lim for assistance with figure design and illustration, Dr. Faraz Pathan for providing images for Fig. 5 and A/Prof. Sam Orde for review of final manuscript.
Funding
No funding was provided.
Author information
Authors and Affiliations
Contributions
EB and AM conceived the article and participated in the design and coordination. EB prepared the final manuscript. EB prepared Tables 1, 2 and 3. EB and AM prepared Figs. 1, 2, 3 and 4. EB prepared Figs. 5, 6 and 7. Both authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Ethical approval and consent were waived for this article.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bowcock, E.M., Mclean, A. Bedside assessment of left atrial pressure in critical care: a multifaceted gem. Crit Care 26, 247 (2022). https://doi.org/10.1186/s13054-022-04115-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04115-9