Abstract
Background
In patients with vasodilatory shock, plasma concentrations of angiotensin I (ANG I) and II (ANG II) and their ratio may reflect differences in the response to severe vasodilation, provide novel insights into its biology, and predict clinical outcomes. The objective of these protocol prespecified and subsequent post hoc analyses was to assess the epidemiology and outcome associations of plasma ANG I and ANG II levels and their ratio in patients with catecholamine-resistant vasodilatory shock (CRVS) enrolled in the Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) study.
Methods
We measured ANG I and ANG II levels at baseline, calculated their ratio, and compared these results to values from healthy volunteers (controls). We dichotomized patients according to the median ANG I/II ratio (1.63) and compared demographics, clinical characteristics, and clinical outcomes. We constructed a Cox proportional hazards model to test the independent association of ANG I, ANG II, and their ratio with clinical outcomes.
Results
Median baseline ANG I level (253 pg/mL [interquartile range (IQR) 72.30–676.00 pg/mL] vs 42 pg/mL [IQR 30.46–87.34 pg/mL] in controls; P < 0.0001) and median ANG I/II ratio (1.63 [IQR 0.98–5.25] vs 0.4 [IQR 0.28–0.64] in controls; P < 0.0001) were elevated, whereas median ANG II levels were similar (84 pg/mL [IQR 23.85–299.50 pg/mL] vs 97 pg/mL [IQR 35.27–181.01 pg/mL] in controls; P = 0.9895). At baseline, patients with a ratio above the median (≥1.63) had higher ANG I levels (P < 0.0001), lower ANG II levels (P < 0.0001), higher albumin concentrations (P = 0.007), and greater incidence of recent (within 1 week) exposure to angiotensin-converting enzyme inhibitors (P < 0.00001), and they received a higher norepinephrine-equivalent dose (P = 0.003). In the placebo group, a baseline ANG I/II ratio <1.63 was associated with improved survival (hazard ratio 0.56; 95% confidence interval 0.36–0.88; P = 0.01) on unadjusted analyses.
Conclusions
Patients with CRVS have elevated ANG I levels and ANG I/II ratios compared with healthy controls. In such patients, a high ANG I/II ratio is associated with greater norepinephrine requirements and is an independent predictor of mortality, thus providing a biological rationale for interventions aimed at its correction.
Trial registration
ClinicalTrials.gov identifier NCT02338843. Registered 14 January 2015.
Similar content being viewed by others
Background
Vasodilatory shock, a form of life-threatening generalized acute circulatory failure [1, 2], affects many patients in intensive care [3] and is associated with high mortality [4]. Vasodilatory shock has many etiologies, including but not limited to sepsis (the most common cause), inflammatory shock without infection (e.g., pancreatitis), postsurgical vasoplegia, endocrine shock, and spinal shock [5]. The primary goal of the hemodynamic treatment of such patients is to restore adequate mean arterial pressure (MAP) [6] with fluid resuscitation and/or vasopressors [7,8,9]. However, some patients are resistant to vasopressor therapy and require high doses to reach target MAP. This catecholamine-resistant vasodilatory shock (CRVS) is associated with adverse events [10, 11] and high mortality rates [12,13,14], but its pathophysiology is not well understood.
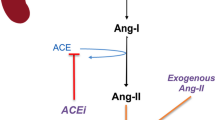
The peptide angiotensin I (ANG I) is an integral part of the renin-angiotensin-aldosterone system, which regulates blood pressure and is converted by the angiotensin-converting enzyme (ACE) to ANG II, making the ANG I/II ratio a marker of ACE function [15, 16]. Low levels of ANG II, a potent vasoconstrictor, are associated with increased mortality in severe sepsis [17], vasodilatory shock [18], and acute respiratory distress syndrome [19], all of which are conditions with endothelial injury, decreased endothelium-bound ACE activity, and decreased capacity to convert ANG I to ANG II [18,19,20]. Thus, the ANG I/II ratio may be elevated in CRVS and predict worse clinical outcomes. These considerations have become increasingly relevant since synthetic human ANG II was approved in the USA and Europe to increase MAP in patients with vasodilatory shock [21].
Accordingly, as part of the randomized, double-blind, phase 3 ATHOS-3 (Angiotensin II for the Treatment of High-Output Shock) trial (ClinicalTrials.gov, NCT02338843), we measured ANG I and II levels of patients with CRVS before initiation of synthetic human ANG II infusion and calculated their ratio. We hypothesized that such patients would have elevated ANG I levels and an increased ANG I/II ratio compared with healthy controls and that a higher ANG I/II ratio would be associated with increased norepinephrine requirements at baseline and with increased mortality.
Methods
Patients
Patients with vasodilatory shock
The ATHOS-3 study protocol, including patient characteristics, has been previously published [22, 23]. In brief, patients with catecholamine-resistant hypotension (defined as those with a total vasopressor dose >0.2 mcg/kg/min for ≥6 h) and high-output shock (defined as central venous oxygen saturation >70% with central venous pressure >8 mmHg or cardiac index >2.3 L/min/m2) were randomized and treated with either ANG II or placebo, plus standard of care. Blood samples were drawn and stored after randomization and prior to administration of study drug. Collected blood was centrifuged (2000 g for 10 min) and stored at –80 °C until shipped for analysis.
Healthy control sera
As part of the ANG I and ANG II assay validation, ANG I and ANG II levels were measured in banked sera donated by healthy volunteers.
ANG I and ANG II assessments
Endogenous serum concentrations of ANG I and ANG II were measured by ultra-performance liquid chromatography with tandem mass spectrometry detection, capable of measuring angiotensin peptide levels as low as 10 pg/mL (inVentiv Health Clinique, Quebec City, Quebec, Canada). Following rapid thawing of the serum, samples were stabilized with a combination of aliskiren, pepstatin A, and o-phenanthroline in acidified dimethyl sulfoxide combined with a mixture of EDTA and 4-(hydroxymercury) benzoic acid in phosphate-buffered saline. All samples were spiked with stable-isotope-labeled internal standards for ANG I and ANG II at a concentration of 50 pg/mL. Following protein precipitation using acetonitrile with 1% formic acid and solid-phase extraction (Oasis MCX; Waters Corporation, Milford, MA, USA) of the supernatant, samples underwent liquid chromatography-tandem mass spectrometry analysis using a reverse-phase analytical column (Acquity CSH C18; Waters Corporation) operating in line with an XEVO TQ-S triple quadrupole mass spectrometer (Waters Corporation) in multiple reaction monitoring. The sum of the signal from three different mass transitions per peptide was measured, and angiotensin concentrations were calculated by relating the ratio of peptide signal to internal standard signal.
Statistical analyses
Analyses of baseline ANG I, ANG II, and ANG I/II ratio and association with survival were prespecified. All other analyses, including comparison to healthy controls, were post hoc. Wilcoxon rank-sum test, Fisher’s exact test for binary outcomes, and chi-square statistic for other categorical outcomes were used for comparisons. Survival from the time of randomization to time of death from any cause was analyzed by the Kaplan–Meier formula. Estimates and confidence intervals were calculated by the product limit method and Greenwood’s formula for the variance and included the difference between treatment arms. For missing data in time-to-event analyses, including mortality at day 28, censored data techniques were utilized. Patients with missing data were censored on the last known survival date up to the specified endpoint (i.e., day 28).
Differences in survival between ANG I/II ratios above and below the median were analyzed by a two-sided log-rank test for mortality to day 28. Multivariate analyses were conducted for mortality to day 28, which included a stratified log-rank test using baseline strata and covariates that were not balanced. To adjust for the impact of multiple comparisons, a P < 0.01 was used to infer statistical significance.
Results
We studied 321 patients with vasodilatory shock. Sera from 24 healthy subjects formed the control group. Baseline ANG I and II levels are summarized in Table 1. In comparison to healthy controls, vasodilatory shock patients had substantially (roughly 6-fold) higher ANG I levels (253 pg/mL [interquartile range (IQR) 72.30–676.00 pg/mL] vs 42 pg/mL [IQR 30.46–87.34 pg/mL]; difference P < 0.0001) and higher ANG I/II ratios (1.63 [IQR 0.98–5.25] vs 0.4 [IQR 0.28–0.64]; difference P < 0.0001). In contrast, ANG II levels were not different between groups (84 pg/mL [IQR 23.85–299.50 pg/mL] vs 97 pg/mL [IQR 35.27–181.01 pg/mL]; difference P = 0.9895). Distribution of baseline ANG I and II levels and ANG I/II ratio for vasodilatory shock patients can be found in Additional file 1: Figures S1–S3 (Table 1).
Angiotensin I/II ratio
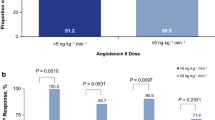
The median ANG I/II ratio across treatment arms at baseline was 1.63 (IQR 0.98–5.25). Patient demographics and disease characteristics by baseline median ANG I/II ratio were largely similar between groups (Table 2). However, recent exposure to ACE inhibitors was significantly more common in patients with a ratio above the median. Moreover, patients with a higher ANG I/II ratio had higher serum albumin concentrations and were receiving a higher dose of vasopressor support (norepinephrine-equivalent dose) at baseline. Baseline ANG I/II ratios were similar between the placebo (n = 139) and ANG II treatment arms (n = 142) (Table 2).
Survival by baseline ANG I/II ratio
Mortality in the trial’s placebo treatment arm was 64.7% in those with baseline ANG I/II ratio above the median and 45.2% in those with a ratio below the median (Fig. 1). In a multivariate analysis of mortality in the placebo arm, the baseline ANG I/II ratio was a significant predictor of overall mortality (hazard ratio 0.54; P = 0.0111) (Table 3, Fig. 1)
Discussion
We measured the plasma concentrations of ANG I and ANG II and calculated their ratio at baseline in patients enrolled in the ATHOS-3 study. We found that, in patients with CRVS, ANG I levels were higher than in healthy controls. We also found that despite much higher ANG I concentrations in the ATHOS-3 patients, ANG II levels were similar to those in healthy controls; this led to increased ANG I/II ratios. These observations suggest that ACE function and the conversion of ANG I to ANG II may be disordered in vasodilatory shock. Moreover, we found that ANG I/II ratios above the median were associated with specific baseline features (i.e., recent use of ACE inhibitor, greater dose of norepinephrine-equivalent administration, and greater severity of illness). Finally, we found that a high ANG I/II ratio predicted increased mortality.
Relationship to previous studies
Previous studies have reported that the baseline ANG I/II ratio averaged 0.38 in otherwise healthy patients with hypertension [15]; this is consistent with the ratio of 0.4 in healthy sera measured. The median ratio value of 1.63 for patients in the present study suggests a possible pathological decrease in conversion of ANG I to ANG II in patients with CRVS. Endothelial injury is common during septic shock. Thus, endothelial membrane–bound ACE activity may be reduced during shock. Logically, reduced ACE activity should lead to decreased ANG I to ANG II conversion and an increased ratio. A significant proportion of ATHOS-3 patients had high ANG I/II ratios, suggesting decreased ACE activity. Low levels of ANG II and ACE activity on day 1 have been previously reported in patients with sepsis and appear associated with a poor prognosis [17]. Decreased ACE activity could be due to an intrinsic defect in ACE function [20] or to small peptides with ACE inhibitory properties [24]. In addition, at least two pro-inflammatory cytokines (tumor necrosis factor-α [TNF-α] and interleukin-1β) downregulate ACE in cultured human endothelial cells [25]. Finally, while not examined in this study, different single-nucleotide polymorphisms of ACE can affect ACE activity and are associated with mortality rates in septic shock [26], possibly through interactions between TNF-α and such polymorphisms [27]. It appears biologically plausible that a high ANG I/II ratio may reflect decreased ACE activity. In keeping with this notion, the recent use of ACE inhibitors was markedly more common in patients with a high ANG I/II ratio in our study.
Another key enzyme, ACE2, can also affect the ANG I/II ratio. ACE2 catalyzes the conversion of ANG II to ANG (1–7) [28], and increased ACE2 activity may also decrease ANG II levels and increase ANG I/II ratios. Therefore, high ACE2 activity may contribute to a high ANG I/II ratio in vasodilatory shock.
Study implications
Our findings suggest that in many patients with CRVS, there is an imbalance between ANG I and ANG II levels. This imbalance may be related to changes in ACE1 and/or ACE2 activity, which may relatively diminish ANG II generation and can be exacerbated by recent ACE inhibitor administration. Moreover, the findings imply that diminished ability to convert ANG I to ANG II may contribute to a catecholamine-resistant vasodilatory state and increase the risk of death. In their aggregate, these findings suggest that there is a biological rationale for the exogenous administration of ANG II in CRVS.
Strengths and limitations
To our knowledge, this is one of the first studies to evaluate serum ANG I and ANG II levels and the ANG I/II ratio in patients with CRVS. Only a single recent pilot study found that increased ANG I levels were correlated with mortality [29]. In comparison, our study was much larger and involved several hundred patients in multiple countries and continents, thus providing a high level of external validity. In addition, this study utilized a double-blind, placebo-controlled, phase 3 registration trial design, assuring that characteristics and outcomes were collected prospectively and were independently monitored; this minimized selection and ascertainment bias. The measurements of ANG I and ANG II were performed by an independent laboratory blinded to clinical characteristics, thus further minimizing bias. Moreover, the analysis of such data followed a prespecified protocol. Finally, the associations observed appear logical and consistent with current knowledge of the physiology and pathophysiology of ANG I, ANG II, and ACE1 and ACE2 activity in inflammatory states.
Our study had limitations. We dichotomized ANG I/II ratios as part of our assessment. Such an approach simplifies comparisons but is insensitive to the continuous nature of biological variables. Thus, the correct specific cutoff point to inform clinical decisions remains unknown. Follow-up was to 28 days only, so implications for longer survival windows could not be made. In addition, ACE activity was not measured directly; rather, ACE activity was inferred from the ratio of ANG I/II in this study. However, patients with prior exposure to ACE inhibitors appeared to be particularly prone to a high baseline ANG I/II ratio, indicating that, in at least some patients, a high baseline ratio very likely resulted from decreased ACE activity. We did not measure the ANG I/II ratio in real time. However, ANG I and II concentrations were collected prospectively as part of a prespecified analysis. We did not measure ACE2 activity as part of the ATHOS-3 study. Thus, our suggestion that increased ACE2 activity may affect the ANG I/II ratio remains speculative. Further studies will require a more detailed assessment of the increasingly complex angiotensin family of molecules and their interaction with ACE1 and ACE2 activity.
Conclusions
In CRVS, both ANG I and the ANG I/II ratio are elevated. High ANG I/II ratios are associated with specific baseline clinical features and predict increased mortality. These observations provide a biological rationale for interventions aimed at correcting such imbalance.
Availability of data and materials
The data that support the findings of this study are available from La Jolla Pharmaceutical Company, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. However, data are available from the authors upon reasonable request and with the permission of La Jolla Pharmaceutical Company.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ANG:
-
Angiotensin
- ATHOS-3:
-
Angiotensin II for the Treatment of High-Output Shock
- CRVS:
-
Catecholamine-resistant vasodilatory shock
- MAP:
-
Mean arterial pressure
- TNF-α:
-
Tumor necrosis factor-α
References
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–15.
Vincent JL, De Backer D. Circulatory shock. New Engl J Med. 2013;369(18):1726–34.
Sakr Y, Reinhart K, Vincent JL, Sprung CL, Moreno R, Ranieri VM, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) study. Crit Care Med. 2006;34(3):589–97.
Bassi E, Park M, Azevedo LC. Therapeutic strategies for high-dose vasopressor-dependent shock. Crit Care Res Pract. 2013;2013:654708.
Standl T, Annecke T, Cascorbi I, Heller AR, Sabashnikov A, Teske W. The nomenclature, definition and distinction of types of shock. Dtsch Arztebl Int. 2018;115(45):757–68.
Leone M, Asfar P, Radermacher P, Vincent JL, Martin C. Optimizing mean arterial pressure in septic shock: a critical reappraisal of the literature. Crit Care. 2015;19:101.
Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45(6):1061–93.
Howell MD, Davis AM. Management of sepsis and septic shock. JAMA. 2017;317(8):847–8.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Avni T, Lador A, Lev S, Leibovici L, Paul M, Grossman A. Vasopressors for the treatment of septic shock: systematic review and meta-analysis. PLoS One. 2015;10(8):e0129305.
Schmittinger CA, Torgersen C, Luckner G, Schroder DC, Lorenz I, Dunser MW. Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 2012;38(6):950–8.
Brown SM, Lanspa MJ, Jones JP, Kuttler KG, Li Y, Carlson R, et al. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143(3):664–71.
Auchet T, Regnier MA, Girerd N, Levy B. Outcome of patients with septic shock and high-dose vasopressor therapy. Ann Intensive Care. 2017;7(1):43. https://doi.org/10.1186/s13613-017-0261-x.
Brand DA, Patrick PA, Berger JT, Ibrahim M, Matela A, Upadhyay S, Spiegler P. Intensity of vasopressor therapy for septic shock and the risk of in-hospital death. J Pain Symptom Manag. 2017;53(5):938–43.
Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertens. 1996;14(6):799–805.
Azizi M, Chatellier G, Guyene TT, Murieta-Geoffroy D, Menard J. Additive effects of combined angiotensin-converting enzyme inhibition and angiotensin II antagonism on blood pressure and renin release in sodium-depleted normotensives. Circulation. 1995;92(4):825–34.
Zhang W, Chen X, Huang L, Lu N, Zhou L, Wu G, Chen Y. Severe sepsis: low expression of the renin-angiotensin system is associated with poor prognosis. Exp Ther Med. 2014;7(5):1342–8.
Rice CL, Kohler JP, Casey L, Szidon JP, Daise M, Moss GS. Angiotensin-converting enzyme (ACE) in sepsis. Circ Shock. 1983;11(1):59–63.
Orfanos SE, Armaganidis A, Glynos C, et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation. 2000;102(16):2011–8.
Chawla LS, Busse LW, Brasha-Mitchell E, Alotaibi Z. The use of angiotensin II in distributive shock. Crit Care. 2016;20(1):137.
Giapreza (angiotensin II) injection for intravenous infusion [package insert]. San Diego, CA: La Jolla Pharmaceutical Company; 2017.
Chawla LS, Russell JA, Bagshaw SM, Shaw AD, Goldstein SL, Fink MP, Tidmarsh GF. Angiotensin II for the Treatment of High-Output Shock 3 (ATHOS-3): protocol for a phase III, double-blind, randomised controlled trial. Crit Care Resusc. 2017;19(1):43–9.
Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017;377(5):419–30.
Kistler E, Li J, Mazor R, Munoz D, Aletti F, Santamaria M. ACE inhibition by circulating peptides formed de novo in experimental hemorrhagic shock. Crit Care Med. 2016;44(12):120.
Saijonmaa O, Nyman T, Fyhrquist F. Downregulation of angiotensin-converting enzyme by tumor necrosis factor-alpha and interleukin-1beta in cultured human endothelial cells. J Vasc Res. 2001;38(4):370–8.
Dou XM, Cheng HJ, Meng L, Zhou LL, Ke YH, Liu LP, Li YM. Correlations between ACE single nucleotide polymorphisms and prognosis of patients with septic shock. Biosci Rep. 2017;37(2). https://doi.org/10.1042/BSR20170145.
Yende S, Quasney MW, Tolley EA, Wunderink RG. Clinical relevance of angiotensin-converting enzyme gene polymorphisms to predict risk of mechanical ventilation after coronary artery bypass graft surgery. Crit Care Med. 2004;32(4):922–7.
Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev. 2018;98(1):505–53.
Reddy R, Asante I, Liu S, Parikh P, Liebler J, Borok Z, et al. Circulating angiotensin peptides levels in acute respiratory distress syndrome correlate with clinical outcomes: a pilot study. PLoS One. 2019;14(3):e0213096.
Acknowledgments
We thank the patients and their families for entrusting us to conduct this study. We also thank the study investigators, study coordinators, and support staff across all sites for their compassion, dedication, and diligence in the conduct of this trial. Medical editorial assistance was provided by Robert J. Schoen, PharmD, of ApotheCom, Yardley, PA, USA. This assistance was funded by La Jolla Pharmaceutical Company, San Diego, CA, USA.
Funding
The original study and the prespecified and post hoc analyses were funded by La Jolla Pharmaceutical Company. La Jolla Pharmaceutical Company was involved in the design of the study; the collection, analysis, and interpretation of data; and the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
RB, AMD, MTM, and TEA contributed to study conception and design. RGW, HS, AMD, AKK, MTM, MO, and TEA contributed to the acquisition of the data. RB, RGW, and LWB contributed to the analysis and/or interpretation of data. All authors contributed to the drafting or critical revision of the article for important intellectual content. All authors gave final approval of the version of the article to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All sites received approval from their respective ethics boards as part of the ATHOS-3 study (Khanna A et al. N Engl J Med. 2017;377(5):419–430.
Consent for publication
Not applicable.
Competing interests
Rinaldo Bellomo, Richard G. Wunderink, Ashish K. Khanna, Marlies Ostermann, and Paul J. Young have nothing to disclose.
Harold Szerlip received personal fees from La Jolla Pharmaceutical Company during the conduct of the study.
Shane W. English’s research center (Ottawa Hospital Research Institute) received per-patient reimbursement from La Jolla Pharmaceutical company for research coordinator time.
Laurence W. Busse has received consulting fees from La Jolla Pharmaceutical Company.
Adam M. Deane’s employer at the time of study (Royal Adelaide Hospital) received per-patient reimbursement to the institution from La Jolla Pharmaceutical Company for research coordinator time.
Michael T. McCurdy has served on the speakers’ bureau for La Jolla Pharmaceutical Company.
Damian R. Handisides and Lakhmir S. Chawla are employees of La Jolla Pharmaceutical Company and own stock.
George F. Tidmarsh was an employee of La Jolla Pharmaceutical Company at the time of the analysis and owns stock.
Timothy E. Albertson received institutional research funding during the conduct of the study and speaking honoraria after the conduct of the study from La Jolla Pharmaceutical Company.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Angiotensin I distribution at baseline. Figure S2. Angiotensin II distribution at baseline. Figure S3. Angiotensin I/II ratio distribution at baseline.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bellomo, R., Wunderink, R.G., Szerlip, H. et al. Angiotensin I and angiotensin II concentrations and their ratio in catecholamine-resistant vasodilatory shock. Crit Care 24, 43 (2020). https://doi.org/10.1186/s13054-020-2733-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-020-2733-x