Abstract
Background
Diabetes mellitus is a common co-existing disease in the critically ill. Diabetes mellitus may reduce the risk of acute respiratory distress syndrome (ARDS), but data from previous studies are conflicting. The objective of this study was to evaluate associations between pre-existing diabetes mellitus and ARDS in critically ill patients with acute hypoxemic respiratory failure (AHRF).
Methods
An ancillary analysis of a global, multi-centre prospective observational study (LUNG SAFE) was undertaken. LUNG SAFE evaluated all patients admitted to an intensive care unit (ICU) over a 4-week period, that required mechanical ventilation and met AHRF criteria. Patients who had their AHRF fully explained by cardiac failure were excluded. Important clinical characteristics were included in a stepwise selection approach (forward and backward selection combined with a significance level of 0.05) to identify a set of independent variables associated with having ARDS at any time, developing ARDS (defined as ARDS occurring after day 2 from meeting AHRF criteria) and with hospital mortality. Furthermore, propensity score analysis was undertaken to account for the differences in baseline characteristics between patients with and without diabetes mellitus, and the association between diabetes mellitus and outcomes of interest was assessed on matched samples.
Results
Of the 4107 patients with AHRF included in this study, 3022 (73.6%) patients fulfilled ARDS criteria at admission or developed ARDS during their ICU stay. Diabetes mellitus was a pre-existing co-morbidity in 913 patients (22.2% of patients with AHRF). In multivariable analysis, there was no association between diabetes mellitus and having ARDS (OR 0.93 (0.78–1.11); p = 0.39), developing ARDS late (OR 0.79 (0.54–1.15); p = 0.22), or hospital mortality in patients with ARDS (1.15 (0.93–1.42); p = 0.19). In a matched sample of patients, there was no association between diabetes mellitus and outcomes of interest.
Conclusions
In a large, global observational study of patients with AHRF, no association was found between diabetes mellitus and having ARDS, developing ARDS, or outcomes from ARDS.
Trial registration
NCT02010073. Registered on 12 December 2013.
Similar content being viewed by others
Background
Acute hypoxemic respiratory failure (AHRF) is a common cause of admission to the intensive care unit (ICU). Many patients with AHRF will meet criteria for acute respiratory distress syndrome (ARDS), whilst those that do not meet the criteria remain at risk of developing it. ARDS remains a common condition in the critically ill and is associated with high mortality [1]. The long-term sequelae of ARDS are considerable, with substantive reductions in long-term quality of life [2, 3].
Despite numerous clinical trials there remain few therapeutic options for patients with ARDS [4]. Recent studies have identified subphenotypes of ARDS, which have a differential response to treatment [5]. The variety of pre-disposing conditions and inciting events for ARDS contributes to the heterogeneity of patients with this condition. It has been hypothesised that this heterogeneity may explain why therapies that were effective in exploratory studies were not shown to be effective in phase III trials [4]. Targeting interventions to prevent ARDS in patients at higher risk may prove more effective, and it is therefore necessary to identify those groups of patients who have a higher risk of ARDS.
Diabetes mellitus is common [6], with 4.4% of the world’s population anticipated to have a diagnosis by 2030 [7]. Observational data suggest that 40% of patients admitted to ICUs have a pre-existing diagnosis of diabetes mellitus [8]. Diabetes mellitus may protect against the development of ARDS. Whilst several studies indicated that patients with diabetes mellitus are less likely to develop ARDS [9,10,11,12,13,14], others did not demonstrate any protective effect of diabetes mellitus [15, 16]. The effect of diabetes mellitus on the risk of mortality in critically ill patients is also unclear, with most [17,18,19], but not all [16], data suggesting that diabetes mellitus is not associated with an increased risk of mortality. No studies have examined this relationship since the introduction of the current Berlin definition of ARDS [20]. Therefore, there remains a need to clarify and define any associations between diabetes mellitus and this syndrome. Any potential association could have significant impact on a clinician’s evaluation of a patient’s anticipated clinical outcome and may help to inform clinical trials evaluating therapies to prevent and treat ARDS.
The Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) undertaken in 459 ICUs in 50 countries [21] provides a unique opportunity to evaluate associations between pre-existing diabetes mellitus and the presence of ARDS in critically ill patients with acute hypoxemic respiratory failure. Secondary objectives of this analysis were to explore for any association of diabetes mellitus with the progression of AHRF to ARDS, and subsequent clinical outcome.
Methods
Study design
An ancillary analysis of LUNG SAFE was performed. This was a global, multi-centre prospective cohort study that enrolled 4499 patients with AHRF in 459 ICUs across 50 countries. The details of this study have previously been described [21]. Briefly, all patients admitted to a participating ICU were screened daily for AHRF (defined as the concurrent presence of (1) a ratio of partial pressure of arterial blood oxygen content to inspired fraction of oxygen (PaO2:FiO2 ratio) ≤ 300 mmHg, (2) acute pulmonary infiltrates identified on chest x-ray or computed tomography and (3) mechanical ventilation with a positive end-expiratory pressure (PEEP) of at least 5 cmH2O) during an enrolment period of 4 consecutive weeks in winter. Exclusion criteria were age below 16 years or inability to obtain informed consent (when required by local regulations).
Data were collected until day 28 (days 1, 2, 3, 5, 7, 14, 21 and 28), ICU discharge or death (whichever occurred earlier). Day 1 was defined as the day in which the patient met criteria for AHRF (baseline). Outcome data were recorded at hospital discharge or at day 90 (whichever occurred earliest). Meaurement of partial pressure of arterial carbon dioxide (PaCO2) was collected daily. Bicarbonate (\( {\mathrm{HCO}}_3^{-} \)) data were not collected, instead the data were derived by the Henderson–Hasselbalch equation:
Using prospectively identified data, ARDS was defined according to the Berlin ARDS definition [20]: (1) presence of AHRF criteria, (2) onset within 1 week of insult or new or worsening respiratory symptoms, (3) bilateral opacities on chest x-ray or computed tomography and (4) cardiac failure not being the primary cause of AHRF. Patients were identified as having diabetes mellitus if it was a documented co-morbidity. The data collection form used in LUNG SAFE did not allow for inclusion of diabetes type, medications taken, or glycaemic control, and therefore this information was not available for this ancillary analysis. Furthermore, data were only collected for patients who met AHRF criteria. Each site investigator in LUNG SAFE was responsible for ensuring data integrity. No specific guidance on the diagnosis of cardiac failure was given to sites, and the method used to determine whether it was the sole explanation for AHRF was not recorded.
Outcomes
The primary outcome was presence of ARDS. This was defined as meeting criteria for ARDS at any time during the follow-up period from meeting AHRF criteria. Secondary outcomes included development of ARDS (defined as occurring after day 2 from meeting AHRF criteria), duration of invasive mechanical ventilation, and hospital mortality (defined as outcome at day 90, or hospital discharge, whichever occurred earliest) in patients with ARDS. Duration of invasive mechanical ventilation was calculated as the number of days between the date of intubation and the date of extubation (or death, if the patient died during invasive mechanical ventilation).
Statistical analysis
Descriptive statistics included calculation of proportions for categorical variables and mean (± standard deviation) for continuous variables. The AHRF population was stratified according to the presence of diabetes mellitus, and the statistical difference between groups (diabetic patients, non-diabetic patients) was evaluated by chi-square test (or Fisher exact test) for discrete variables and by t test or Wilcoxon rank sum test for continuous variables. The Shapiro–Wilks test was applied to assess normality of data distribution.
Patients were excluded from the primary analysis if cardiac failure was the only cause of AHRF. All clinical variables and covariates were entered into multivariable logistic regression model with variable selection based on a stepwise approach (forward and backward selection combined with a significance level of 0.05 both for entry and retention) to identify a set of independent variables associated with having ARDS at any time during follow up and after the second day after meeting AHRF criteria and with hospital mortality. A documented co-morbidity of diabetes mellitus (“Diabetes diagnosis”) was locked into the multivariable model as it was the primary exposure of interest. As most patients in LUNG SAFE met ARDS criteria within 48 h of AHRF onset [21], those patients who met ARDS criteria after 48 h were studied separately, and were deemed to have developed ARDS. If diabetes mellitus was not detected as a statistically significant predictor in the final multivariable logistic regression model, for each outcome the entry of this variable was forced into the model. We also evaluated in the models the interaction term “diabetes and presence of a pulmonary ARDS risk factor” in order to assess a possible different effect of diabetes mellitus on outcomes in patients with or without a pulmonary ARDS risk factor. Results of logistic models are shown as odds ratios (ORs) with 95% confidence intervals (CIs) and p value. No assumptions were made for missing data.
To account for the differences in baseline characteristics between patients with AHRF, propensity score was used to match (1:1 without replacement) diabetic and non-diabetic patients. Propensity score was estimated using a logistic regression model that had “presence of diabetes” as the response variable and that contained as predictors sex, age, body mass index (BMI), non-pulmonary Sequential Organ Failure Assessment (SOFA) score (adjusted for missing values), co-morbidities (chronic liver failure, chronic renal failure, chronic heart failure, haematologic neoplasm, immunosuppression, active neoplasm, chronic obstructive pulmonary disease (COPD) or home ventilation), ARDS risk factors and ventilator variables at baseline. The balance in measured variables between groups (diabetic and non-diabetic subjects) has been assessed using standardised difference and a value of less than 0.10 likely denoted a negligible imbalance. The association between diabetes mellitus and outcomes of interest was assessed in matched samples using McNemar’s test for dichotomous outcomes and Wilcoxon signed ranks test for continuous outcomes.
Kaplan–Meier analysis was applied to estimate the probability of hospital mortality within 90 days of AHRF onset. It was assumed that patients discharged alive from hospital before 90 days were alive at day 90. The difference in survival curves between diabetic and non-diabetic patients was evaluated using the log-rank test.
All p values were two-sided, and a p value less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA) and with R, version 3.3.3. (R Project for Statistical Computing, https://www.r-project.org/).
Results
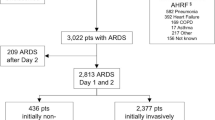
AHRF was identified in 4499 patients: 392 patients (8.7%) were excluded from the primary analysis because their respiratory failure was fully explained by cardiac failure (Additional file 1: Figure S1). Of the remaining 4107 patients with AHRF, 3022 patients (73.6%) fulfilled ARDS criteria within 2 days of AHRF onset (N = 2813; 93.1%) or developed ARDS during their ICU admission (N = 209; 6.9%). A total of 913 patients (22.2%) had diabetes mellitus and of these, 657 (72.0%) met ARDS criteria during their ICU admission (Additional file 1: Table S1). The baseline characteristics of patients with AHRF are summarised in Table 1. Patients with diabetes mellitus were older, had a higher BMI, received mechanical ventilation with larger tidal volumes and higher plateau pressures, had more co-morbidities but were less likely to have a risk factor for ARDS. Active neoplasm, haematological neoplasm and immunosuppression occurred more frequently in non-diabetic patients.
Presence of ARDS (at any time)
There was no difference in the incidence of ARDS between patients with and without diabetes mellitus (72% with vs. 74% without diabetes mellitus; p = 0.21) (Additional file 1: Table S1). In multivariable analysis, there was no association between diabetes mellitus and having ARDS (OR 0.93 (0.78–1.11); p = 0.39). Haematological neoplasm, baseline PaO2:FiO2 ratio, respiratory rate, PEEP and peak inspiratory pressure were significantly associated with having ARDS, whilst COPD or home ventilation were associated with a reduced likelihood of having ARDS (Table 2). No difference in the relationship between diabetes and incidence of ARDS was detected between patients with or without a pulmonary risk factor (p = 0.99, data not shown).
Development of ARDS (after day 2)
A total of 209 patients developed ARDS after 2 days from meeting AHRF criteria. The proportion of patients who developed ARDS after day 2 was similar between the diabetic and non-diabetic cohort (4.8% vs. 5.2%; p = 0.67) (Additional file 1: Table S1).
Patients with diabetes mellitus who developed ARDS after day 2 were older and had more frequent co-existing chronic renal failure, than non-diabetic patients (Additional file 1: Table S2).
In patients who remained at risk of developing ARDS after day 2 from meeting AHRF criteria (N = 1294), there was no association identified in multivariable analysis between diabetes mellitus and developing ARDS (OR 0.79 (0.54–1.15); p = 0.22). Baseline peak inspiratory pressure, age, pulmonary risk factors and the combination of pulmonary and non-pulmonary risk factors were all associated with increased likelihood of developing ARDS after day 2 from meeting AHRF criteria (Table 3).
Outcomes in ARDS
A total of 3022 patients had ARDS at any time during the follow-up period (Additional file 1: Table S1). Patients with diabetes mellitus who developed ARDS were older, had a higher BMI, received mechanical ventilation at baseline with higher tidal volumes and plateau pressure, and had more co-morbidities than non-diabetic patients. Documented immunosuppression (unrelated to diabetes mellitus) was more prevalent in those without diabetes mellitus (Additional file 1: Table S6). The duration of invasive mechanical ventilation was similar between the two groups overall (13.4 ± 17.0 days vs. 12.5 ± 13.4 days; p = 0.70) and in survivors and non-survivors. Hospital mortality was similar between the two groups (41.6% with vs. 38.8% without diabetes mellitus; p = 0.19) (Additional file 1: Table S7). There was no difference in survival probability between patients with and without diabetes mellitus (log-rank test, p = 0.28) (Fig. 1).
There was no association between diabetes mellitus and hospital mortality in those that had ARDS at any time when analysed using a univariate (Additional file 1: Table S8) or multivariate approach (OR 1.15 (0.93–1.42); p = 0.19) (Table 4). No difference in the relationship between diabetes mellitus and hospital mortality was detected between patients with or without a pulmonary ARDS risk factor (p = 0.26, data not shown). In patients who developed ARDS after day 2, there was no association between diabetes mellitus and hospital mortality (OR 1.07 (0.53–2.17); p = 0.84) (Additional file 1: Table S10).
Assessing the impact of diabetes mellitus using propensity score matching
In a matched sample of patients with and without diabetes mellitus there was no difference in baseline characteristics (Additional file 1: Table S11). In this cohort of patients, diabetes mellitus was not associated with having ARDS (72.0% with vs. 72.8% without diabetes mellitus; p = 0.77), nor with developing ARDS after day 2 (5.2% vs. 5.7%; p = 0.52), nor with hospital mortality (40.3% vs. 38.3%; p = 0.48) (Additional file 1: Table S12).
Presence of diabetes mellitus and respiratory muscle dysfunction
Additional file 1: Figure S2, shows blood PaCO2 (panel A) and bicarbonate (panel B) over time in diabetic and non-diabetic patients with AHRF. At day 5 after meeting AHRF criteria, patients with diabetes mellitus had a higher blood bicarbonate level, but no difference in blood PaCO2. In contrast, at day 14, patients with diabetes mellitus had a higher PaCO2 but did not have any difference in blood bicarbonate, compared to patients without diabetes mellitus.
Discussion
In this large, global, multi-centre prospective observational study of patients with AHRF, pre-existing diabetes mellitus was not associated with the presence of ARDS in patients who have AHRF not fully explained by cardiac failure. In patients who remained at risk of developing ARDS after 48 h from having AHRF, there was no association between diabetes mellitus and developing ARDS. Finally, in both cohorts, diabetes mellitus did not modify outcomes.
Previous studies have evaluated whether there is an association between diabetes mellitus and risk of ARDS [9,10,11,12,13,14,15,16, 22], although the results are inconsistent. The LUNG SAFE cohort includes all patients meeting ARDS criteria and provide more robust data than prior observational studies because of the frequency of clinician under-recognition of ARDS [21]. Moreover, that LUNG SAFE comprises 3022 patients with ARDS from 459 ICUs in 50 countries means the findings are more generalizable. Although there are fewer patients with diabetes mellitus, this cohort included many more patients with ARDS than were included in a meta-analysis of this topic [23]. The findings presented in this study are relevant to clinicians evaluating the risk of a patient with a pre-existing history of diabetes mellitus developing ARDS in the setting of AHRF, as well as their outcome.
The limitations of this study include those inherent in observational studies. Epidemiological studies suggest that 5–15% of patients admitted to the ICU have unrecognised diabetes mellitus [8]. Given that data were not collected during LUNG SAFE to identify patients with unrecognised diabetes mellitus, a proportion of patients will be incorrectly categorised. Furthermore, this was not a pre-specified analysis in the LUNG SAFE study, and therefore does not include all the variables that would be of interest when investigating diabetes mellitus and ARDS. As a consequence, it is only possible to adjust for confounding factors that were collected as part of LUNG SAFE [21]. Like most of the previously published data, this cohort was not separated into different types of diabetes mellitus. Although patients with diabetes mellitus had a significantly higher BMI compared with non-diabetic patients (Table 1), we are unable to confirm if this reflects the majority of patients with diabetes mellitus being diagnosed as having type 2 diabetes mellitus. This is potentially important because of the different pathophysiological processes between the various forms of diabetes mellitus that may exert different effects in ARDS. However, in an exploratory analysis of a previous observational study, it was identified that both type 1 and type 2 diabetes mellitus were independently associated with reduced development of ARDS [14]. The results presented in this study are hypothesis generating. Any risk or protective effect associated with diabetes mellitus may be modified by the magnitude of pre-existing glucose intolerance, chronic end-organ complications of diabetes mellitus and chronic glucose-lowering drugs [24, 25]. Accordingly, future investigations of the relationship between diabetes mellitus and ARDS should aim to have a more rigorous screening process for diabetes mellitus and should consider evaluating an association between different types of diabetes mellitus and ARDS, as well as the confounding effects of treatment.
In LUNG SAFE, the criteria for AHRF determined that patients who were included in the analysis had significant lung injury at the point of entry into the study. Whilst this describes a significant proportion of patients who develop ARDS, the cohort of patients “at risk” of ARDS include those with less significant physiological derangement (e.g. patients with pneumonia but a PaO2:FiO2 ratio > 300 mmHg), and therefore does not include all patients at risk of ARDS. The LUNG SAFE study did not collect detailed data on all screened patients (Additional file 1: Figure S1). Therefore, it was not possible to assess the relationship between diabetes mellitus and ARDS in all patients at risk. This includes patients with respiratory failure who were not receiving ventilatory support, both within and outside the ICU environment, therefore limiting the applicability of these findings. Finally, it is possible that the sample of patients developing ARDS after day 2 was too small to detect an association with diabetes mellitus.
In this analysis of the LUNG SAFE cohort, patients who had AHRF only due to cardiac failure were excluded, providing a clearer description of the relationship between diabetes mellitus, ARDS and outcomes of AHRF. When these patients were included in the analysis, diabetes mellitus was associated with a reduced risk of developing ARDS (data not shown). This is explained by more patients with diabetes mellitus having their AHRF fully explained by cardiac failure (11.6% with vs. 7.8% without diabetes mellitus; p = 0.0002). Most prior observational studies that demonstrated a protective effect of diabetes mellitus on ARDS, did not exclude patients who developed cardiac failure from their at-risk population [9, 10, 12, 14]. In one study that excluded patients with cardiogenic pulmonary oedema at the onset of septic shock, patients with diabetes mellitus developed ARDS less frequently than those patients without diabetes mellitus; however, in a final multiple logistic regression analysis diabetes mellitus was not significantly associated with ARDS [16]. This demonstrates that combining patients who develop cardiac failure with those that have neither ARDS nor cardiac failure can cause a bias in the estimates of the effect of diabetes mellitus on ARDS [16], and the results presented in this analysis of LUNG SAFE support that finding. Future studies evaluating exposure risk in ARDS should consider excluding patients who develop acute hypoxaemic respiratory failure due to cardiac failure in their risk analysis.
Most patients met ARDS criteria within the first 48 h of having AHRF. In those patients that developed ARDS (i.e. after day two from AHRF), there was no association between diabetes mellitus and ARDS. This expands on the understanding gathered from previous observational studies. Patients with diabetes mellitus received more injurious mechanical ventilation at baseline (Table 1), and it is possible that ventilator-induced lung injury negated any protective effect that diabetes mellitus has upon the development of ARDS. There was no statistically significant association between diabetes mellitus and reduction in mortality in those who developed ARDS, a finding that supports prior observational data [9, 10, 12, 14].
The results of most previous studies have demonstrated that diabetes mellitus is not associated with increased ICU mortality [17,18,19]. However, in some circumstances diabetes mellitus may have a negative effect upon patients’ health and outcome from disease. For example, during the 2009 H1N1 influenza pandemic, diabetes mellitus was associated with more severe infection and with a greater risk of death [26]. Similarly, diabetes mellitus is associated with an increased risk of hospitalisation from community-acquired pneumonia and bacterial infection [27], with patients < 40 years of age at the highest risk when compared with age-matched controls [28]. ARDS can be sub-divided into “direct” and “indirect” based on the underlying insult, and both influenza and community-acquired pneumonia would be considered direct insults. Given the association between diabetes mellitus and more severe disease, it is plausible that there is a harmful association between diabetes mellitus and ARDS in patients who have direct risk factors. However, previous data have demonstrated that diabetes mellitus is independently associated with reduced risk of mortality in direct ARDS (defined as ARDS associated with gastric aspiration or pneumonia) [29]. Interestingly, in this ancillary analysis of LUNG SAFE, there was no association between diabetes mellitus and ARDS or hospital mortality among patients who had at least one pulmonary risk factor. This difference may be explained by the difference between the included patients, as LUNG SAFE included inhalational injury, pulmonary contusion, pulmonary vasculitis and drowning, alongside gastric aspiration and pneumonia, as direct risk factors.
Diabetes mellitus may act as a confounding factor for receiving insulin therapy, and it may be that the previously observed protective effects of diabetes mellitus in ARDS may reflect an immune-modulatory effect of medications such as insulin [30]. Pre-clinical studies have demonstrated protective benefits of insulin therapy in lung injury secondary to trauma [31], and when used to maintain euglycaemia in ARDS secondary to endotoxaemia [32]. However, other data suggest that immune hypo-responsiveness in diabetes mellitus is reversed by insulin therapy, and associated with alveolar neutrophil infiltration and increased alveolar concentration of pro-inflammatory cytokines in lipopolysaccharide (LPS)-induced lung injury [33], suggesting that insulin may restore immune function and therefore could reverse some of the protective effects of diabetes mellitus in relation to ARDS. Other medications that have been identified as having potential immuno-modulatory effects, including metformin [34, 35], aspirin [36] ace-inhibitors [4] and statins [37] may act as confounding factors in this study. Diabetic polyneuropathy has been demonstrated to affect respiratory neuromuscular function in patients with type 2 diabetes mellitus [38], but it is unknown whether diabetic polyneuropathy has a significant impact in patients with AHRF. In an assessment of blood PaCO2 and bicarbonate over time in patients with and without diabetes mellitus, there was no trend identified to suggest that respiratory muscle insufficiency was greater in either cohort. However, this analysis is limited, and further assessment of this important, potential confounding factor, may be warranted.
Patients with diabetes mellitus had significant differences in their baseline co-morbidities when compared to patients without diabetes mellitus. To account for this, a post-hoc propensity score was used to match patients with and without diabetes mellitus. This analysis did not demonstrate an association between diabetes mellitus and outcomes of interest, suggesting that the absence of an effect of diabetes mellitus in the wider AHRF population is unlikely to have been due to baseline population imbalance.
Conclusions
In this global, multi-centre, prospective observational study of mechanically ventilated patients with AHRF, there was no association identified between diabetes mellitus and ARDS or outcomes. These findings are hypothesis generating and may inform clinicians about the risk of developing ARDS and the outcomes of patients with diabetes mellitus and AHRF. Further research is required to establish if there is an effect between the type and severity of diabetes mellitus, as well as the confounding effect of treatment for this condition, and the development and outcome of ARDS.
Abbreviations
- AHRF:
-
Acute hypoxemic respiratory failure
- ARDS:
-
Acute respiratory distress syndrome
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- ICU:
-
Intensive care unit
- LUNG SAFE:
-
Large observational study to understand the global impact of severe acute respiratory failure
- NYHA:
-
New York Heart Association
- OR:
-
Odds ratio
- PaCO2 :
-
Partial pressure of arterial carbon dioxide
- PaO2:FiO2 ratio:
-
Ratio of partial pressure of arterial blood oxygen content to inspired fraction of oxygen
- PEEP:
-
Positive end-expiratory pressure
- SOFA:
-
Sequential Organ Failure Assessment
References
Mac Sweeney R, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416–30.
Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304.
Pfoh ER, Wozniak AW, Colantuoni E, Dinglas VD, Mendez-Tellez PA, Shanholtz C, et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–66.
Boyle AJ, Mac Sweeney R, McAuley DF. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med. 2013;11:166.
Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–20.
The Lancet. The diabetes pandemic. The Lancet. 2011;378:99.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53.
Kar P, Jones KL, Horowitz M, Deane AM. Management of critically ill patients with type 2 diabetes: the need for personalised therapy. World J Diabetes. 2015;6:693–706.
Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28:2187–92.
Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–8.
Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, et al. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Crit Care Med. 2008;36:1518–22.
Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13:R18.
Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, Kojicic M, Kashyap R, Thakur S, et al. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J. 2011;37:604–9.
Yu S, Christiani DC, Thompson BT, Bajwa EK, Gong MN. Role of diabetes in the development of acute respiratory distress syndrome. Crit Care Med. 2013;41:2720–32.
Kor DJ, Warner DO, Alsara A, Fernández-Pérez ER, Malinchoc M, Kashyap R, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. 2011;115:117–28.
Koh GCKW, Vlaar APJ, Hofstra JJ, de Jong HK, van Nierop S, Peacock SJ, et al. In the critically ill patient, diabetes predicts mortality independent of statin therapy but is not associated with acute lung injury: a cohort study. Crit Care Med. 2012;40:1835–43.
Vincent J-L, Preiser J-C, Sprung CL, Moreno R, Sakr Y. Insulin-treated diabetes is not associated with increased mortality in critically ill patients. Crit Care. 2010;14:R12.
Stegenga ME, Vincent J-L, Vail GM, Xie J, Haney DJ, Williams MD, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med. 2010;38:539–45.
Graham BB, Keniston A, Gajic O, Trillo Alvarez CA, Medvedev S, Douglas IS. Diabetes mellitus does not adversely affect outcomes from a critical illness. Crit Care Med. 2010;38:16–24.
The ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33.
Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800.
Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, et al. Early identification of patients at risk of acute lung injury. Am J Respir Crit Care Med. 2011;183:462–70.
Gu W-J, Wan Y-D, Tie H-T, Kan Q-C, Sun T-W. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: a meta-analysis. PLoS One. 2014;9:e90426.
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–55.
Luethi N, Cioccari L, Biesenbach P, Lucchetta L, Kagaya H, Morgan R, et al. Liberal glucose control in ICU patients with diabetes: a before-and-after study. Crit Care Med. 2018;46:935–42.
Kerkhove MDV, Vandemaele KAH, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Risk factors for severe outcomes following 2009 influenza a (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053.
Hamilton EJ, Martin N, Makepeace A, Sillars BA, Davis WA, Davis TME. Incidence and predictors of hospitalization for bacterial infection in community-based patients with type 2 diabetes: the Fremantle diabetes study. PLoS One. 2013;8:e60502.
Kornum JB, Thomsen RW, Riis A, Lervang H-H, Schønheyder HC, Sørensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care. 2008;31:1541–5.
Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, et al. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151:755–63.
Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit Care Med. 2009;37:2455–64.
Donnelly M, Condron C, Murray P, Bouchier-Hayes D. Modulation of the glycemic response using insulin attenuates the pulmonary response in an animal trauma model. J Trauma. 2007;63:351–7.
Chen HI, Yeh DY, Liou H-L, Kao S-J. Insulin attenuates endotoxin-induced acute lung injury in conscious rats. Crit Care Med. 2006;34:758–64.
de Oliveira MJ, Meyer-Pflug AR, Alba-Loureiro TC, Melbostad H, da Cruz JWM C, Coimbra R, et al. Modulation of lipopolysaccharide-induced acute lung inflammation: role of insulin. Shock. 2006;25:260–6.
Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, et al. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med. 2008;178:168–79.
Jian M-Y, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305:L844–55.
Boyle AJ, Gangi SD, Hamid UI, Mottram L-J, McNamee L, White G, et al. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: a prospective analysis. Crit Care. 2015;19:109.
Shyamsundar M, McAuley DF, Shields MO, MacSweeney R, Duffy MJ, Johnston JR, et al. Effect of simvastatin on physiological and biological outcomes in patients undergoing esophagectomy: a randomized placebo-controlled trial. Ann Surg. 2014;259:26–31.
Kabitz H-J, Sonntag F, Walker D, Schwoerer A, Walterspacher S, Kaufmann S, et al. Diabetic polyneuropathy is associated with respiratory muscle impairment in type 2 diabetes. Diabetologia. 2008;51:191–7.
Acknowledgements
The authors thank all study sites, investigators, coordinators and national associations who contributed to LUNG SAFE.
National coordinators for LUNG SAFE
Argentina: Fernando Rios; Australia/New Zealand: Frank Van Haren; Bangladesh: Mohammad Omar Faruq; Belgium: Sottiaux T, Depuydt P; Bolivia: Fredy S Lora; Brazil: Luciano Cesar Azevedo; Canada: Eddy Fan; Chile: Guillermo Bugedo; China: Haibo Qiu; Colombia: Marcos Gonzalez; Costa Rica: Juan Silesky; Czech Republic: Vladimir Cerny; Denmark: Jonas Nielsen; Ecuador: Manuel Jibaja; France: Tài Pham; Germany: Hermann Wrigge; Greece: Dimitrios Matamis; Guatemala: Jorge Luis Ranero; Hong Kong: Charles Gomersall; India: Pravin Amin; Iran: S.M. Hashemian; Ireland: Kevin Clarkson; Italy: Giacomo Bellani; Japan: Kiyoyasu Kurahashi; Korea: Younsuck Koh; Mexico: Asisclo Villagomez; Morocco: Amine Ali Zeggwagh; Netherlands: Leo M Heunks; Norway: Jon Henrik Laake; Pakistan: Waqar Kashif; Panama: Jorge Synclair; Philippines: Jose Emmanuel Palo; Portugal: Antero do Vale Fernandes; Romania: Dorel Sandesc; Saudi Arabia: Yaasen Arabi; Serbia: Vesna Bumbasierevic; Spain: Nicolas Nin, Jose A Lorente; Sweden: Anders Larsson; Switzerland: Lise Piquilloud; Thailand: Boonsong Patjanasoontorn; Tunisia: Fekri Abroug; United Kingdom: Daniel F McAuley, Lia McNamee; Uruguay: Javier Hurtado; USA: Ed Bajwa; Venezuela: Gabriel Démpaire.
National Societies/Networks Endorsing LUNG SAFE
ANZICS Clinical Trials Group, Réseau Européen de Recherche en Ventilation Artificielle (ReVA Network); Irish Critical Care Trials Group; Société de Réanimation de Langue Française (SRLF); Société Française d’Anesthésie et de Réanimation (SFAR); Società Italiana Anestesia, Analgesia, Rianimazione e Terapia Intensiva (SIAARTI); The Japanese Society of Intensive Care Medicine (JSICM); Nonprofit Organisation Japanese Society of Education for Physicians and Trainees in Intensive Care (JSEPTIC); UK Intensive Care Society.
Study coordination
Guy M Francois (European Society of Intensive Care Medicine, Brussels, Belgium).
Data revision and management
Francesca Rabboni (University Of Milan-Bicocca, Monza, Italy), Fabiana Madotto (University Of Milan-Bicocca, Monza, Italy), Sara Conti (University Of Milan-Bicocca, Monza, Italy).
Site investigators by country
Albania: Uhc Mother Theresa (Tirana): Hektor Sula, Lordian Nunci; University Hospital Shefqet Ndroqi (Tirana): Alma Cani.
Argentina: Clinica De Especialidades (Villa Maria): Alan Zazu; Hospital Dr Julio C Perrando (Resistencia): Christian Dellera, Carolina S Insaurralde; Sanatorio Las Lomas (San Isidro, Buenos Aires): Risso V Alejandro; Sanatorio De La Trinidad San Isidro (San Isidro): Julio Daldin, Mauricio Vinzio; Hospital Español De Mendoza (Godoy Cruz - Mendoza): Ruben O Fernandez; Hospital Del Centenario (Rosario): Luis P Cardonnet, Lisandro R Bettini; San Antonio (Gualeguay (Entre Rios)): Mariano Carboni Bisso, Emilio M Osman; Cemic (Buenos Aires): Mariano G Setten, Pablo Lovazzano; Hospital Universitrario Austral (Pilar): Javier Alvarez, Veronica Villar; Hospital Por + Salud (Pami) Dr Cesar Milstein (Buenos Aires): Norberto C Pozo, Nicolas Grubissich; Sanatorio Anchorena (Buenos Aires): Gustavo A Plotnikow, Daniela N Vasquez; Sanatorio De La Trinidad Mitre (Buenos Aires): Santiago Ilutovich, Norberto Tiribelli; Hospital Luis Lagomaggiore (Mendoza): Ariel Chena, Carlos A Pellegrini; H.I.G.A San Martín (La Plata): María G Saenz, Elisa Estenssoro; Hospital Misericordia (Cordoba): Matias Brizuela, Hernan Gianinetto; Sanatorio Juncal (Temperley): Pablo E Gomez, Valeria I Cerrato; Hospital D. F. Santojanni (Buenos Aires): Marco G Bezzi, Silvina A Borello; Hospital Alejandro Posadas (Buenos Aires): Flavia A Loiacono, Adriana M Fernande.
Australia: St. Vincents Hospital, Sydney (Darlinghurst): Serena Knowles, Claire Reynolds; St George Public Hospital (Kogarah): Deborah M Inskip, Jennene J Miller; Westmead Hospital (Westmead): Jing Kong, Christina Whitehead; Flinders Medical Centre (Bedford Park, South Australia): Shailesh Bihari; John Hunter Hospital (Newcastle): Aylin Seven, Amanda Krstevski; Canberra Hospital (Garran): Helen J Rodgers, Rebecca T Millar; Calvary Mater Newcastle (Waratah): Toni E Mckenna, Irene M Bailey; Cabrini Hospital (Melbourne): Gabrielle C Hanlon; Liverpool Hospital (Liverpool): Anders Aneman, Joan M Lynch; Coffs Harbour Health Campus (Coffs Harbour): Raman Azad, John Neal; Sir Charles Gairdner Hospital (Nedlands): Paul W Woods, Brigit L Roberts; Concord Hospital (Concord): Mark R Kol, Helen S Wong.
Austria: General Hospital Of Vienna/Medical University Of Vienna (Vienna): Katharina C Riss, Thomas Staudinger;
Belgium: Cliniques universitaires St Luc, UCL (Brussels): Xavier Wittebole, Caroline Berghe; CHU Dinant-Godinne (Yvoir): Pierre A Bulpa, Alain M Dive; AZ Sint Augustinus Veurne (Veurne): Rik Verstraete, Herve Lebbinck; Ghent University Hospital (Ghent): Pieter Depuydt, Joris Vermassen;; University Hospitals Leuven (Leuven): Philippe, Meersseman, Helga Ceunen.
Brazil: Hospital Renascentista (Pouso Alegre): Jonas I Rosa, Daniel O Beraldo; Vitoria Apart Hospital (Serra): Claudio Piras, Adenilton M Rampinelli; Hospital Das Clinicas (São Paulo): Antonio P Nassar Jr.; Hospital Geral Do Grajaù (São Paulo): Sergio Mataloun, Marcelo Moock; Evangelical Hospital (Cachoeiro De Itapemirim/Espírito Santo): Marlus M Thompson, Claudio H Gonçalves-,; Hospital Moinhos De Vento (Porto Alegre): Ana Carolina P Ant ônio, Aline Ascoli; Hospital Alvorada Taguatinga (Taguatinga): Rodrigo S Biondi, Danielle C Fontenele; Complexo Hospitalar Mngabeira Tarcisio Burity (Joao Pessoa): Danielle Nobrega, Vanessa M Sales.
Brunei Darussalam: Raja Isteri Pengiran Anak Saleha (Ripas) Hospital (Bandar Seri Begawan): Dr Suresh Shindhe, Dr Dk Maizatul Aiman B Pg Hj Ismail.
Canada: Medical-Surgical ICU of St Michael’s Hospital (Toronto): John Laffey, Francois Beloncle; St. Josephs Health Centre (Toronto): Kyle G Davies, Rob Cirone; Sunnybrook Health Sciences Center (Toronto): Venika Manoharan, Mehvish Ismail; Toronto Western Hospital (Toronto): Ewan C Goligher, Mandeep Jassal; Medical Surgical ICU of the Toronto General Hospital (Toronto): Niall D. Ferguson, Erin Nishikawa, Areej Javeed; Cardiovascular ICU of St Michael’s Hospital (Toronto): Gerard Curley, Nuttapol Rittayamai; Cardiovascular ICU of the Toronto General Hospital (Toronto): Matteo Parotto, Mount Sinai Hospital (Toronto): Sangeeta Mehta, Jenny Knoll; Trauma-Neuro ICU of St Michael’s Hospital (Toronto): Antoine Pronovost, Sergio Canestrini.
Chile: Hospital Clínico Pontificia Universidad Católica De Chile (Santiago): Alejandro R Bruhn, Patricio H Garcia; Hospital Militar De Santiago (Santiago): Felipe A Aliaga, Pamela A Farías; Clinica Davila (Santiago): Jacob S Yumha; Hospital Guillermo Grant Benavente (Concepcion): Claudia A Ortiz, Javier E Salas; Clinica Las Lilas (Santiago): Alejandro A Saez, Luis D Vega; Hospital Naval Almirante Nef (Viña Del Mar): Eduardo F Labarca, Felipe T Martinez; Hospital Luis Tisné Brousse (Penanolen): Nicolás G Carreño, Pilar Lora.
China: The Second Affiliated Hospital Of Harbin Medical University (Harbin): Haitao Liu; Nanjing Zhong-Da Hospital, Southeast University (Nanjing): Haibo Qiu, Ling Liu; The First Affiliated Hospital Of Anhui Medical University (Hefei): Rui/Tang, Xiaoming Luo; Peking University People’s Hospital (Beijing): Youzhong An, Huiying Zhao; Fourth Affiliated Hospital Of Harbin Medical University (Harbin): Yan - Gao, Zhe - Zhai; Nanjing Jiangbei Peoples Hospital Affiliated To Medical School Of Southeast University (Nanjing): Zheng L Ye, Wei Wang; The First Affiliated Hospital Of Dalian Medical Unvercity (Dalian): Wenwen Li, Qingdong Li; Subei Peoples Hospital Of Jiangsu Province (Yanghzou): Ruiqiang Zheng; Jinling Hospital (Nanjing): Wenkui Yu, Juanhong Shen; Urumqi General Hospital (Urumqi): Xinyu Li; Intensive Care Unit, First Affiliated Hospital Of Wanna Medical College, Yijishan Hospital, (Wuhu): Tao Yu, Weihua Lu; Sichuan Provincial Peoples Hospital (Chengdu): Ya Q Wu, Xiao B Huang; Hainan Province Peoples Hospital (Haikou): Zhenyang He; Peoples Hospital Of Jiangxi Province (Nanchang): Yuanhua Lu; Qilu Hospital Of Shandong University (Jinan): Hui Han, Fan Zhang; Zhejiang Provincial Peoples Hospital (Hangzhou): Renhua Sun; The First Affiliated Hospital Of Bengbu Medical College (Bengbu, Anhui): Hua X Wang, Shu H Qin; Nanjing Municipal Government Hospital (Nanjing): Bao H Zhu, Jun Zhao; The First Hospital Of Lanzhou University (Lanzhou): Jian / Liu, Bin/Li; The First Affiliated Hospital Of Chongqing University Of Medical Science (Chongqing): Jing L Liu, Fa C Zhou; Xuzhou Central Hospital, Jiangsu Province, China (Xuzhou): Qiong J Li, Xing Y Zhang; The First Peoples Hospital Of Foshan (Foshan): Zhou Li-Xin, Qiang Xin-Hua; The First Affiliated Hospital Of Guangxi Medical University (Nanning): Liangyan Jiang; Renji Hospital, Shanghai Jiao Tong University School Of Medicine (Shanghai): Yuan N Gao, Xian Y Zhao; First Hospital Of Shanxi Medical University (Taiyuan): Yuan Y Li, Xiao L Li; Shandong Provincial Hospital (Jinan): Chunting Wang, Qingchun Yao; Fujian Provincial Hospital (Fuzhou): Rongguo Yu, Kai Chen; Henan Provincial People’s Hospital (Zhengzhou): Huanzhang Shao, Bingyu Qin; The Second Affiliated Hospital Of Kunming Medical University (Kunming City): Qing Q Huang, Wei H Zhu; Xiangya Hospital, Central South University (Changsha): Ai Y Hang, Ma X Hua; The First Affiliated Hospital Of Guangzhou Medical University (Guangzhou): Yimin Li, Yonghao Xu; Peoples Hospital of Hebei Province (Shijiazhuang): Yu D Di, Long L Ling; Guangdong General Hospital (Guangzhou): Tie H Qin, Shou H Wang; Beijing Tongren Hospital (Beijing): Junping Qin; Jiangsu Province Hospital (Nanjing): Yi Han, Suming Zhou.
Colombia: Fundación Valle Del Lili (Cali): Monica P Vargas.
Costa Rica: Hospital San Juan De Dios: Juan I Silesky Jimenez, Manuel A González Rojas; Hospital San Juan De Dios (San José): Jaime E Solis-Quesada, Christian M Ramirez-Alfaro.
Czech Republic: University Hospital Of Ostrava (Ostrava): Jan Máca, Peter Sklienka;
DENMARK: Aarhus Universitetshospital (Aarhus N): Jakob Gjedsted, Aage Christiansen; Rigshopitalet: Jonas Nielsen.
Ecuador: Hospital Militar (Quito): Boris G Villamagua, Miguel Llano.
France: Clinique du Millénaire (Montpellier): Philippe Burtin, Gautier Buzancais; Centre Hospitalier (Roanne): Pascal Beuret, Nicolas Pelletier; CHU d’Angers (Angers): Satar Mortaza, Alain Mercat; Hôpital Marc Jacquet (Melun): Jonathan Chelly, Sébastien Jochmans; CHU de Caen (Caen): Nicolas Terzi, Cédric Daubin; Henri Mondor Hospital (Créteil): Guillaume Carteaux, Nicolas de Prost; Cochin Hospital (Paris): Jean-Daniel Chiche, Fabrice Daviaud; CHU Tenon (Paris): Tài Pham, Muriel Fartoukh; CH Mulhouse-Emile Muller (Mulhouse): Guillaume Barberet, Jerome Biehler; Archet 1 University Hospital (Nice): Jean Dellamonica, Denis Doyen; Hopital Sainte Musse (Toulon): Jean-Michel Arnal, Anais Briquet; Hopital Nord - Réanimation des Détresses Respiratoires et Infections Sévères (Marseille): Fanny Klasen, Laurent Papazian; HEGP (Paris): Arnaud Follin; Louis Mourier Hospital (Colombes): Damien Roux, Jonathan Messika; Centre Hospitalier de Dax (Dax): Evangelos Kalaitzis; Réanimation Médicale, GH Pitié-Salpêtrière (Paris): Laurence Dangers, Alain Combes; Ap-Hp Ambroise Paré (Boulogne-Billancourt): Siu-Ming Au; University Hospital Rouen (Rouen): Gaetan Béduneau, Dorothée Carpentier; CHU Amiens (Amiens - Salouel): Elie H Zogheib, Herve Dupont; Centre Hospitalier Intercommunal Robert Ballanger (Aulnay Sous Bois): Sylvie Ricome, Francesco L Santoli; Centre Hospitalier René Dubos (Pontoise): Sebastien L Besset; CHI Portes de l’Oise (Beaumont Sur Oise): Philippe Michel, Bruno Gelée; Archet 2 University Hospital (Nice): Pierre-Eric Danin, Bernard Goubaux; Centre Hospitalier Pierre Oudot (Bourgoin Jallieu): Philippe J Crova, Nga T Phan; CH Dunkerque (Dunkerque): Frantz Berkelmans; Centre Hospitalier de Belfort Montbéliard (Belfort): Julio C Badie, Romain Tapponnier; Centre Hospitalier Emile Muller (Mulhouse): Josette Gally, Samy Khebbeb; Hôpital de Hautepierre-Hôpitaux Universitaires de Strasbourg (Strasbourg): Jean-Etienne Herbrecht, Francis Schneider; Centre Hospitalier de Dieppe (Dieppe): Pierre-Louis M Declercq, Jean-Philippe Rigaud; Bicêtre (Le Kremin-Bicetre): Jacques Duranteau, Anatole Harrois; CHU Gabriel Montpied (Clermont-Ferrand): Russell Chabanne, Julien Marin; CHU Estaing (Clermont-Ferrand): Jean-Michel Constantin, Sandrine Thibault; CHI Eure-Seine Evreux (Evreux): Mohammed Ghazi, Messabi Boukhazna; Centre Hospitalier de Châlons en Champagne (Châlons en Champagne): Salem Ould Zein; CH Beauvais (Beauvais): Jack R Richecoeur, Daniele M Combaux; Centre Hospitalier Le Mans (Le Mans): Fabien Grelon, Charlene Le Moal; Hôpital Fleyriat (Bourg en Bresse): Elise P Sauvadet, Adrien Robine; Hôpital Saint Louis (Paris): Virginie Lemiale, Danielle Reuter; Pneumologie et Réanimation Médicale, Hôpital Pitié-Salpêtrière (Paris): Martin Dres, Alexandre Demoule; Centre Hospitalier Gonesse (Gonesse): Dany Goldgran-Toledano; Hôpital Croix Rousse (Lyon): Loredana Baboi, Claude Guérin.
Germany: St. Nikolaus-Stiftshospital (Andernach): Ralph Lohner; Fachkrankenhaus Coswig Gmbh (Coswig):Jens Kraßler, Susanne Schäfer; University Hospital Frankfurt (Frankfurt am Main): Kai D Zacharowski, Patrick Meybohm; Department of Anaesthesia & Intensive Care Medicine, University Hospital of Leipzig (Leipzig): Andreas W Reske, Philipp Simon; Asklepios Klinik Langen (Langen): Hans-Bernd F Hopf, Michael Schuetz; Städtisches Krankenhaus Heinsberg (Heinsberg): Thomas Baltus.
Greece: Hippokrateion General Hospital Of Athens (Athens): Metaxia N Papanikolaou, Theonymfi G Papavasilopoulou; Gh Ahepa (Thessaloniki): Giannis A Zacharas, Vasilis Ourailogloy; Hippokration General Hospital of Thessaloniki (Thessaloniki): Eleni K Mouloudi, Eleni V Massa; Hospital General of Kavala (Kavala): Eva O Nagy, Electra E Stamou; Papageorgiou General Hospital (Thessaloniki): Ellada V Kiourtzieva, Marina A Oikonomou.
Guatemala: Hospital General De Enfermedades, Instituto Guatemalteco De Seguridad Social (Ciudad De Guatemala): Luis E Avila; Centro Médico Militar (Guatemala): Cesar A Cortez, Johanna E Citalán.
India: Deenanath Mangeshkar Hospital And Research Center (Pune): Sameer A Jog, Safal D Sable; Care Institute Of Medical Sciences (CIMS) Hospital (Ahmedabad): Bhagyesh Shah; Sanjay Gandhi Postgraduate Institute Of Medical Sciences (SGPGIMS) (Lucknow): Mohan Gurjar, Arvind K Baronia; Rajasthan Hospital (Ahmedabad): Mohammedfaruk Memon; National Institute Of Mental Health And Neuro Sciences (NIMHANS) (Bangalore): Radhakrishnan Muthuchellappan, Venkatapura J Ramesh; Anaesthesiology Unit of the Kasturba Medical College & Dept of Respiratory Therapy, SHOAS, Manipal University (Manipal): Anitha Shenoy, Ramesh Unnikrishnan; Sanjeevan Hospital (Pune): Subhal B Dixit, Rachana V Rhayakar; Apollo Hospitals (Chennai): Nagarajan Ramakrishnan, Vallish K Bhardwaj; Medicine Unit of the Kasturba Medical College & Dept of Respiratory Therapy, SHOAS, Manipal University (Manipal): Heera L Mahto, Sudha V Sagar; G Kuppuswamy Naidu Memorial Hospital (Coimbatore): Vijayanand Palaniswamy, Deeban Ganesan;
IRAN: NRITLD/Masih Daneshvari (Tehran): Seyed Mohammadreza Hashemian, Hamidreza Jamaati; Milad Hospital (Tehran): Farshad Heidari.
Ireland: St Vincent’s University Hospital (Dublin): Edel A Meaney, Alistair Nichol; Mercy University Hospital (Cork): Karl M Knapman, Donall O’Croinin; Cork University Hospital (Cork): Eimhin S Dunne, Dorothy M Breen; Galway University Hospital (Galway): Kevin P Clarkson, Rola F Jaafar; Beaumont Hospital (Dublin): Rory Dwyer, Fahd Amir; Mater Misericordiae University Hospital (Dublin): Olaitan O Ajetunmobi, Aogan C O’Muircheartaigh; Tallaght Hospital (Dublin): Colin S Black, Nuala Treanor; Saint James’s Hospital (Dublin): Daniel V Collins, Wahid Altaf.
Italy: Santa Maria delle Croci Hospital (Ravenna): Gianluca Zani, Maurizio Fusari; Arcispedale Sant’Anna Ferrara. (Ferrara): Savino Spadaro, Carlo A Volta; Ospedale Profili (Fabriano) (An): Romano Graziani, Barbara Brunettini; Umberto I Nocera Inferiore (Nocera Inferiore Salerno): Salvatore Palmese; Azienda Ospedaliera San Paolo - Polo Universitario - Università degli Studi di Milano (Milan): Paolo Formenti, Michele Umbrello; Sant’Anna (San Fermo Della Battaglia (Co)): Andrea Lombardo; Spedali Civili Brescia (Brescia): Elisabetta Pecci, Marco Botteri; Fondazione Irccs Ca Granda, Ospedale Maggiore Policlinico (Milan): Monica Savioli, Alessandro Protti; University Campus Bio-Medico of Rome (Rome): Alessia Mattei, Lorenzo Schiavoni; Azienda Ospedaliera “Mellino Mellini” (Chiari (Bs)): Andrea Tinnirello, Manuel Todeschini; Policlinico P. Giaccone, University of Palermo (Palermo): Antonino Giarratano, Andrea Cortegiani; Niguarda Cà Granda Hospital (Milan): Sara Sher, Anna Rossi; A. Gemelli University Hospital (Rome): Massimo M Antonelli, Luca M Montini; Ospedale “Sandro Pertini” (Rome): Paolo Casalena, Sergio Scafetti; ISMeTT IRCCS UPMC (Palermo): Giovanna Panarello, Giovanna Occhipinti; Ospedale San Gerardo (Monza): Nicolò Patroniti, Matteo Pozzi; Santa Maria Della Scaletta (Imola): Roberto R Biscione, Michela M Poli; Humanitas Research Hospital (Rozzano): Ferdinando Raimondi, Daniela Albiero; Ospedale Desio - Ao Desio-Vimercate (Desio): Giulia Crapelli, Eduardo Beck; Pinetagrande Private Hospital (Castelvolturno): Vincenzo Pota, Vincenzo Schiavone; Irccs San Martino Ist (Genova): Alexandre Molin, Fabio Tarantino; Ospedale San Raffaele (Milano): Giacomo Monti, Elena Frati; Ospedali Riuniti Di Foggia (Foggia): Lucia Mirabella, Gilda Cinnella; Azienda Ospedaliera Luigi Sacco - Polo Universitario (Milano): Tommaso Fossali, Riccardo Colombo; A.O.U. Città della Salute e della Scienza di Torino (Turin): Pierpaolo Terragni Ilaria Pattarino; Università degli Studi di Pavia-Fondazione IRCCS Policlinico San Matteo (Pavia): Francesco Mojoli, Antonio Braschi; Ao Ospedale Civile Legnano (Legnano): Erika E Borotto; Arnas Ospedale Civico Di Cristina Benfratelli (Palermo): Andrea N Cracchiolo, Daniela M Palma; Azienda Ospedaliera Della Provincia Di Lecco - Ospedale “A. Manzoni” (Lecco): Francesco Raponi, Giuseppe Foti; A.O. Provincia Di Lecco - Ospedale Alessandro Manzoni (Lecco): Ettore R Vascotto, Andrea Coppadoro; Cliniche Universitarie Sassari (Sassari): Luca Brazzi, Leda Floris; IRCCS Policlinico San Matteo (Pavia): Giorgio A Iotti, Aaron Venti.
Japan: Yokohama City University Hospital (Yokohama): Osamu Yamaguchi, Shunsuke Takagi; Toyooka Hospital (Toyooka City, Hyogo Prefecture): Hiroki N Maeyama; Chiba University Hospital (Chiba City): Eizo Watanabe, Yoshihiro Yamaji; Okayma University Hospital (Okayama): Kazuyoshi Shimizu, Kyoko Shiozaki; Japanese Foundation for Cancer Research, Cancer Institute Hospital, Department Of Emergency Medicine And Critical Care (Tokyo): Satoru Futami; Ibaraki Prefectural Central Hospital (Kasama): Sekine Ryosuke; Tohoku University Hospital (Sendai-Shi): Koji Saito, Yoshinobu Kameyama; Tokyo Medical University Hachioji Medical Center (Hachioji, Tokyo): Keiko Ueno; Tokushima University Hospital (Tokushima): Masayo. Izawa, Nao Okuda; Maebashi Red Cross Hospital (Gunma Maebashi): Hiroyuki Suzuki, Tomofumi Harasawa; Urasoe General Hospital (Urasoe): Michitaka Nasu, Tadaaki Takada; Ohta General Hospital Foundation Ohta Nishinouchi Hospital (Fukushima): Fumihito Ito; Jichi Medical University Hospital (Shimotsuke): Shin - Nunomiya, Kansuke - Koyama; Mito Kyodo General Hospital, Tsukuba University Hospital Mito Medical Center (Mito): Toshikazu Abe; Sendai City Hospital (Sendai): Kohkichi Andoh, Kohei Kusumoto; Ja Hiroshima General Hospital (Hatsukaichi City, Hiroshima): Akira Hirata, Akihiro Takaba; Yokohama Rosai Hospital (Yokohama): Hiroyasu Kimura; Nagasaki University Hospital (Nagasaki): Shuhei Matsumoto, Ushio Higashijima; Niigata University Medical & Dental Hospital (Niigata): Hiroyuki Honda, Nobumasa Aoki; Mie University Hospital (Tsu, Mie): Hiroshi Imai; Yamaguchi University Hospital (Ube, Yamaguchi): Yasuaki Ogino, Ichiko Mizuguchi; Saiseikai Kumamoto Hospital (Kumamoto City): Kazuya Ichikado; Shinshu University School Of Medecine (Matsumoto City): Kenichi Nitta, Katsunori Mochizuki; Kuki General Hospital (Kuki): Tomoaki Hashida; Kyoto Medical Center (Kyoto): Hiroyuki Tanaka; Fujita Health University (Toyoake): Tomoyuki Nakamura, Daisuke Niimi; Rakwakai Marutamachi Hospital (Kyoto): Takeshi Ueda; Osaka University Hospital (Suita City, Osaka Prefecture): Yozo Kashiwa, Akinori Uchiyama.
Latvia: Paul Stradins Clinical University Hospital (Riga): Olegs Sabelnikovs, Peteris Oss.
Lebanon: Kortbawi Hospital (Jounieh): Youssef Haddad.
Malaysia: Hospital Kapit (Kapit): Kong Y Liew.
Mexico: Instituto Nacional De Cancerología, México (Mexico City): Silvio A Ñamendys-Silva, Yves D Jarquin-Badiola; Hospital De Especialidades “Antonio Fraga Mouret” Centro Medico Nacional La Raza IMSS (Mexico City): Luis A Sanchez-Hurtado, Saira S Gomez-Flores; Hospital Regional 1° De Octubre (Mexico City): Maria C Marin, Asisclo J Villagomez; Hospital General Dr Manuel Gea Gonzalez (Mexico City): Jordana S Lemus, Jonathan M Fierro; Hospital General De Zona No. 1 Instituto Mexicano Del Seguro Social Tepic Nayarit (Tepic): Mavy Ramirez Cervantes, Francisco Javier Flores Mejia; Centro Medico Dalinde (Mexico D.F.): Dulce Dector, Dulce M Dector; Opd Hospital Civil De Guadalajara Hospital Juan I Menchaca (Guadalajara): Daniel R Gonzalez, Claudia R Estrella; Hospital Regional De Ciudad Madero Pemex (Ciudad Madero): Jorge R Sanchez-Medina, Alvaro Ramirez-Gutierrez; Centro Médico ABC (Mexico D.F.): Fernando G George, Janet S Aguirre; Hospital Juarez De Mexico (Mexico City): Juan A Buensuseso, Manuel Poblano.
Morocco: Mohammed V University, University Teaching Ibn Sina Hospital (Rabat): Tarek Dendane, Amine Ali Zeggwagh; Hopital Militaire D’Instruction Mohammed V (Rabat): Hicham Balkhi; Errazi (Marrakech): Mina Elkhayari, Nacer Samkaoui; University Teaching Hospital Ibn Rushd (Casablanca): Hanane Ezzouine, Abdellatif Benslama; Hôpital des Spécialités de Rabat (HSR) (Rabat): Mourad Amor, Wajdi Maazouzi.
Netherlands: Tjongerschans (Heerenveen): Nedim Cimic, Oliver Beck; Cwz (Nijmegen): Monique M Bruns, Jeroen A Schouten; Rijnstate Hospital (Arnhem): Myra - Rinia, Monique Raaijmakers; Radboud Umc (Nijmegen): Leo M Heunks, Hellen M Van Wezel; Maastricht University Medical Centre (Maastricht): Serge J Heines, Ulrich Strauch; Catharinaziekenhuis (Eindhoven): Marc P Buise; Academic Medical Center (Amsterdam): Fabienne D Simonis, Marcus J Schultz.
New Zealand: Tauranga Hospital (Tauranga): Jennifer C Goodson, Troy S Browne; Wellington Hospital (Wellington): Leanlove Navarra, Anna Hunt; Dunedin Hospital (Dunedin): Robyn A Hutchison, Mathew B Bailey; Auckland City Hospital (Auckland): Lynette Newby, Colin Mcarthur; Whangarei Base Hospital (Whangarei): Michael Kalkoff, Alex Mcleod; North Shore Hospital (Auckland): Jonathan Casement, Danielle J Hacking.
Norway: Ålesund Hospital (Ålesund): Finn H Andersen, Merete S Dolva; Oslo University Hospital - Rikshospitalet Medical Centre (Oslo): Jon H Laake, Andreas Barratt-Due; Stavanger University Hospital (Stavanger): Kim Andre L Noremark, Eldar Søreide; Haukeland University Hospital (Bergen): Brit Å Sjøbø, Anne B Guttormsen.
Peru: Hospital Nacional Edgardo Rebagliati Martins (Lima): Hector H Leon Yoshido; Clínica Ricardo Palma (Lima): Ronald Zumaran Aguilar, Fredy A Montes Oscanoa.
Philippines: The Medical City (Pasig): Alain U Alisasis, Joanne B Robles; Chong Hua Hospital (Cebu): Rossini Abbie B Pasanting-Lim, Beatriz C Tan.
Poland: Warsaw University Hospital (Warsaw): Pawel Andruszkiewicz, Karina Jakubowska;
PORTUGAL: Centro Hospitalar Da Cova Da Beira (Covilhã): Cristina M Coxo; Hospital Santa Maria, Chln (Lisboa): António M Alvarez, Bruno S Oliveira; Centro Hospitalar Trás-Os-Montes E Alto Douro - Hospital De S. Pedro -Vila Real (Vila Real): Gustavo M Montanha, Nelson C Barros; Hospital Beatriz Ângelo (Loures): Carlos S Pereira, António M Messias; Hospital De Santa Maria (Lisboa): Jorge M Monteiro; Centro Hospitalar Médio Tejo - Hospital De Abrantes (Abrantes): Ana M Araujo, Nuno T Catorze; Instituto Português De Oncologia De Lisboa (Lisboa): Susan M Marum, Maria J Bouw; Hospital Garcia De Orta (Almada): Rui M Gomes, Vania A Brito; Centro Hospitalar Do Algarve (Faro): Silvia Castro, Joana M Estilita; Hpp Hospital De Cascais (Alcabideche): Filipa M Barros; Hospital Prof. Doutor Fernando Fonseca Epe (Amadora): Isabel M Serra, Aurelia M Martinho.
Romania: Fundeni Clinical Institute (Bucharest): Dana R Tomescu, Alexandra Marcu; Emergency Clinical County Hospital Timisoara (Timisoara): Ovidiu H Bedreag, Marius Papurica; Elias University Emergency Hospital (Bucharest): Dan E Corneci, Silvius Ioan Negoita.
Russian Federation: University Hospital (Kemerovo): Evgeny Grigoriev; Krasnoyarsk Regional Hospital, Krasnoyarsk State Medical University (Krasnoyarsk): Alexey I Gritsan, Andrey A Gazenkampf.
Saudi Arabia: GICU of PSMMC (Riyadh): Ghaleb Almekhlafi, Mohamad M Albarrak; SICU of PSMMC (Riyadh): Ghanem M Mustafa;; King Faisal Hospital And Research Center (Riyadh): Khalid A Maghrabi, Nawal Salahuddin; King Fahad Hospital (Baha): Tharwat M Aisa; King Abdulaziz Medical City (Riyadh): Ahmed S Al Jabbary, Edgardo Tabhan; King Abdulaziz Medical City (Riyadh): Yaseen M Arabi; King Abdulaziz Medical City (Riyadh): Yaseen M Arabi, Olivia A Trinidad; King Abdulaziz Medical City (Riyadh): Hasan M Al Dorzi, Edgardo E Tabhan.
South Africa: Charlotte Maxeke Johannesburg Academic Hospital (Johannesburg): Stefan Bolon, Oliver Smith.
Spain: Hospital Sant Pau (Barcelona): Jordi Mancebo, Hernan Aguirre-Bermeo; Hospital Universitari Bellvitge (L Hospitalet De Llobregat (Barcelona)): Juan C Lopez-Delgado, Francisco Esteve; Hospital Son Llatzer (Palma De Mallorca): Gemma Rialp, Catalina Forteza; Sabadell Hospital, CIBER Enfermedades Respiratorias (Sabadell): Candelaria De Haro, Antonio Artigas; Hospital Universitario Central De Asturias (Oviedo): Guillermo M Albaiceta, Sara De Cima-Iglesias; Complejo Hospitalario Universitario A Coruña (A Coruña): Leticia Seoane-Quiroga, Alexandra Ceniceros-Barros; Hospital Universitario Miguel Servet (Zaragoza): Antonio L Ruiz-Aguilar, Luis M Claraco-Vega; Morales Meseguer University Hospital (Murcia): Juan Alfonso Soler, Maria del Carmen Lorente; Hospital Universitario del Henares (Coslada): Cecilia Hermosa, Federico Gordo; Complejo Asistencial De Palencia. Hospital Rio Carrión (Palencia): Miryam - Prieto-González, Juan B López-Messa; Fundación Jiménez Díaz (Madrid): Manuel P Perez, Cesar P Perez; Hospital Clínico Universitario Lozano Blesa (Zaragoza): Raquel Montoiro Allue; Hospital Verge de la Cinta (Tortosa): Ferran Roche-Campo, Marcos Ibañez-Santacruz; Hospital Universitario 12 De Octubre (Madrid): Susana - Temprano; Hospital Universitario Príncipe De Asturias (Alcalá De Henares, Madrid): Maria C Pintado, Raul De Pablo; Hospital Universitari Germans Trias I Pujol (Badalona): Pilar Ricart Aroa Gómez; Hospital Universitario Arnau De Vilanova De Lleida (Lleida): Silvia Rodriguez Ruiz, Silvia Iglesias Moles; Cst Terrassa (Barcelona): Mª Teresa Jurado, Alfons Arizmendi; Hospital Universitari Mútua Terrassa (Terrassa): Enrique A Piacentini; Hospital Universitario De Móstoles (Mostoles): Nieves Franco, Teresa Honrubia; Complejo Asistencial De Salamanca (Salamanca): Meisy Perez Cheng, Elena Perez Losada; Hospital General Universitario De Ciudad Real (Ciudad Real): Javier - Blanco, Luis J Yuste; Torrecardenas (Almeria): Cecilia Carbayo-Gorriz, Francisca G Cazorla-Barranquero; Hospital Universitario Donostia (San Sebastian): Javier G Alonso, Rosa S Alda; Hospital Universitario De Torrejón (Madrid): Ángela Algaba, Gonzalo Navarro; Hospital Universitario De La Princesa (Madrid): Enrique Cereijo, Esther Diaz-Rodriguez; Hospital Universitario Lucus Augusti (Lugo): Diego Pastor Marcos, Laura Alvarez Montero; Hospital Universitario Santa Lucia (Cartagena): Luis Herrera Para, Roberto Jimenez Sanchez; Hospital Universitario Severo Ochoa, Leganes (Madrid): Miguel Angel Blasco Navalpotro, Ricardo Diaz Abad; University Hospital Of Ntra. Sra. De Candelaria (Santa Cruz De Tenerife): Raquel Montiel Gonz á lez, D á cil Parrilla Toribio; Hospital Universitario Marques De Valdecilla (Santander): Alejandro G Castro, Maria Jose D Artiga; Hospital Infanta Cristina (Parla, Madrid): Oscar Penuelas; Hospital General De Catalunya (Sant Cugat Del Valles): Tomas P Roser, Moreno F Olga; San Pedro De Alcántara (Cáceres): Elena Gallego Curto, Rocío Manzano Sánchez; Sant Joan De Reus (Reus): Vallverdu P Imma, Garcia M Elisabet; Hospital Joan XXIII (Tarragona): Laura Claverias, Monica Magret; Hospital Universitario De Getafe (Madrid): Ana M Pellicer, Lucia L Rodriguez; Hospital Universitario Río Hortega (Valladolid): Jesús Sánchez-Ballesteros, Ángela González-Salamanca; Hospital Arquitecto Marcide (Ferrol, La Coruña): Antonio G Jimenez, Francisco P Huerta; Hospital General Universitario Gregorio Marañón (Madrid): Juan Carlos J Sotillo Diaz, Esther Bermejo Lopez; Hospital General De Segovia (Segovia): David D Llinares Moya, Alec A Tallet Alfonso; Hospital General Universitario Reina Sofia (Murcia): Palazon Sanchez Eugenio Luis, Palazon Sanchez Cesar; Complejo Hospitalario Universitario De Albacete (Albacete): Sánchez I Rafael, Corcoles G Virgilio; Hospital Infanta Elena (Valdemoro): Noelia N Recio.
Sweden: Sahlgrenska University Hospital (Gothenburg): Richard O Adamsson, Christian C Rylander; Karolinska University Hospital (Stockholm): Bernhard Holzgraefe, Lars M Broman; Akademiska Sjukhuset Uppsala (Uppsala): Joanna Wessbergh, Linnea Persson; Vrinnevisjukhuset (Norrköping): Fredrik Schiöler, Hans Kedelv; Linkoping University Hospital (Linköping): Anna Oscarsson Tibblin, Henrik Appelberg; Skellefteå Lasarett (Skellefteå): Lars Hedlund, Johan Helleberg; Karolinska University Hospital Solna (Stockholm): Karin E Eriksson, Rita Glietsch; Umeå University Hospital (Umeå): Niklas Larsson, Ingela Nygren; Danderyd Hospital (Stockholm): Silvia L Nunes, Anna-Karin Morin; Lund University Hospital (Lund): Thomas Kander, Anne Adolfsson.
Switzerland: Chuv (Centre Hospitalier Universitaire Vaudois) (Lausanne): Lise Piquilloud; Hôpital neuchâtelois - La Chaux-De-Fonds (La Chaux-De-Fonds): Hervé O. Zender, Corinne Leemann-Refondini.
Tunisia: Hopital Taher Sfar Mahdia (Mahdia): Souheil Elatrous; University Hospital Farhat Hached Sousse (Sousse): Slaheddine Bouchoucha, Imed Chouchene; CHU F Bourguiba (Monastir): Islem Ouanes; Mongi Slim University Hospital, La Marsa (La Marsa): Asma Ben Souissi, Salma Kamoun;
TURKEY: Cerrahpasa Medical Faculty Emergency Intensive Care Unit (Istanbul): Oktay Demirkiran; Cerrahpasa Medical Faculty Sadi Sun Intensive Care Unit (Istanbul): Mustafa Aker, Emre Erbabacan; Uludag University Medical Faculty (Bursa): Ilkay Ceylan, Nermin Kelebek Girgin; Ankara University Faculty of Medicine, Reanimation 3nd level ICU (Ankara): Menekse Ozcelik, Necmettin Ünal; Ankara University Faculty of Medicine, 2nd level ICU-postoperative ICU (Ankara): Basak Ceyda Meco; Istanbul Kartal Egitim Ve Arastirma Hastanesi (Istanbul): Onat O Akyol, Suleyman S Derman.
United Kingdom: Papworth Hospital (Cambridge): Barry Kennedy, Ken Parhar; Royal Glamorgan Hospital (Llantrisant): Latha Srinivasa; Royal Victoria Hospital-Belfast (Belfast): Lia McNamee, Danny McAuley; Jack Steinberg ICU of the King’s College (London): Phil Hopkins, Clare Mellis; Frank Stansil ICU of the King’s College Hospital (London): Vivek Kakar; Liver ICU of the King’s College (London): Dan Hadfield; Christine Brown ICU of the King’s College (London): Andre Vercueil; West Suffolk Hospital (Bury St Edmunds): Kaushik Bhowmick, Sally K Humphreys; Craigavon Area Hospital (Portadown): Andrew Ferguson, Raymond Mckee; Barts Health NHS Trust, Whipps Cross Hospital (Leytonstone): Ashok S Raj, Danielle A Fawkes; Kettering General Hospital, Foundation NHS Trust (Northamptonshire): Philip Watt, Linda Twohey; Barnet General Hospital (Barnet): Rajeev R JhaMatthew Thomas, Alex Morton, Varsha Kadaba; Rotherham General Hospital (Rotherham): Mark J Smith, Anil P Hormis; City Hospital, (Birmingham): Santhana G Kannan, Miriam Namih; Poole Hospital NHS Foundation Trust (Poole): Henrik Reschreiter, Julie Camsooksai; Weston General Hospital (Weston-Super-Mare): Alek Kumar, Szabolcs Rugonfalvi; Antrim Area Hospital (Antrim): Christopher Nutt, Orla Oneill; Aintree University Hospital (Liverpool): Colette Seasman, Ged Dempsey; Northern General Hospital (Sheffield): Christopher J Scott, Helen E Ellis; John Radcliffe Hospital (Oxford): Stuart Mckechnie, Paula J Hutton; St Georges Hospital (London): Nora N Di Tomasso, Michela N Vitale; Hillingdon Hospital (Uxbridge): Ruth 0 Griffin, Michael N Dean; The Royal Bournemouth & Christchurch NHS Foundation Trust (Bournemouth, Dorset): Julius H Cranshaw, Emma L Willett; Guys And St Thomas NHS Foundation Trust (London): Nicholas Ioannou, Gstt Severe Respiratory Failure Service; Whittington Hospital (London): Sarah Gillis; Wexham Park Hospital (Slough): Peter Csabi; Western General Hospital (Edinburgh): Rosaleen Macfadyen, Heidi Dawson; Royal Preston Hospital (Preston): Pieter D Preez, Alexandra J Williams; Brighton And Sussex University Hospitals NHS Trust (Brighton): Owen Boyd, Laura Ortiz-Ruiz De Gordoa; East And North Herts NHS Trust (Stevenage): Jon Bramall, Sophie Symmonds; Barnsley Hospital (Barnsley): Simon K Chau, Tim Wenham; Prince Charles Hospital (Merthyr Tydfil): Tamas Szakmany, Piroska Toth-Tarsoly; University Hospital Of South Manchester NHS Foundation Trust (Manchester): Katie H Mccalman, Peter Alexander; Harrogate District Hospital (Harrogate): Lorraine Stephenson, Thomas Collyer; East And North Herts NHS Trust (Welwyn Garden City): Rhiannon Chapman, Raphael Cooper; Western Infirmary (Glasgow): Russell M Allan, Malcolm Sim; Dumfries And Galloway Royal Infirmary (Dumfries): David W Wrathall, Donald A Irvine; Charing Cross Hospital (London): Kim S Zantua, John C Adams; Worcestershire Royal Hospital (Worcester): Andrew J Burtenshaw, Gareth P Sellors; Royal Liverpool University Hospital (Liverpool): Ingeborg D Welters, Karen E Williams; Royal Alexandra Hospital (Glasgow): Robert J Hessell, Matthew G Oldroyd; Morriston Hospital (Swansea): Ceri E Battle, Suresh Pillai; Frimley Park Hospital (Frimley): Istvan - Kajtor, Mageswaran - Sivashanmugavel; Altnagelvin Hospital (Derry): Sinead C Okane, Adrian Donnelly; Buckinghamshire Healthcare NHS Trust (High Wycombe, Buckinghamshire): Aniko D Frigyik, Jon P Careless; Milton Keynes Hospital (Milton Keynes): Martin M May, Richard Stewart; Ulster Hospital (Belfast): T John Trinder, Samantha J Hagan; University Hospital of Wales (Cardiff): Matt P Wise, Jade M Cole; Freeman Hospital (Newcastle Upon Tyne): Caroline C MacFie, Anna T Dowling.
Uruguay: Hospital Español (Montevideo): Javier Hurtado, Nicolás Nin; Cudam (Montevideo): Javier Hurtado; Sanatorio Mautone (Maldonado): Edgardo Nuñez; Sanatorio Americano (Montevideo): Gustavo Pittini, Ruben Rodriguez; Hospital De Clínicas (Montevideo): María C Imperio, Cristina Santos; Circulo Católico Obreros Uruguay- Sanatorio JPII (Montevido: Ana G. França, Alejandro EBEID; CASMU (Montevideo): Alberto Deicas, Carolina Serra.
USA: Saint Louis University Hospital (St Louis): Aditya Uppalapati, Ghassan Kamel; Beth Israel Deaconess Medical Center (Boston): Valerie M Banner-Goodspeed, Jeremy R Beitler; Memorial Medical Center (Springfield): Satyanarayana Reddy Mukkera, Shreedhar Kulkarni; Massachusetts General Hospital (Boston): Jarone Lee, Tomaz Mesar; University Of Cincinnati Medical Center (Cincinnati): John O Shinn Iii, Dina - Gomaa; Massachusetts General Hospital (Boston): Christopher Tainter, Jarone Lee; Massachusetts General Hospital (Boston): Tomaz Mesar, Jarone Lee; R Adams Cowley Shock Trauma Center (Baltimore): Dale J Yeatts, Jessica Warren; Intermountain Medical Center (Murray, Utah): Michael J Lanspa, Russel R Miller; Intermountain Medical Center (Murray, Utah): Colin K Grissom, Samuel M Brown; Mayo Clinic (Rochester): Philippe R Bauer; North Shore Medical Center (Salem): Ryan J Gosselin, Barrett T Kitch; Albany Medical Center (Albany): Jason E Cohen, Scott H Beegle; John H Stoger Hospital Of Cook County (Chicago, Il): Renaud M Gueret, Aiman Tulaimat; Albany Medical Center (Albany): Shazia Choudry; University of Alabama at Birmingham (UAb) (Birmingham, AL): William Stigler, Hitesh Batra; Duke University Hospital (Durham): Nidhi G Huff; Iowa Methodist Medical Center (Des Moines, Iowa): Keith D Lamb, Trevor W Oetting; Surgical & Neurosciences Intensive Care Unit of the University Of Iowa Hospitals And Clinics (Iowa City, Iowa): Nicholas M Mohr, Claine Judy; Medical Center of Louisiana at New Orleans (New Orleans, Louisiana): Shigeki Saito, Fayez M Kheir; Tulane University (New Orleans): Fayez Kheir; Critical Care Unit of the University Of Iowa Hospitals And Clinics (Iowa City, Iowa): Adam B Schlichting, Angela Delsing; University Of California, San Diego Medical Center (San Diego, CA): Daniel R Crouch, Mary Elmasri; Uc San Diego Thornton Hospital (La Jolla): Daniel R Crouch, Dina Ismail; University Hospital (Cincinnati): Kyle R Dreyer, Thomas C Blakeman; University Hospital (Cincinnati): Kyle R Dreyer, Dina Gomaa; Tower 3B Medical ICU of Brigham and Women’s Hospital (Boston): Rebecca M Baron, Carolina Quintana Grijalba; Tower 8C Burn/Trauma ICU of Brigham and Women’s Hospital (Boston): Peter C Hou; Tower 8D Surgical ICU of Brigham and Women’s Hospital (Boston): Raghu Seethala; Tower 9C Neurosurgical ICU of Brigham and Women’s Hospital (Boston): Imo Aisiku; Tower 9D Neurological ICU of Brigham and Women’s Hospital (Boston): Galen Henderson; Tower 11C Thoracic ICU of Brigham and Women’s Hospital (Boston): Gyorgy Frendl; Shapiro 6 W Cardiac Surgery ICU of Brigham and Women’s Hospital (Boston): Sen-Kuang Hou; Shapiro 9E Coronary Care Unit of Brigham and Women’s Hospital (Boston): Robert L Owens, Ashley Schomer.
Serbia: Clinical Center of Serbia (Belgrade): Vesna Bumbasirevic, Bojan Jovanovic; Military Medical Academy (Belgrade): Maja Surbatovic, Milic Veljovic.
Funding
This work was supported by the European Society of Intensive Care Medicine (ESICM), Brussels, Belgium who funded the original LUNG SAFE study.
Availability of data and materials
The data that support the findings of this study were made available by the European Society of Intensive Care Medicine. Restrictions apply to the availability of these data, which were used after approval was granted by the executive committee for the OPEN-LUNG SAFE initiative. Further details about accessing these data can be found online (https://www.esicm.org/research/trials/trials-group-2/lung-safe/).
Author information
Authors and Affiliations
Consortia
Contributions
AJB, AMD and DFM conceived and designed this ancillary analysis of LUNG SAFE. JGL, GB, TP, AP and BTT conceived, designed and coordinated LUNG SAFE. FM performed data analysis. AJB, FM, CMO, AMD and DFM were involved in data interpretation. AB, FM, AMD and DFM drafted the first version of the manuscript, and all authors critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is an ancillary analysis of the LUNG SAFE database. All ICUs participating in LUNG SAFE obtained ethical approval, patient consent or ethics committee waiver of consent [21]. No further data were collected for this ancillary analysis.
Consent for publication
Not applicable.
Competing interests
Prof McAuley reports personal fees from consultancy for GlaxoSmithKline, SOBI, Peptinnovate, Boehringer Ingelheim and Bayer, funds to his institution from grants from the UK NIHR and others and from GlaxoSmithKline for undertaking bronchoscopy as part of a clinical trial. In addition, Prof McAuley has a patent application issued to his institution. Dr O’Kane reports a travel grant from AstraZeneca and that her spouse has received personal fees from consultancy for GlaxoSmithKline, SOBI, Peptinnovate, Boehringer Ingelheim, and Bayer. Dr O’Kane’s institution has also received funds from grants from the Northern Ireland Health and Social Care Research and Development office for studies outside of the submitted work. All other authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Supplementary analysis, figures and tables. Contains supplementary results and data from this analysis of the LUNG SAFE database. Included are results relating to outcomes from patients who developed ARDS after day 2, a flowchart describing the study population, and tables supplementary to the results presented in the main manuscript. (DOCX 832 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Boyle, A.J., Madotto, F., Laffey, J.G. et al. Identifying associations between diabetes and acute respiratory distress syndrome in patients with acute hypoxemic respiratory failure: an analysis of the LUNG SAFE database. Crit Care 22, 268 (2018). https://doi.org/10.1186/s13054-018-2158-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-018-2158-y