Abstract
Background
The early prediction of acute kidney injury (AKI) can facilitate timely intervention and prevent complications. We aimed to understand the predictive value of urinary liver-type fatty-acid binding protein (L-FABP) levels on admission to medical (non-surgical) cardiac intensive care units (CICUs) for AKI, both independently and in combination with serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels.
Methods
We prospectively investigated the predictive value of L-FABP and NT-proBNP for AKI in a large, heterogeneous cohort of patients treated in medical CICUs. Baseline urinary L-FABP and serum NT-proBNP were measured on admission. AKI was diagnosed according to the Kidney Disease: Improving Global Outcomes criteria. We studied 1273 patients (mean age, 68 years), among whom 46% had acute coronary syndromes, 38% had acute decompensated heart failure, 5% had arrhythmia, 3% had pulmonary hypertension, 2% had acute aortic syndrome, 2% had infective endocarditis, and 1% had Takotsubo cardiomyopathy.
Results
Urinary L-FABP levels correlated with serum NT-proBNP levels (r = 0.17, p < 0.0001). AKI occurred in 224 patients (17.6%), including 48 patients with stage 2 or 3 disease. Patients who developed AKI had higher one-week and 6-month mortality than those who did not develop AKI (p = 0.0002 and p = 0.003, respectively). In the multivariate logistic analysis, both L-FABP (p < 0.0001) and NT-proBNP (p = 0.006) were independently associated with the development of AKI. Adding L-FABP and NT-proBNP to a baseline model that included established risk factors further improved reclassification (p < 0.001) and discrimination (p < 0.01) beyond that of the baseline model or any single biomarker individually.
Conclusions
Urinary L-FABP and serum NT-proBNP levels on admission are independent predictors of AKI, and when used in combination, improve early prediction of AKI in patients hospitalized at medical CICUs.
Similar content being viewed by others
Background
Acute kidney injury (AKI) is a common clinical syndrome that affects critically ill patients and is strongly associated with increases in morbidity, mortality, and long-term loss of kidney function [1,2,3].
Serum creatinine is unable to identify early renal tubular injury before the decrease in glomerular filtration rate [4, 5], so an early and reliable biomarker of AKI is still needed to facilitate timely intervention and to prevent complications. However, it is also possible that a single biomarker may inadequately assess the risk of AKI because the syndrome is complex and multifactorial [4]. Combining different biomarkers in a single assessment could improve early prediction of AKI in the critically ill.
Liver-type fatty acid-binding protein (L-FABP), a marker of renal tubular injury, is an endogenous antioxidant protein spanning 14 kDa that is expressed in proximal tubular epithelial cells [6, 7]. Found in the cytoplasm of tubular cells, L-FABP is rapidly released into the tubular lumen in response to ischemia [8] or oxidative stress [8, 9]. To date, urinary L-FABP has been shown to be useful for the early prediction of AKI after surgery (particularly cardiac) [10, 11], sepsis [12], and emergency coronary angiography or percutaneous coronary intervention for acute coronary syndrome [13, 14]. However, there is limited information about the clinical utility of urinary L-FABP for predicting AKI in a heterogeneous cohort of patients treated in medical cardiac intensive care units (CICUs).
Another important and potentially relevant marker is N-terminal pro-B-type natriuretic peptide (NT-proBNP), a marker of hemodynamic stress [15]. High serum levels of NT-proBNP reflect hemodynamic deterioration, myocardial wall stress, myocardial ischemia, derangements in volume loading conditions, activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, and renal dysfunction, all of which may lead to the development of AKI [16,17,18,19,20]. Several studies have also explored the association of NT-proBNP with the development of AKI after cardiac surgery [21] and coronary angiography or percutaneous coronary intervention [16, 22]. However, few studies have examined the clinical utility of NT-proBNP in medical CICUs.
In the present study, we prospectively investigated the predictive value of urinary L-FABP on admission, both independently and in combination with serum NT-proBNP, for predicting AKI in patients hospitalized to medical CICUs.
Methods
Study design
This prospective study was conducted at the Department of Cardiology, Fujita Health University School of Medicine (Toyoake, Japan). We enrolled patients hospitalized at medical CICUs at the Fujita Health University Hospital, 1435-bed tertiary-care academic medical center, from January 2016 to July 2017. Our medical CICU, a 10-bed unit, is staffed daily by eight cardiovascular care-trained attending physicians and three internal medicine residents. The nursing to patient ratio was 1:4. The study was approved by the Ethics Committee of Fujita Health University and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Patients with cardiovascular disease requiring hospitalization as determined by the attending physician of the medical CICUs were eligible for enrollment. We obtained urinary and blood samples for baseline biomarker measurements on admission. The following patients were excluded: patients (1) under 18 years of age, (2) undergoing cardiac surgery, (3) experiencing trauma, (4) having end-stage renal disease (ESRD), (5) receiving percutaneous cardiopulmonary support before admission, (6) having active malignant disease being treated with chemotherapy or radiation, and (7) having autoimmune diseases. Independent physicians blinded to urinary L-FABP levels were free to select therapy as indicated. Clinical characteristics were obtained from patients’ medical records on enrollment, and patients were followed up for 6 months.
Definitions and calculations
AKI was diagnosed according to the “Kidney Disease: Improving Global Outcomes” criteria, as an increase in serum creatinine by ≥ 0.3 mg/dL within 48 h or an increase in serum creatinine to ≥ 1.5 times the baseline within 1 week [23]. We used the lowest known serum creatinine value during the past 3 months as the baseline creatinine. For patients without known baseline, we used the lowest creatinine value within 7 days after admission at medical CICUs. The creatinine-based estimated glomerular filtration rate (eGFR) was calculated using the Modification of diet in renal disease study equation, as recommended by the Japan Chronic Kidney Disease Initiative [24]. ESRD was defined as receiving hemodialysis or having an eGFR < 15 mL/min/1.73 m2. Chronic kidney disease (CKD) was defined as an eGFR < 60 mL/min/1.73 m2 or a history of kidney transplantation.
Diabetes mellitus was defined as a history of or current diabetes mellitus and/or a fasting plasma glucose level ≥ 126 mg/dL, a hemoglobin A1c value ≥ 6.5%, or the presence of diabetic retinopathy. Hypertension was defined by systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or a history of antihypertensive treatment. Dyslipidemia was defined as total cholesterol ≥ 220 mg/dL or a history of lipid-lowering therapy. For the smoking history, patients were defined as either current smokers or ex-smokers. We routinely performed two-dimensional echocardiography, from which the left ventricular ejection fraction (LVEF) was calculated using the modified Simpson method.
Outcomes
The primary endpoint was the development of AKI. Urinary criteria were not used to diagnose AKI because of the inconsistent data recorded and the potential alterations in urine volume induced by medical therapy.
Biomarker measurement
Urinary and blood samples were collected in nonheparinized tubes immediately after admission and centrifuged at 1000 g at 4 °C for 15 min, before being stored at − 80 °C until assayed. Urinary L-FABP was measured by a latex turbidimetric immunoassay, using a Norudia® L-FABP (Sekisui Medical CO., Ltd., Tokyo, Japan), which had a lower detection limit of 1.5 ng/mL. Urinary L-FABP values below the lower detection limit of the assay were defined as 0.75 ng/mL. Serum NT-proBNP and high-sensitivity troponin T (hs-TnT) were measured using an electrochemiluminescence immunoassay and a Cobas® e601 system (Roche Diagnostics, Tokyo, Japan). Serum high-sensitivity C-reactive protein (hs-CRP) was measured using a latex-enhanced hsCRP immunoassay (N-Latex CRP II, Siemens Healthineers, Tokyo, Japan). Finally, the serum creatinine concentration was determined by an enzyme method, using the Liquitech® Creatinine PAP II (Roche Diagnostics, Tokyo, Japan) on admission, daily until day 3, and then on day 7.
Data analysis
Statistical analyses were performed using StatFlex version 6 (Artech Co. Ltd. Osaka, Japan). Normally distributed variables are expressed as mean values ± standard deviations, and nonparametric data are presented as medians and interquartile ranges. Given the skewed distributions of the urinary L-FABP and serum NT-proBNP, hs-TnT, and hs-CRP data, analyses were performed after log-transformation to meet the criteria for use in normalized statistical approaches (after statistical confirmation). Intergroup differences were evaluated by one-way analysis of variance or the Kruskal–Wallis test for continuous variables and by the chi-square test for categorical variables. Intergroup differences in survival were examined using the Kaplan–Meier method and compared by the log-rank test. The odds ratios and 95% confidence intervals were calculated for each factor by logistic analysis: all baseline variables with p values <0.05 in the univariate analyses were entered into the multivariate logistic model to determine the independent predictors of AKI.
We calculated the C-index, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) to assess whether the accuracy of predicting AKI would improve after adding L-FABP and NT-proBNP, or either factor in isolation, into a baseline model with established risk factors. The established risk factors were as follows: age, sex, hypertension, dyslipidemia, diabetes mellitus, smoking status, CKD, paroxysmal or persistent atrial fibrillation, acute decompensated heart failure, previous history of myocardial infarction, previous coronary revascularization, systolic blood pressure, heart rate, emergent coronary angiography, or percutaneous coronary intervention before admission, mechanical ventilation before admission, and intraaortic balloon pump before admission. The C-index was defined as the area under the receiver operating characteristic curves between individual predictive probabilities for AKI and the incidence of AKI, and it was compared with both the baseline model (established risk factors only) and the enriched models (baseline model plus L-FABP, NT-proBNP, or both) [25]. The NRI was a relative indicator of how many patients had an improvement in the predicted probability of AKI, whereas the IDI indicated the average improvement in the predicted probability of AKI after adding variables into the baseline model [26]. We considered p values <0.05 as statistically significant.
Results
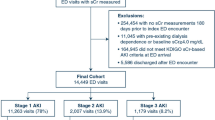
We enrolled 1273 patients aged 21–86 years and have summarized their demographics and clinical characteristics in Table 1. In total, 547 patients (43%) were diagnosed with CKD, with no case of kidney transplantation. A total of 588 patients (46%) were admitted because of acute coronary syndrome (241 patients had ST-segment elevation myocardial infarction, 292 had non-ST-segment elevation myocardial infarction, and 55 had unstable angina), 485 (38%) because of acute decompensated heart failure (244 patients with reduced ejection fraction (LVEF < 40%), 80 with mid-range ejection fraction (40% ≤ LVEF < 50%), and 161 with preserved ejection fraction (LVEF ≥ 50%)), 59 (5%) because of arrhythmia, 35 (3%) because of pulmonary hypertension, 27 (2%) because of acute aortic syndrome, 22 (2%) because of infective endocarditis, 15 (1%) because of Takotsubo cardiomyopathy, and 42 (3%), due to other causes (Table 2). Before admission, emergency coronary angiography or percutaneous coronary intervention was performed in 36%, mechanical ventilation was required for respiratory insufficiency in 2.1%, and intraaortic balloon pumps were needed for hemodynamic instability in 9% of patients.
Urinary L-FABP levels correlated with age (r = 0.06, p = 0.02), hemoglobin (r = − 0.06, p = 0.03), eGFR (r = − 0.28, p < 0.0001), hs-CRP (r = 0.21, p < 0.0001), NT-proBNP (r = 0.17, p < 0.0001), and hs-TnT levels (r = 0.26, p < 0.0001), and LVEF (r = − 0.07, p = 0.01). Serum NT-proBNPlevels correlated with age (r = 0.27, p < 0.0001), hemoglobin (r = − 0.31, p < 0.0001), eGFR (r = − 0.48, p < 0.0001), and hs-CRP levels (r = 0.44, p < 0.0001), and LVEF (r = − 0.46, p < 0.0001).
Of the 1273 patients, 224 (17.6%) developed AKI (48 had stage 2 or 3 disease). Compared with the non-AKI group, the patients in the AKI group were older; had higher heart rate; higher white blood cell count; higher levels of glucose, hs-CRP, NT-proBNP, hs-TnT, and L-FABP; and lower levels of hemoglobin, eGFR, and LVEF; more patients in the AKI group had the following: a history of intraaortic balloon pump or diuretic therapy; hypertension or diabetes mellitus; and acute decompensated heart failure (Table 1).
The median length of CICU stay in the AKI group (4.0 (3.0–6.0) days) was longer than that in the non-AKI group (3.0 (2.0–4.0) days) (p < 0.001). During the 6-month follow-up period, there were 94 all-cause deaths (7.4%), of which 66 were cardiovascular related. During the first week after admission, 24 patients died, of which all deaths were related to cardiovascular issues. Patients with AKI had higher risk of 1-week mortality compared to those who did not (4.9% vs 1.2%, p = 0.0002). Patients with AKI had higher risks of all-cause death and cardiovascular death than patients without AKI (both, p = 0.0003) (Fig. 1).
Patients were divided into tertiles according to their urinary L-FABP and serum NT-proBNP levels. In the multivariate logistic analyses, both L-FABP and NT-proBNP were independent predictors of AKI when assessed as either continuous variables (p < 0.0001 and p = 0.006, respectively) or variables categorized by tertile (p < 0.0001 and p = 0.009, respectively, when comparing the third tertile with the first tertile) (Table 3). Intraaortic balloon pump use and the eGFR also remained significantly associated with AKI. Moreover, Fig. 2 shows that the incidence of AKI gradually increased as the L-FABP and NT-proBNP tertile increased. As shown in Fig. 3, patients in whom both markers were in the highest tertile had the highest risk of AKI, whereas those who had both biomarkers in the lowest tertile had the lowest risk of AKI (p < 0.0001).
Finally, we looked at the effect of adding L-FABP, NT-proBNP, or both to a baseline model of established risk factors. As shown in Table 4, the significant increase in the C-index indicated that adding L-FABP improved the prediction of AKI beyond that of the baseline model alone. Furthermore, adding both L-FABP and NT-proBNP significantly improved the reclassification of patients beyond that of adding any single biomarker and beyond that of the baseline model alone (all p < 0.001); the IDI improved similarly after adding both L-FABP and NT-proBNP (all p < 0.01).
Discussion
The main findings of this study were as follows. First, admission levels of both urinary L-FABP and serum NT-proBNP were independent predictors of developing AKI in patients treated in medical CICUs. Second, patients with L-FABP and NT-proBNP in the upper tertiles were most strongly associated with an increased risk of AKI. Third, combining L-FABP and NT-proBNP improved the predictive value for AKI beyond that achieved with any single biomarker or baseline model alone, as demonstrated by the NRI and IDI. Fourth and finally, patients who developed AKI had a higher risk of death at 1 week and at 6 months than patients who did not develop AKI. These findings indicate that assessing both urinary L-FABP and serum NT-proBNP levels on admission may help with the early risk stratification of AKI in patients admitted to medical CICUs.
Medical CICUs have evolved from their origins of focusing exclusively on patients with acute coronary syndromes and now provide comprehensive critical care to patients with a range of acute cardiovascular illnesses and complex comorbidities [27, 28]. These include patients with diabetes mellitus, hypertension, dyslipidemia, and CKD, and those who use therapeutic devices. Recent prospective studies have shown that, as with patients treated in surgical intensive care units, AKI is commonly and is strongly associated with increased mortality [27]. However, the utility of urinary L-FABP for predicting AKI has not been fully evaluated in medical CICU settings, and there was a need to confirm the reliability and generalizability of L-FABP in heterogeneous populations before its clinical use could be advocated. Thus, we, for the first time, demonstrated that the combined use of urinary L-FABP and serum NT-proBNP, which are individually independent predictors of AKI, could improve the early prediction of AKI in a large (n = 1273), heterogeneous cohort of patients treated at medical CICUs not including cardiac surgery cases.
Type 1 cardiorenal syndrome reflects an abrupt worsening of cardiac function leading to AKI [29]. The combination of urinary L-FABP, a marker of renal tubular injury, and NT-proBNP, a marker of hemodynamic stress, to assess for the development of the cardiorenal syndrome is conceptually attractive. The data presented herein not only support both of these as independent predictors of AKI but also show that combining L-FABP and NT-proBNP can improve the risk reclassification and discrimination for AKI among medical patients in the CICU. Indeed, patients whose results for both markers were in the highest tertile were approximately eightfold more likely to develop AKI than those whose results for both markers were in the lowest tertile. It is crucial that these biomarkers benefit from being readily measurable, easily accessible, relatively inexpensive, and reproducible with high sensitivity and specificity. Following confirmation of our data in other independent cohorts, this combination approach to screening could be included in an algorithm for predicting AKI on admission to a CICU.
In the present study, urinary L-FABP was weakly (r = 0.17), although statistically significantly, correlated with NT-proBNP. This weak correlation suggests that they may target two different pathophysiological dysregulations of AKI: renal tubular injury and hemodynamic stress. Also, the coefficient for correlation between urinary L-FABP and age and between urinary L-FABP hemoglobin was less than 0.2, which was consistent with the results of previous studies [30, 31].
Recently, the predictive value of combining urinary L-FABP with other urinary AKI biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL) [32, 33], interleukin-18 [34], or kidney injury molecule-1 [11], for the development of AKI after cardiac surgery have been studied. Especially, a combination of urinary L-FABP and NGAL may be the most promising strategy for predicting AKI. With respect to the plasma NGAL evaluation along with B-type natriuretic peptide (BNP) in acutely decompensated heart failure, the GALLANT trial showed prognostic values for plasma NGAL, alone and in combination with BNP in acutely decompensated heart failure [35]. These findings lead us to speculate that a biomarker panel consisting of L-FABP, BNP or NT-proBNP, and urinary or plasma NGAL may improve the early prediction of AKI in patients admitted to medical CICUs. Further investigation is desired to confirm the usefulness of this approach.
It should be noted that we measured urinary L-FABP only at the point of admission to the CICU. The decision for this was based on reports of whether this is the best time for predicting AKI in patients with acute decompensated heart failure [36] or whether it is after cardiac surgery [37]. Thus, although we showed that admission measurements of urinary L-FABP may help physicians predict AKI, we do not know whether L-FABP, NT-proBNP, or a combination of both could also be used for monitoring markers. Indeed, we do not know whether improvements in these biomarkers would have affected the study outcomes.
The treatments in this study were also not randomized or controlled, making it difficult to evaluate the effects of treatments on the progression of AKI. For example, AKI was significantly associated with the frequent use of diuretics, whereas some patients underwent emergency coronary angiography or percutaneous coronary intervention and intraaortic balloon pump insertion before samples were obtained. These differences in treatment strategy may have had potentially significant confounding effects on the study results. However, even when we entered these factors into our multivariate logistic analyses, both L-FABP and NT-proBNP remained valid, significant, independent predictors of AKI. Thus, we believe that these did not contribute to significant confounding.
Observational studies suggest an association between AKI and the subsequent development of CKD and ESRD, even following apparent renal recovery [3, 38]. Thus, it is interesting to see if urinary L-FABP could predict CKD and ESRD. However, we could not evaluate this issue because of the study design focused on the early prediction of AKI. Nevertheless, when we retrospectively collected serum creatinine data between 3 and 6 months after discharge in 926 patients (73%) from the study group, the progression to ESRD occurred in 29 patients, including 7 patients who received hemodialysis. The incidence rate for the progression to ESRD in patients with AKI was significantly higher than in patients without AKI (15.6% vs 0.5%, p < 0.0001). Patients in the third tertile of L-FABP had the highest risk of the progression to ESRD (p < 0.0001, when compared with patients in both the first or second tertile; 8.8% vs 0.6% or 0.7%), speculating that higher levels of urinary L-FABP may be associated with the development of ESRD. A larger prospective study is needed to clarify this issue.
There are a few other limitations in the present study. First, it was conducted at a single institution. Second, we evaluated urinary L-FABP as the absolute concentration, but did not use urinary creatinine correction, because the urinary creatinine excretion rate may change over time under non-steady-state conditions [39]. Nevertheless, when we analyzed L-FABP using urinary creatinine correction, the results were comparable with those obtained without creatinine correction (data not shown). Further investigation is necessary to determine the optimal normalization for urinary L-FABP. Third, AKI was only defined using the elevation of serum creatinine because of the inconsistent data recorded and the potential alterations in urine volume induced by medical therapy. This may lead to the neglect of a part of the renal insult, which may be determined by urine output. Finally, the incidence of AKI in this study was 17.6%, which was lower than that reported in two recent studies (28.7% and 31.6%) [40, 41]. This difference may be attributable to differences in the severity of patients’ disease, with higher frequencies of mechanical ventilation and higher values for NT-proBNP in the previous research.
Conclusion
Combining measurements for two independent predictors of AKI, namely, urinary L-FABP and serum NT-proBNP, when patients are admitted to a CICU, improves the early prediction of AKI beyond that achieved when using either predictor in isolation.
Abbreviations
- AKI:
-
Acute kidney injury
- BNP:
-
B-type natriuretic peptide
- CICUs:
-
Cardiac intensive care units
- CKD:
-
Chronic kidney disease
- eGFR:
-
Creatinine-based estimated glomerular filtration rate
- ESRD:
-
End-stage renal disease
- hs-CRP:
-
High-sensitivity C-reactive protein
- hs-TnT:
-
High-sensitivity troponin T
- IDI:
-
Integrated discrimination improvement
- L-FABP:
-
Liver-type fatty acid-binding protein
- LVEF:
-
Left ventricular ejection fraction
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- NRI:
-
Net reclassification improvement
- NSTEMI:
-
Non–ST-segment elevation myocardial infarction
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- STEMI:
-
ST-segment elevation myocardial infarction
References
Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70.
Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8.
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2011;81:442–8.
Vanmassenhove J, Kielstein J, Jörres A, et al. Management of patients at risk of acute kidney injury. Lancet. 2017;389:2139–51.
Kellen M, Aronson S, Roizen MF, et al. Predictive and diagnostic tests of renal failure: a review. Anesth Analg. 1994;78:134–42.
Yamamoto T, Noiri E, Ono Y, et al. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–902.
Xu Y, Xie Y, Shao X, et al. L-FABP: a novel biomarker of kidney disease. Clin Chim Acta. 2015;445:85–90.
Noiri E, Doi K, Negishi K, et al. Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296:F669–F79.
Ek-Von Mentzer BA, Zhang F, Hamilton JA. Binding of 13-HODE and 15-HETE to phospholipid bilayers, albumin, and intracellular fatty acid binding proteins. Implications for transmembrane and intracellular transport and for protection from lipid peroxidation. J Biol Chem. 2001;276:15575–80.
Katagiri D, Doi K, Honda K, et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann Thorac Surg. 2012;93:577–83.
Parikh CR, Thiessen-Philbrook H, Garg AX, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8:1079–88.
Doi K, Noiri E, Maeda-Mamiya R, et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit Care Med. 2010;38:2037–42.
Susantitaphong P, Siribamrungwong M, Doi K, et al. Performance of urinary liver-type fatty acid binding protein in acute kidney injury: a meta-analysis. Am J Kidney Dis. 2013;61:430–9.
Torregrosa I, Montoliu C, Urios A, et al. Urinary KIM-1, NGAL and L-FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessel. 2015;30:703–11.
Wiese S, Breyer T, Dragu A, et al. Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation. 2000;102:3074–9.
Goussot S, Mousson C, Guenancia C, et al. N-terminal fragment of pro B-type natriuretic peptide as a marker of contrast-induced nephropathy after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 2015;116:865–71.
Jarai R, Dangas G, Huber K, et al. B-type natriuretic peptide and risk of contrast-induced acute kidney injury in acute ST-segment-elevation myocardial infarction: a substudy from the HORIZONS-AMI trial. Circ Cardiovasc Interv. 2012;5:813–20.
Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–44.
Luchner A, Hengstenberg C, Löwel H, et al. N-terminal pro-brain natriuretic peptide after myocardial infarction: a marker of cardio-renal function. Hypertension. 2002;39:99–104.
Yamashita T, Seino Y, Ogawa A, et al. N-terminal pro-BNP is a novel biomarker for integrated cardio-renal burden and early risk stratification in patients admitted for cardiac emergency. J Cardiol. 2010;55:377–83.
Parikh CR, Puthumana J, Shlipak MG, et al. Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. J Am Soc Nephrol. 2017;28:3699–707.
Liu Y, He YT, Tan N, et al. Preprocedural N-terminal pro-brain natriuretic peptide (NT-proBNP) is similar to the Mehran contrast-induced nephropathy (CIN) score in predicting CIN following elective coronary angiography. J Am Heart Assoc. 2015; https://doi.org/10.1161/JAHA.114.001410.
Kidney Disease: Improving global outcomes (KDIGO) acute kidney injury work group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138.
Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1998;44:837–45.
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. (discussion 207–12)
Holland EM, Moss TJ. Acute noncardiovascular illness in the cardiac intensive care unit. J Am Coll Cardiol. 2017;69:1999–2007.
Na SJ, Chung CR, Jeon K, et al. Association between presence of a cardiac intensivist and 8Mortality in an adult cardiac care unit. J Am Coll Cardiol. 2016;68:2637–48.
Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39.
von Eynatten M, Baumann M, Heemann U, et al. Urinary L-FABP and anaemia: distinct roles of urinary markers in type 2 diabetes. Eur J Clin Investig. 2010;40:95–102.
Maeda Y, Suzuki A, Ishii J, et al. Level of urinary liver-type fatty acid-binding protein is associated with cardiac markers and electrocardiographic abnormalities in type-2 diabetes with chronic kidney disease stage G1 and G2. Heart Vessel. 2015;30:362–8.
Liu S, Che M, Xue S, et al. Urinary L-FABP and its combination with urinary NGAL in early diagnosis of acute kidney injuryafter cardiac surgery in adult patients. Biomarkers. 2013;18:95–101.
Zeng XF, Li JM, Tan Y, et al. Performance of urinary NGAL and L-FABP in predicting acute kidney injury and subsequent renal recovery: a cohort study based on major surgeries. Clin Chem Lab Med. 2014;52:671–8.
Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–9.
Maisel AS, Mueller C, Fitzgerald R, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL evaluation along with B-type natriuretic peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846–51.
Hishikari K, Hikita H, Nakamura S, et al. Urinary liver-type fatty acid-binding protein level as a predictive biomarker of acute kidney injury in patients with acute decompensated heart failure. Cardiorenal Med. 2017;7:267–75.
Matsui K, Kamijo-Ikemori A, Sugaya T, et al. Usefulness of urinary biomarkers in early detection of acute kidney injury after cardiac surgery in adults. Circ J. 2012;76:213–20.
Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis. 2016;67:742–52.
Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–94.
Chen TH, Chang CH, Lin CY, et al. Acute kidney injury biomarkers for patients in a coronary care unit: a prospective cohort study. PLoS One. 2012;7:e32328.
Parr SK, Clark AJ, Bian A, et al. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2015;87:640–8.
Funding
This work was supported by JSPS KAKENHI (17 K08995).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
HN conceived, designed, and coordinated the study, performed data acquisition and analyses, and drafted the manuscript. JI exerted a major impact on the interpretation of data and critical appraisal of the manuscript. HT supported the statistical analysis. FK contributed to measuring samples in this study. HN, HK, TM, MH, AY, SM, SM, MH, EM, EW, HI, and YO contributed to data interpretation and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of Fujita Health University approved this study (HM16–362).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Naruse, H., Ishii, J., Takahashi, H. et al. Predicting acute kidney injury using urinary liver-type fatty-acid binding protein and serum N-terminal pro-B-type natriuretic peptide levels in patients treated at medical cardiac intensive care units. Crit Care 22, 197 (2018). https://doi.org/10.1186/s13054-018-2120-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-018-2120-z