Abstract
This article is one of ten reviews selected from the Annual Update in Intensive Care and Emergency medicine 2017. Other selected articles can be found online at http://ccforum.com/series/annualupdate2017. Further information about the Annual Update in Intensive Care and Emergency Medicine is available from http://www.springer.com/series/8901.
Similar content being viewed by others
Background
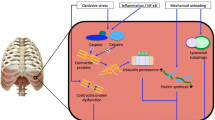
The respiratory muscles drive ventilation. Under normal conditions, the diaphragm is the main muscle for inspiration, whereas expiration is largely passive, driven by relaxation of the inspiratory muscles and elastic recoil pressure of the lung. A disturbance in the balance between capacity and loading of the inspiratory muscles will result in respiratory failure. This may occur in the course of numerous disorders that increase loading of the diaphragm, such as pneumonia, acute respiratory distress syndrome (ARDS), acute exacerbation of chronic obstructive pulmonary disease (COPD) and trauma. On the other hand, capacity of the respiratory muscles may be impaired due to congenital myopathies or acquired muscle dysfunction (e. g., in COPD or cancer cachexia). Mechanical ventilation can partially or completely unload the respiratory muscles. Despite being life‐saving, studies published in the last decade have demonstrated that respiratory muscle function may further deteriorate in ventilator bound intensive care unit (ICU) patients. Respiratory muscle weakness in ventilated ICU patients is associated with prolonged duration of ventilator weaning [1] and increased risk of ICU and hospital readmission [2]. Therefore, it seems of crucial importance to limit the detrimental effects of critical illness, and in particular mechanical ventilation, on the respiratory muscles. The aim of this chapter is to describe recent insights into the pathophysiology of diaphragm dysfunction in ICU patients and how these new findings may affect clinical care for our patients. We will focus on studies performed in humans.
Effects of critical illness on diaphragm structure and function
Effects of critical illness on diaphragm structure
Less than a decade ago, Levine et al. were the first to describe a rapid loss of diaphragm muscle mass in patients on controlled mechanical ventilation [3]. Biopsies were obtained from 14 brain‐dead organ donors on controlled mechanical ventilation for 18 to 69 h before organ harvest. The cross‐sectional area (CSA) of diaphragm fibers was significantly lower (53 and 57% for fast‐ and slow‐twitch fibers respectively) compared to fibers obtained from patients referred for elective lung cancer surgery. Interestingly, the severity of atrophy was less pronounced in the pectoralis muscle, indicating that the diaphragm is much more sensitive to the effects of disuse. In subsequent studies, it was demonstrated that the decrease in diaphragm fiber CSA was proportional to the duration of mechanical ventilation (Pearson r 2 = 0.28) [4]. Diaphragm inactivity was associated with oxidative stress, active caspase‐3 expression and upregulation of mRNAs coding for ligands related to the proteolytic ubiquitin‐proteasome pathway (muscle atrophy F‐box [MAFBx] and muscle ring finger 1 [MuRF‐1]). In addition, activation of the lysosomal‐autophagy pathway was demonstrated in the diaphragm of these brain‐dead patients ventilated for ±60 h [5]. Very recently, Matecki et al. demonstrated increased oxidation, nitrosylation and phosphorylation of the diaphragm sarcoplasmic reticulum ryanodine calcium release channel in ventilated brain‐dead patients [6]. These molecular modifications were associated with enhanced calcium leak from the channel (increased open probability). Additional experiments in mice indicated that leaky ryanodine channels are associated with diaphragm weakness.
Collectively, these data demonstrate that inactivity of the diaphragm under controlled mechanical ventilation is associated with rapid posttranslational protein modifications, activation of proteolytic pathways and muscle fiber atrophy. However, these early studies used biopsies of the diaphragm of brain‐dead patients. This might be an excellent model for diaphragm disuse associated with mechanical ventilation, but differences in treatment and underlying pathophysiology in more representative ICU patients should be recognized. Hooijman et al. were the first to study structural, biochemical and functional modifications of biopsies obtained from the diaphragm of ventilated ICU patients (n = 22, mean duration on the ventilator: 7 days, range 14 to 607 h) [7]. These authors found that both fast‐ and slow‐twitch diaphragm fibers had a CSA that was approximately 25% smaller than that of fibers from the diaphragm of patients referred for elective surgery. Biochemical analysis revealed activation of the proteolytic ubiquitin‐proteasome pathway. Histological analysis demonstrated that the number of inflammatory cells, including neutrophils and macrophages, was significantly increased in the diaphragm of ICU patients; this supports a role for inflammatory mediators in the development of atrophy or injury. Interestingly, van Hees et al. demonstrated that plasma from septic shock patients, but not from healthy subjects, induced atrophy in (healthy) cultured skeletal muscle myotubes [8]. This indicates that plasma from septic shock patients contains molecules with catabolic properties. Additional experiments presented in that paper suggest that interleukin (IL)‐6 plays a role in the development of muscle atrophy in sepsis.
Effect of critical illness on diaphragm function
Structural modifications of the respiratory muscles as described above may have functional implications. Single muscle fibers isolated from the diaphragm provide an excellent model to study contractile protein function. The force generated by single fibers from the diaphragm of ICU patients was significantly reduced compared to fibers from non‐ICU patients [7]. Reduction in force resulted from loss of contractile proteins (atrophy) and contractile protein dysfunction.
The gold standard to evaluate in vivo diaphragm contractile function in ventilated patients is to assess the change in endotracheal tube pressure induced by magnetic stimulation of the phrenic nerves during airway occlusion (Ptr,magn). The major advantage of this technique is that it can be performed at the bedside and does not require patient cooperation. Demoule et al. measured Ptr,magn within 24 h of mechanical ventilation in a population of 85 critically ill patients [9]. Of this group, 54 (64%) patients were diagnosed with diaphragm dysfunction defined as a Ptr,magn < 11 cmH2O. More recently, the same investigators confirmed these earlier findings in 43 ventilated patients [10]; 23 (53%) of these patients exhibited diaphragm dysfunction at ICU admission. These are important findings, as they indicate that other factors besides disuse play a role in the pathophysiology of respiratory muscle weakness in ICU patients. In fact, in their earlier study [9], the authors reported that diaphragm dysfunction was independently associated with sepsis. Consistent with that study, Supinski et al. [11] found that measures for in vivo contractile force were affected more in ventilated patients with infection than in ventilated patients without infection.
In a follow up study, Jaber et al. measured Ptr,magn every 24 to 36 h in ICU patients (n = 6) ventilated for > 5 days [4]. Ptr,magn rapidly declined by approximately 30% in the first 5 to 6 days of controlled mechanical ventilation. The recent study by Demoule et al. [10] also assessed Ptr,magn during the ICU stay. In that study, 61% of patients fulfilling criteria for diaphragm dysfunction at admission had persistent respiratory muscle weakness while in the ICU. Of the patients with normal diaphragm function at ICU admission, 55% developed weakness while on the ventilator. This study demonstrates that 80% of all patients fulfilling their inclusion criteria (i. e., > 5 days on mechanical ventilation), develop respiratory muscle weakness at some time while on the ventilator.
Goligher et al. described the evolution of diaphragmatic thickness during mechanical ventilation and its impact on diaphragm function, assessed with ultrasound [12]. Changes in diaphragm thickness were found in 56% of the study population (n = 128). Both loss and gain of diaphragm thickness were observed during the first week of ventilation, in 44 and 12% of the patients, respectively. Contractile activity of the diaphragm was estimated as the diaphragm thickening fraction during maximal inspiratory effort. There was a significant correlation between the mean diaphragm thickening fraction during the first 3 days of ventilation and changes in diaphragm thickness (p = 0.01): a loss of diaphragm muscle mass was associated with lower contractile activity, while higher contractile activity was found for patients exhibiting increases in diaphragm thickness. Both increased and decreased diaphragm thickness seemed to be modulated by the intensity of respiratory muscle work performed by the patient, because the change in diaphragm thickness was inversely correlated with the driving pressure applied by the ventilator over the first 72 h of ventilation (p = 0.04, after removal of one outlier).
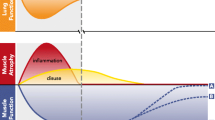
In conclusion, in the last decade our knowledge of the effects of critical illness and mechanical ventilation on respiratory muscle function has markedly improved. We have gained more insight into the pathophysiology, including molecular pathways, associated with dysfunction. In addition, it appears that the most important clinical risk factors for development of dysfunction include disuse and inflammation/sepsis.
Clinical implications of current knowledge
Monitoring diaphragm function in the ICU
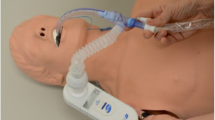
As described above, disuse is proposed to be an important risk factor for the development of atrophy and dysfunction of the diaphragm in ventilated patients. Therefore, a reasonable clinical goal is to limit the duration of respiratory muscle inactivity by using partially assisted modes, such as pressure support ventilation as soon as feasible and safe (fulfilling criteria for lung‐protective ventilation). It should be recognized that even in partially assisted modes, full unloading of the diaphragm may occur ([13]; Fig. 1). Moreover, this study demonstrated that clinicians are often unable to recognize diaphragm inactivity based on pressure and flow signals on the ventilator screen. Therefore, there may be a role for more advanced respiratory muscle monitoring techniques [14, 15]. Recently, we reviewed clinically available methods to monitor respiratory muscle function in ventilated ICU patients [14].
Ventilator screen of a patient in pressure support mode. Despite the fact that this patient was in a partial assist mode, the diaphragm was inactive as demonstrated by the absence of diaphragm electrical activity (lower tracing). Technical issues for absence of diaphragm electrical activity were excluded
Today, the state of the art technique for monitoring diaphragm activity is assessment of the transdiaphragmatic pressure (Pdi). This can be estimated by simultaneous measurement of esophageal pressure (Pes) and gastric pressure (Pga) as surrogates for the pleural and abdominal pressures, respectively. Recently, recommendations for the use of Pes monitoring in ICU patients were published [16, 17]. Although no reference values for Pdi are available for critically ill patients, monitoring will prevent unwanted periods of diaphragm inactivity due to over‐assist in partially supported modes. In addition, Pes monitoring enables the clinician to detect patient‐ventilator asynchronies.
Binding of acetylcholine to the skeletal muscle acetylcholine receptor will result in depolarization of the plasma membrane, which in turn opens calcium release channels that activate contractile proteins, resulting in the generation of force. Depolarization of the plasma membranes can be visualized by electromyography (EMG). Recently, continuous monitoring of diaphragm electrical activity (EAdi) has become available for ICU patients. Neurally‐adjusted ventilator assist (NAVA) is a relatively new mode for partially assisted ventilation that uses EAdi to control the ventilator. EAdi is acquired using a dedicated nasogastric feeding tube with nine electrodes positioned at the level of the diaphragm muscle. The electrical activity is shown real‐time on the ventilator screen (Servo‐i/U, Maquet Sweden), even when the patient is ventilated in another mode than NAVA. Therefore, EAdi may be an alternative to esophageal and gastric balloons to monitor diaphragm activity (Fig. 2). Indeed, absence of EAdi is consistent with inactivity of the diaphragm, when technical issues have been excluded. However, conversion of EAdi (in microvolt) into pressure has not yet been well validated. Bellani et al. proposed a proportionality coefficient (pressure‐electrical activity index, PEi) that allows calculation of pressure generated by the diaphragm from EAdi [18]. Another index derived from the EAdi is the patient‐ventilator breath contribution (PVBC) index, which provides an estimation of the fraction of breathing effort that is generated by the patient compared to the total work of breathing (ventilator + patient). Liu et al. demonstrated in 12 patients ventilated using the NAVA mode that PVBC predicts the contribution of the inspiratory muscles versus the pressure delivered by the ventilator [19]. Although both PEi and PVBC may be helpful to limit the risk of ventilator over‐assist, additional studies are required before clinical implementation is justified.
Strategies to prevent diaphragm dysfunction
Although respiratory muscle weakness may already be present at ICU admission [9], many patients develop weakness while in the ICU [10]. Sepsis is a recognized risk factor for development of respiratory muscle weakness [10], but apart from appropriate treatment of sepsis, no specific interventions can be applied to protect the respiratory muscles. Certain drugs have been associated with the development of respiratory muscle weakness, in particular corticosteroids and neuromuscular blockers. The effect of both drugs, together or separately, on muscle dysfunction is complex and beyond the scope of this paper. We refer to an excellent recent review on this subject [20]. However, it should be noticed that short periods (48 h) of full neuromuscular blockade are not associated with the development of clinically relevant respiratory muscle weakness, because ARDS patients treated with a neuromuscular blocker were liberated faster from mechanical ventilation than patients in a placebo group [21].
As discussed earlier, inactivity appears to be an important risk factor for the development of respiratory muscle dysfunction. No clinical studies have compared the effects of controlled and partially supported modes on the development of respiratory muscle dysfunction in humans. Sassoon et al. [22] investigated the effect of controlled mechanical ventilation or partially supported modes of mechanical ventilation on diaphragm force‐velocity relationships in vitro in rabbits. The loss of diaphragmatic force‐generating capacity was significantly less in rabbits on partially supported modes compared to those receiving controlled mechanical ventilation. Despite the absence of evidence in humans, from a physiological perspective it is reasonable to apply partially assisted ventilator modes when feasible and safe. Recently, it was demonstrated that assisted ventilation in patients with mild‐to‐moderate ARDS improved patient‐ventilator interaction and preserved respiratory variability, while maintaining lung‐protective ventilation in terms of tidal volume and lung‐distending pressure [23].
Nevertheless, in some critically ill patients admitted to the ICU partially assisted modes are not feasible, for instance due to very high respiratory drive. In these patients, high levels of sedation may be required, resulting in full unloading of the respiratory muscles. An interesting hypothesis is that in these patients electrical activation of the muscles (‘pacing’) may limit development of dysfunction. Pavlovic and Wendt were the first to hypothesize that electrical pacing of the diaphragm may prevent disuse atrophy and contractile dysfunction in mechanically ventilated patients [24]. This hypothesis was based on evidence that electrical pacing of limb skeletal muscle is an effective therapy to maintain muscle activity without patient cooperation. For example, multiple studies have shown that non‐invasive pacing of the quadriceps in COPD patients improves muscle performance and exercise capacity [25,26,27]. Based on the effectiveness of these studies, there is a growing interest for pacing the respiratory muscles in the critically ill ventilated patient.
Today, the major clinical application of non‐invasive pacing of the phrenic nerve has been as a diagnostic tool, using brief transcutaneous stimuli at the neck for the evaluation of phrenic nerve conduction time, diaphragm contractility and fatigue [28, 29]. However, this method is not feasible for therapeutic purposes, because prolonged transcutaneous pacing is uncomfortable for patients and correct positioning of the stimulator (coil) is cumbersome in an ICU setting because of its selectiveness: minor changes in the location of the stimulus can result in submaximal activation of the phrenic nerve and co‐activation of other anatomically related nerves.
Although there is no clinical evidence for therapeutic pacing to reduce diaphragm atrophy and dysfunction during mechanical ventilation, a few studies have been conducted. A very novel technique, using transvenous phrenic nerve pacing, has been investigated recently [30]. To activate the phrenic nerves bilaterally, a pacing catheter is introduced in the left subclavian vein and advanced into the superior vena cava. The preclinical study in mechanically ventilated pigs showed that loss of diaphragm muscle mass was attenuated in pigs subjected to phrenic nerve pacing. Future studies should evaluate the feasibility of this device in humans.
Strategies to restore respiratory muscle dysfunction
ICU‐acquired respiratory muscle weakness may prolong duration of mechanical ventilation. Therefore, in difficult‐to‐wean patients, strategies that improve respiratory muscle strength are of potential clinical importance. Surprisingly, very few studies have evaluated strategies to enhance respiratory muscle function in these patients. We will discuss the role of inspiratory muscle training and pharmacological interventions.
Inspiratory muscle training
We have recently discussed the rationale for inspiratory muscle training in ICU patients [31]. Briefly, few studies have evaluated the effects of inspiratory muscle training in ICU patients. In the largest trial [32], 69 patients admitted to a long‐term weaning facility were randomized to inspiratory muscle strength training or sham training. In both groups, endurance training was accomplished by progressive spontaneous breathing trials. Inspiratory muscle strength training significantly improved inspiratory muscle strength and facilitated weaning from the ventilator. Importantly, no adverse effects of inspiratory strength training were reported.
Inspiratory muscle weakness after extubation is associated with increased risk of ICU and hospital readmission within 6 weeks after ICU discharge [2]. Therefore, the recently published trial by Bissett and colleagues [33], in which 70 ICU patients ventilated for > 7 days were randomized to inspiratory muscle strength training or usual care after successful extubation (>48 h), is of particular relevance. Training was performed five days/week for two weeks. The primary endpoint, respiratory muscle strength, was significantly improved in the training group (training: 17% versus control: 6%, p = 0.02), but this did not result in clinical benefits, such as length of stay or risk of readmission.
In conclusion, based on these preliminary studies, inspiratory muscle strength training appears to be effective in enhancing inspiratory muscle strength and is safe in ICU patients. Future studies are required to determine the optimal moment to start inspiratory muscle training (early versus late in ICU admission) and to investigate the optimal training protocol.
Pharmacotherapy
In contrast to cardiac muscle dysfunction, surprisingly no drug is approved to improve respiratory muscle function. We briefly discuss the role of anabolic hormones and of the cardiac inotrope, levosimendan. For more extensive discussion we refer to an earlier review by our group [31].
Anabolic steroids increase bodyweight, fat‐free mass and muscle strength in healthy people [34]. Although many clinical trials have assessed the effects of anabolic steroids in patients with chronic diseases, they do not have sufficient statistical power for clinically relevant endpoints. Pan et al. [35] conducted a meta‐analysis and selected eight randomized controlled trials investigating the effects of anabolic steroids in patients with COPD. They concluded that anabolic steroids improve fat‐free mass and quality of life, but do not influence inspiratory muscle strength, although some high quality studies did demonstrate that anabolic hormones improved inspiratory muscle strength when administered during a rehabilitation program [36]. No randomized studies have been performed in weak difficult‐to‐wean ICU patients. Notably, Takala et al. reported that early administration of growth hormone increased mortality in ICU patients [37]. In conclusion, anabolic hormones should not be routinely used in ICU patients to enhance muscle strength. Whether they may have a role for difficult‐to‐wean patients with severe respiratory muscle weakness remains to be investigated.
Levosimendan is a relatively novel cardiac inotrope, which enhances the binding of calcium to troponin and improves contractile efficiency of the muscle fibers. It is approved for the treatment of heart failure, but exerts effects in other organs as well [38]. Levosimendan has been shown to enhance contractile efficiency of muscle fibers isolated from the diaphragm of healthy subjects and patients with COPD [39]. Doorduin et al. demonstrated in healthy subjects that levosimendan reversed fatigue and improved neuromechanical efficiency of the diaphragm in vivo [40]. Future studies should evaluate the effects of levosimendan on respiratory muscle function in difficult to wean patients.
Conclusion
In the last decade, our understanding of the pathophysiology of ICU‐acquired respiratory muscle weakness has improved considerably. Molecular mechanisms include activation of different proteolytic pathways and posttranslational protein modifications, in particular of calcium release channels. This results in atrophy of respiratory muscle fibers and dysfunction of the remaining contractile proteins. Weakness is associated with prolonged mechanical ventilation and increased risk of ICU and hospital readmission. Risk factors for the development of respiratory muscle weakness include sepsis and disuse. In particular, duration of disuse can be limited in selected patients by applying appropriate monitoring techniques that help reduce over‐assist by the ventilator. Future studies should reveal the optimal monitoring techniques and the precise goals of monitoring. In patients who have developed ICU‐acquired respiratory muscle weakness, no intervention has been shown to improve outcome. Nevertheless, several studies have demonstrated that inspiratory muscle strength training improves strength. No drug has been approved to improve respiratory muscle function. As respiratory muscle weakness has detrimental effects on patient outcomes, clinically effective treatment strategies are urgently needed.
References
De Jonghe B, Bastuji-Garin S, Durand MC, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–15.
Adler D, Dupuis-Lozeron E, Richard JC, Janssens JP, Brochard L. Does inspiratory muscle dysfunction predict readmission after intensive care unit discharge? Am J Respir Crit Care Med. 2014;190:347–50.
Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35.
Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183:364–71.
Hussain SN, Mofarrahi M, Sigala I, et al. Mechanical ventilation-induced diaphragm disuse in humans triggers autophagy. Am J Respir Crit Care Med. 2010;182:1377–86.
Matecki S, Dridi H, Jung B, et al. Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc Natl Acad Sci U S A. 2016;113:9069–74.
Hooijman PE, Beishuizen A, Witt CC, et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191:1126–38.
van Hees HW, Schellekens WJ, Linkels M, et al. Plasma from septic shock patients induces loss of muscle protein. Crit Care. 2011;15:R233.
Demoule A, Jung B, Prodanovic H, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact – a prospective study. Am J Respir Crit Care Med. 2013;188:213–9.
Demoule A, Molinari N, Jung B, et al. Patterns of diaphragm function in critically ill patients receiving prolonged mechanical ventilation: a prospective longitudinal study. Ann Intensive Care. 2016;6:75.
Supinski GS, Westgate P, Callahan LA. Correlation of maximal inspiratory pressure to transdiaphragmatic twitch pressure in intensive care unit patients. Crit Care. 2016;20:77.
Goligher EC, Fan E, Herridge MS, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192:1080–8.
Colombo D, Cammarota G, Alemani M, et al. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med. 2011;39:2452–7.
Doorduin J, van Hees HW, van der Hoeven JG, Heunks LM. Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med. 2013;187:20–7.
Heunks LM, Doorduin J, van der Hoeven JG. Monitoring and preventing diaphragm injury. Curr Opin Crit Care. 2015;21:34–41.
Akoumianaki E, Maggiore SM, Valenza D, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–31.
Mauri T, Eronia N, Turrini C, et al. Bedside assessment of the effects of positive end-expiratory pressure on lung inflation and recruitment by the helium dilution technique and electrical impedance tomography. Intensive Care Med. 2016;42:1576–87.
Bellani G, Mauri T, Coppadoro A, et al. Estimation of patient’s inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41:1483–91.
Liu L, Liu S, Xie J, et al. Assessment of patient-ventilator breath contribution during neurally adjusted ventilatory assist in patients with acute respiratory failure. Crit Care. 2015;19:43.
Annane D. What Is the evidence for harm of neuromuscular blockade and corticosteroid use in the intensive care unit? Semin Respir Crit Care Med. 2016;37:51–6.
Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:107–16.
Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170:626–32.
Doorduin J, Sinderby CA, Beck J, et al. Assisted ventilation in patients with acute respiratory distress syndrome: Lung-distending pressure and patient-ventilator interaction. Anesthesiology. 2015;123:181–90.
Pavlovic D, Wendt D. Diaphragm pacing during prolonged mechanical ventilation of the lungs could prevent from respiratory muscle fatigue. Med Hypotheses. 2003;60:398–403.
Maddocks M, Nolan CM, Man WD, et al. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:27–36.
Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest. 2003;124:292–6.
Abdellaoui A, Prefaut C, Gouzi F, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J. 2011;38:781–8.
Luo YM, Lyall RA, Lou HM, Rafferty GF, Polkey MI, Moxham J. Quantification of the esophageal diaphragm electromyogram with magnetic phrenic nerve stimulation. Am J Respir Crit Care Med. 1999;160:1629–34.
Cattapan SE, Laghi F, Tobin MJ. Can diaphragmatic contractility be assessed by airway twitch pressure in mechanically ventilated patients? Thorax. 2003;58:58–62.
Reynolds SC, Meyyappan R, Thakkar V, et al. Mitigation of ventilator-induced diaphragm atrophy by transvenous phrenic nerve stimulation. Am J Respir Crit Care Med. 2016. Epub ahead of print
Schellekens WJ, van Hees HW, Doorduin J, et al. Strategies to optimize respiratory muscle function in ICU patients. Crit Care. 2016;20:103.
Martin AD, Smith BK, Davenport PD, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15:R84.
Bissett BM, Leditschke IA, Neeman T, Boots R, Paratz J. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax. 2016;71:812–9.
Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7.
Pan L, Wang M, Xie X, Du C, Guo Y. Effects of anabolic steroids on chronic obstructive pulmonary disease: a meta-analysis of randomised controlled trials. PLoS One. 2014;9:e84855.
Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152:1268–74.
Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–92.
Farmakis D, Alvarez J, Gal TB, et al. Levosimendan beyond inotropy and acute heart failure: Evidence of pleiotropic effects on the heart and other organs: An expert panel position paper. Int J Cardiol. 2016;222:303–12.
van Hees HW, Dekhuijzen PN, Heunks LM. Levosimendan enhances force generation of diaphragm muscle from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:41–7.
Doorduin J, Sinderby CA, Beck J, et al. The calcium sensitizer levosimendan improves human diaphragm function. Am J Respir Crit Care Med. 2012;185:90–5.
Acknowledgements
Not applicable.
Funding
Publication costs were funded by the VU University Medical Centre Amsterdam.
Availability of data and materials
Not applicable.
Authors’ contributions
All authors contributed to writing of the manuscript. LH revised latest version. All authors read and approved the final manuscript.
Competing interests
LH has received institutional research funding from Orion Pharma, Finland and Liberate Medical USA. LH has received speakers fee from Orion Pharma, Finland, Maquet Critical Care Sweden. LH is a member of the clinical advisory board of Maquet Critical Care Sweden. AJ and DJ have no disclosures.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Jonkman, A.H., Jansen, D. & Heunks, L.M.A. Novel insights in ICU-acquired respiratory muscle dysfunction: implications for clinical care. Crit Care 21, 64 (2017). https://doi.org/10.1186/s13054-017-1642-0
Published:
DOI: https://doi.org/10.1186/s13054-017-1642-0