Abstract
Background
The aim was to test the primary hypothesis that in patients suffering from shock, treatment with N-acetylcysteine (NAC) plus deferoxamine (DFX) decreases the incidence of acute kidney injury (AKI).
Methods
A double-blind, randomized, placebo-controlled trial was conducted in a general intensive care unit in an academic hospital. Patients were included if they had new-onset hypotension, defined as mean arterial blood pressure <60 mmHg or requirement for vasopressor medication. A loading dose of NAC or placebo of 50 mg/kg in 4 h was administered intravenously. After the loading dose, patients received 100 mg/kg/day for the next 48 h. DFX or placebo was administered once at 1000 mg at a rate of 15/mg/kg/h. The primary outcome was the incidence of AKI.
Results
A total of 80 patients were enrolled in the study. The incidence of AKI was 67 % in the placebo arm and 65 % in the treatment group (relative risk (RR) 0.89 (0.35–2.2)). Furthermore, NAC plus DFX was effective in decreasing the severity and duration of AKI, and patients in the treatment group had lower serum creatinine levels at discharge. No severe adverse event associated with treatment was reported. The effects of NAC plus DFX could be secondary to the attenuation of early inflammatory response and oxidative damage.
Conclusion
The administration of NAC plus DFX to critically ill patients who had a new episode of hypotension did not decrease the incidence of AKI.
Trial registration
Clinicaltrials.gov NCT00870883 (Registered 25 March 2009.)
Similar content being viewed by others
Background
Oxidative stress and inflammation are related events and occur in a variety of illnesses, including critical illness [1]. Both oxidative damage and inflammatory biomarkers are associated with experimental and clinical kidney injury [2–5], and are independently associated with worse outcomes in patients with shock [6, 7]. These processes may mediate poor clinical outcomes through endothelial damage, oxidation of lipid membranes and proteins, nitric oxide scavenging and vasoconstriction, and cell apoptosis, and all these factors can be associated with the development of acute kidney injury (AKI) [8, 9].
In this context antioxidants could have therapeutic value to prevent AKI in the critically ill patient. N-acetylcysteine (NAC) scavenges oxidant species and donates sulfhydryl groups to the synthesis of glutathione. In animal studies, NAC attenuates oxidative damage and kidney injury caused by shock [10]. In patients with shock, NAC administration prevents oxidative damage and can attenuate some markers of disease severity [11], but its protective effect is controversial [12]. However, NAC has some limitations when used as an antioxidant. Larger doses of NAC can have oxidant effects, probably because it interacts with iron [13]. This may also account for some of the negative results in clinical trials of NAC. In this context, we and others have demonstrated that the addition of an iron chelator (deferoxamine - DFX) to NAC treatment improves its protective effects [14–18]. In a pilot trial we demonstrated that NAC plus DFX decrease plasma levels of both oxidative and inflammatory parameters in patients with shock [19].
On the basis of these data we conducted a randomized, double-blind, placebo-controlled, parallel-group trial to test the hypothesis that treatment with NAC plus DFX is superior to placebo to improve renal function in patients with shock. We further hypothesized that the protective effect of these drugs could be related to its antioxidant and anti-inflammatory effects.
Methods
Patients
Patients were enrolled in the São José Hospital intensive care unit (ICU), Criciúma, Brazil. The trial was approved by the São José Hospital Institutional Review Board and was registered on www.clinicaltrials.gov (NCT00870883). Written informed consent was obtained from all patients or their surrogates. A safety monitor board reviewed all adverse events. All the procedures were in accordance with the Declaration of Helsinki. The trial was conducted in accordance to the original protocol, and the full protocol can be accessed on request.
The same inclusion and exclusion criteria as were used in the NEPHRON clinical trial [20] were adopted to allow indirect comparison with another study in which NAC was used. Patients were included if they had new onset of 30 consecutive minutes of hypotension, defined as mean arterial blood pressure <60 mmHg that did not improve after fluid infusion or requirement for vasopressor medication (dopamine drip at 10 μg/kg/min or norephinephrine, ephinephrine, or vasopressin drip at any concentration). Patients who had an episode of hypotension within the previous 48 h were excluded. The first dose of NAC plus DFX, or placebo, had to be given within 12 h of meeting the inclusion criteria. Patients were excluded if they met any of the following exclusion criteria: age <18 years, participating in another research study, allergy to NAC or DFX, received high-dose NAC for acetaminophen overdose in the previous 48 h, pregnant or lactating, received activated charcoal in the previous 48 h, serum creatinine >3.5 mg/dL or already on hemodialysis, patients with hemoglobin <6.5 mg/dl, weight <40 kg, advanced malignancy, or a “do not resuscitate” order.
A research nurse generated the allocation sequence in a ratio of 1:1, based on a simple randomization scheme (http://graphpad.com/quickcalcs/randomize1). The same nurse assigned participants to study groups, and after informed consent was obtained enrolled patients to receive NAC plus DFX, or placebo. Both placebo and treatment solutions were prepared and infused by the research nurse, the only person to know the group allocation; the patients, intensive care team, and researchers were blinded to group allocation. The appearance of the flasks containing the treatment or placebo was the same. As NAC has a peculiar smell, after the infusion the research nurse discharged the flasks outside the ICU to try and maintain the masking of the treatment arm.
A loading dose of 50 mg/kg NAC diluted in 250 mL of glucose 5 % was administered intravenously at an infusion rate of 62.5 ml/h. After the loading dose, patients received a continuous infusion of NAC 100 mg/kg/day diluted in 250 mL glucose 5 % for two consecutive days. DFX was administered once at a dose of 1000 mg dissolved in 250 mL glucose 5 % at a rate of 3.75 mL/kg/h. The placebo group received the same volume and procedural infusion of glucose 5 %.
Data collection
After entry into the study, demographic information was collected for each patient. Preexisting medical problems were recorded and the Charlson comorbidity score was calculated. The patient’s baseline creatinine for the study was the most recent measurement of creatinine before entry into the study. The Acute Physiology and Chronic Health Evaluation (APACHE) II score was computed for the 24-h period before study inclusion. Sequential Organ Failure Assessment (SOFA) scores were computed during the first 3 days after inclusion. Sepsis was defined according to internationally agreed criteria [21]. Cardiogenic shock was defined by persistent hypotension, and poor perfusion with reduction in cardiac index and adequate or elevated filling pressure. Doppler echocardiography was used in patients without a pulmonary artery catheter, to determine whether shock was of cardiac origin.
For the duration of the ICU hospitalization, serum creatinine (SCr) and urine output was recorded daily, and from this information the incidence of AKI was defined as a new or worsening AKI stage as suggested by the Kidney Disease Improving Global Outcomes (KIDGO) Guideline for Acute Kidney Injury [22], and was the primary study outcome. Several secondary outcomes were examined including the severity of AKI (stages 2 and 3 defined according to the KIDGO Guideline for Acute Kidney Injury), number of days with AKI, time to development of the first episode of AKI, SOFA score at day 3, SCr levels at discharge defined as the last creatinine level before hospital discharge, ICU and hospital length of stay, and requirement for renal replacement therapy (RRT) and mortality.
Inflammatory and oxidative damage parameters
Blood was collected on enrollment and 24 and 48 h after. Blood samples were placed on ice and processed within 10 minutes of collection by centrifugation at 3000 rpm for 10 minutes at 4 °C, and then plasma was stored at –80 °C. Plasma interleukin (IL)-6, IL-10, IL-8, thiobarbituric acid reactive species (TBARS) and protein carbonyls were measured in singulate. Interleukins were measured using commercial ELISA kits (R&D System), and were expressed as pg/mL plasma. The determination of carbonyl groups in proteins provides a convenient technique to detect and quantify oxidative modification in proteins. The method for determination of carbonyl content used was based on the reaction of carbonyl groups with 2,4-dinitrophenylhydrazine to form a 2,4-dinitrophenylhydrazonedetected at 280 nm. Protein carbonyls levels were expressed as nmol/mL plasma. As evidence of oxidative damage the formation of malondialdehyde (MDA) − thiobarbituric acid complex was assessed using the TBARS reaction. The amount of TBARS was determined by measuring the absorbance at 532 nm. TBARS concentration was expressed as MDA equivalents (nmol)/mL plasma).
Statistical analysis
The expected incidence of AKI incidence was 60 % and the expected absolute risk reduction was 20 %; thus, it was determined that 70 patients would need to be enrolled in each arm to have 80 % power to detect this difference using a two-sided test at a significance level 0.05. However, the enrollment rate was slower than expected. In May of 2015, 80 patients had been enrolled, and the data and safety monitoring board recommended stopping enrollment at this time.
All analyses were performed according to the intention-to-treat principle. The primary outcome was analyzed using the chi-square test. Continuous secondary outcomes were analyzed by Student’s t test or Mann-Whitney U test, depending on the data distribution. Dichotomous secondary outcomes were assessed using the chi-square test. Variables that could confound the effect of treatment upon the outcomes were entered into forward multivariate logistic regression analysis. Thus, as prespecified, a model was created that included treatment group, age, SOFA score at enrollment and sepsis. The Hosmer–Lemeshow goodness-of-fit test was used to evaluate the agreement between the observed and expected outcome. The area under the receiver-operating characteristic curve (AROC) was used to assess the model discrimination. A linear mixed model was used to determine the effect of treatment on the temporal variations of biomarker levels. Statistical significance was defined as a p value <0.05.
Results
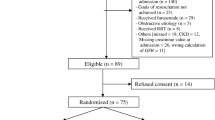
A total of 382 patients were assessed for eligibility from February 2012 to May 2015, of whom 80 were enrolled in the study (Fig. 1). Baseline data were similar among the study groups (Table 1). There was no early termination of the treatment protocol. The ICU team did not report any severe adverse events associated with treatment. On statistical analysis there was no significant effect of NAC plus DFX on the incidence of AKI (Table 2). Only 4 patients (10 %) in the placebo group, and 5 (12.5 %) in the NAC plus DFX group had AKI defined based on SCr levels. The vast majority were categorized as AKI on the basis of urine output or urine output plus SCr criteria. Only age and SOFA at study admission were independently associated with the incidence of AKI in our sample. When secondary outcomes were analyzed, NAC plus DFX was effective in decreasing the severity and duration of AKI, and patients in the treatment group had lower SCr at discharge. No other significant differences were observed (Tables 2 and 3).
The effects of NAC plus DFX could be secondary to the attenuation of early inflammatory response and oxidative damage. Baseline inflammatory and oxidative damage parameters were similar between groups. Both TBARS and IL-6 increased after allocation in the placebo group (Fig. 2a and b). However, NAC plus DFX treatment reversed this trend. In addition, NAC plus DFX, but not placebo, decreased IL-8 (Fig. 2c). There was no significant effect of NAC plus DFX treatment on IL-10 and protein carbonyls (Fig. 2d and e).
Oxidative and inflammatory parameters. Blood was collected on enrollment and on the morning of the subsequent 2 days for the measurement of thiobarbituric acid reactive species (TBARS) (a), interleukin (IL)-6 (b), IL-8 (c), IL-10 (d), and protein carbonyls (e). *Different from time 0, same group. #Difference between groups at the same time point NAC + DFX N-acetylcysteine plus deferoxamine, MDA malondialdehyde
Discussion
We have demonstrated that the incidence of AKI did not decrease after treatment with NAC plus DFX in patients with new onset of sustained hypotension. However, there was a significant difference in the severity of AKI and patients in the treatment group had lower SCr at discharge. In addition, NAC plus DFX treatment decreased blood oxidative and inflammatory parameters.
Oxidative damage can be detected in plasma in a number of critical illnesses, such as sepsis (6), acute respiratory distress syndrome (ARDS) [23], and hemorrhagic shock [24]. In all of these conditions, oxidative damage has been associated with poor clinical outcomes. Despite this, antioxidant strategies aimed at improving clinically significant outcomes give controversial results [11, 12]. To date, NAC is one of the most common antioxidant strategy that has been studied in critically ill patients. The inconsistency of results using NAC could be secondary to the range of dosing across the studies. No optimal dose for any indication has been established.
Prophylaxis for contrast-induced nephropathy doses ranges from 1200 to 2400 mg/day, and patients with acetaminophen overdose require up to 1000 mg/kg. Komisarof and colleagues studied a population similar our treated patients, and administered an oral loading dose of 3000 mg, followed by 4500 mg/day for the next 3 days and 2400 mg/day for the last 4 days [22]. We used a loading intravenous dose of 3500 mg (for a patient weighing 70 kg), followed by a continuous infusion of 14,000 mg (for a patient weighing 70 kg) in the next 2 days, which is higher than in the Komisarof study, and the route of administration was also different (oral as compared to intravenous). Thus, it is difficult to directly compare different studies. We chose this dose because it is similar to the dose used in our pre-clinical model (equivalent to 6–8 g/day) [14, 15]. In addition, the study of Komisarof and colleagues suggests that higher doses must be used in the context of hypotension [20]: they observed a trend towards a lower SOFA score during the first 3 days of NAC administration, but this trend disappeared shortly after the reduction of the NAC dose [20]. It is unknown whether higher doses, such as the doses used for acetaminophen intoxication, would result in better outcomes, mainly with the concomitant administration of DFX.
The timing of NAC therapy may also be relevant. When administered 24 h after admission, NAC could worsen organ dysfunction [12]. We used the same approach of Komisarof and colleagues, giving NAC plus DFX within 12 h of the occurrence of hypotension. We had previously demonstrated that NAC plus DFX was effective in an animal model of sepsis when administered 3 h after induction of sepsis [14]. The protective effect was lost when administered 12 h after induction of sepsis (unpublished data). As oxidative stress results in chain reactions that could amplify the initiation reaction, it is reasonable to suppose that interventions aimed at decreasing oxidative damage should have its greater effect early after initiation of disease. This narrow therapeutic window, allied with the higher NAC dose, and the addition of DFX could explain our partially positive results. In addition, this would impact on inflammatory response, and both antioxidant and anti-inflammatory effects could partially explain some of the protective effects of NAC + DFX. However, the effects of NAC + DFX upon these biomarkers could alternatively reflect less severe kidney injury, as some of these are cleared by the kidney [25].
Our findings are consistent with results from pre-clinical models, whereby we and others demonstrated that the association of NAC plus DFX was superior to decrease inflammation, oxidative stress and organ failure when compared to the isolated use of NAC [14–18]. During states of shock, iron could be released from several different sources, and free iron could drive free radical formation trough the Fenton reaction [26], besides having several other detrimental effects [27, 28]. In addition, NAC could have pro-oxidant effects in the presence of iron [13]. Thus, it seems rational to add an iron chelator to improve the antioxidant and protective effects of NAC. We used the iron chelator as a single dose as it has been previously in pre-clinical studies [14–18]. This points to a major role for NAC in this context, but as NAC has a potential pro-oxidant effect, the addition of DFX seems to be relevant. Moving from bench to bedside a single dose of DFX was maintained with the theoretical objective of decreasing the pro-oxidant effect mainly of the loading dose of NAC and narrowing the possibility of adverse effects of DFX administration in critically ill patients.
Iron seems to have a pivotal role in injury, specifically in the kidney. Preloading animals with iron has been shown to worsen ischemic damage to the kidney [29], and the use of iron chelators is effective in different animal models of kidney injury [30, 31]. Iron could have direct toxic effects in the kidney, such as increased vascular resistance, and direct damage to tubular cells. One of the most relevant examples of the role of iron in kidney damage is neutrophil gelatinase-associated lipocalin (NGAL) [32]. NGAL is highly upregulated after injury, and serum or urine NGAL can be used as an early marker of kidney injury in humans [33, 34]. NGAL scavenges iron ions, and the protective effects of NGAL administration in animal models of kidney injury seem to involve this function [35–37].
This study has some limitations. First, this was a single-center study. Second, our small sample size resulted in less robust results because small changes in the number of events or non-events would have produced different results [38]; for this reason a larger trial will be needed to confirm these results. The narrow time window to the administration of the drugs could restrict its clinical use, and restricts the inclusion of several patients in our trial (patients otherwise eligible, but unable to obtain consent and start drug administration within the first 12 h of hypotension), which could decrease its external validity. Third, a more in depth characterization of the patients’ volemic status at enrollment, and parameters of fluid responsiveness would help to better understand our results, but unfortunately this information is not consistently available to all included patients. Fourth, to ascertain that DFX is really acting as an iron chelator as was hypothesized, we should measure the iron pool that can be chelated by DFX. As DFX permeates into cells relatively slowly and does not mobilize significant amounts of iron from transferrin it probably binds non-transferrin-bound iron in the serum [39]. This specific iron pool is more labile than transferrin-bound iron and therefore is a potential catalytic iron source that is available to chelation by DFX [39].
Conclusion
The early administration of NAC and DFX to critically ill patients with new-onset hypotension is not associated with lower incidence of AKI, but is related to less severe AKI and lower SCr levels at discharge. These effects at least seem to be linked to the antioxidant and anti-inflammatory effects of the treatment.
Abbreviations
- AKI:
-
Acute kidney injury
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- AROC:
-
Area under the receiver-operating characteristic curve
- DFX:
-
Deferoxamine
- ICU:
-
Intensive care unit
- IL:
-
Interleukin
- KIDGO:
-
Kidney Disease Improving Global Outcomes
- MDA:
-
Malondialdehyde
- NAC:
-
N-acetylcysteine
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- RRT:
-
Renal replacement therapy
- SCr:
-
Serum creatinine
- SOFA:
-
Sequential Organ Failure Assessment
- TBARS:
-
Thiobarbituric acid reactive species
References
Lemineur T, Deby-Dupont G, Preiser JC. Biomarkers of oxidative stress in critically ill patients: what should be measured, when and how? Curr Opin Clin Nutr Metab Care. 2006;9:704–10.
Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–65.
Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11.
Kasuno K, Shirakawa K, Yoshida H, Mori K, Kimura H, Takahashi N, Nobukawa Y, Shigemi K, Tanabe S, Yamada N, Koshiji T, Nogaki F, Kusano H, Ono T, Uno K, Nakamura H, Yodoi J, Muso E, Iwano M. Renal redox dysregulation in AKI: application for oxidative stress marker of AKI. Am J Physiol Renal Physiol. 2014;307:F1342–51.
Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest. 2014;124:4989–5001.
Guerreiro MO, Petronilho F, Andrades M, Constantino L, Mina FG, Moreira JC, Dal-Pizzol F, Ritter C. Plasma superoxide dismutase activity and mortality in septic patients. J Trauma. 2010;69:E102–6.
Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49.
Huen SC, Parikh CR. Molecular phenotyping of clinical AKI with novel urinary biomarkers. Am J Physiol Renal Physiol. 2015;309:F406–13.
Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20:588–95.
Dare AJ, Phillips AR, Hickey AJ, Mittal A, Loveday B, Thompson N, Windsor JA. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic Biol Med. 2009;47:1517–25.
Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, Napoli C. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406.
Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012;9:CD006616.
Sprong RC, Winkelhuyzen-Janssen AM, Aarsman CJ, van Oirschot JF, van der Bruggen T, van Asbeck BS. Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am J Respir Crit Care Med. 1998;157:1283–93.
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med. 2004;32:342–9.
Ritter C, da Cunha AA, Echer IC, Andrades M, Reinke A, Lucchiari N, Rocha J, Streck EL, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med. 2006;34:471–7.
Ritter C, Reinke A, Andrades M, Martins MR, Rocha J, Menna-Barreto S, Quevedo J, Moreira JC, Dal-Pizzol F. Protective effect of N-acetylcysteine and deferoxamine on carbon tetrachloride-induced acute hepatic failure in rats. Crit Care Med. 2004;32:2079–83.
Petronilho F, Constantino L, de Souza B, Reinke A, Martins MR, Fraga CM, Ritter C, Dal-Pizzol F. Efficacy of the combination of N-acetylcysteine and desferrioxamine in the prevention and treatment of gentamicin-induced acute renal failure in male Wistar rats. Nephrol Dial Transplant. 2009;24:2077–82.
Bulucu F, Oktenli C, Kenar L, Ocal R, Koc B, Inal V, Yamanel L, Yaman H, Sanisoglu YS, Aydin A. Efficacy of deferoxamine, N-acetylcysteine and selenium treatments in rats with Adriamycin-induced nephrotic syndrome. J Nephrol. 2008;21:576–83.
Fraga CM, Tomasi CD, Biff D, Topanotti MF, Felisberto F, Vuolo F, Petronilho F, Dal-Pizzol F, Ritter C. The effects of N-acetylcysteine and deferoxamine on plasma cytokine and oxidative damage parameters in critically ill patients with prolonged hypotension: a randomized controlled trial. J Clin Pharmacol. 2012;52:1365–72.
Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal. 2008;10:739–53.
Szabó C, Módis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010;34 Suppl 1:4–14.
Komisarof JA, Gilkey GM, Peters DM, Koudelka CW, Meyer MM, Smith SM. N-acetylcysteine for patients with prolonged hypotension as prophylaxis for acute renal failure (NEPHRON). Crit Care Med. 2007;35:435–41.
Levy MM, Fink MP, Marshall JC. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6.
KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138.
Thomson AW, Lotze MT. The Cytokine Handbook. Fourth edn. London: Elsevier; 2003.
Lasocki S, Gaillard T, Rineau E. Iron is essential for living! Crit Care. 2014;18:678.
Zeng C, Chen Q, Zhang K, Song S, Fang X. Hepatic hepcidin protects against polymicrobial sepsis in mice by regulating host iron status. Anesthesiology. 2015;122:374–86.
Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A. 2014;111:E4110–8.
Wu ZL, Paller MS. Iron loading enhances susceptibility to renal ischemia in rats. Ren Fail. 1994;16:471–80.
Paller MS, Hedlund BE. Extracellular iron chelators protect kidney cells from hypoxia/reoxygenation. Free Radic Biol Med. 1994;17:597–603.
Vries B, Walter SJ, von Bonsdorff L, Wolfs TG, van Heurn LW, Parkkinen J, Buurman WA. Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia–reperfusion injury. Transplantation. 2004;77:669–75.
Paragas N, Qiu A, Hollmen M, Nickolas TL, Devarajan P, Barasch J. NGAL-Siderocalin in kidney disease. Biochim Biophys Acta. 2012;1823:1451–8.
Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–24.
Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61.
Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15:3073–82.
Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43.
Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin–siderophore–iron complex rescues the kidney from ischemia–reperfusion injury. J Clin Invest. 2005;115:610–21.
Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, Molnar AO, Dattani ND, Burke A, Guyatt G, Thabane L, Walter SD, Pogue J, Devereaux PJ. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014;67:622–8.
Breuer W, Ronson A, Abramov A, Slotki I, Hershko H, Cabantchik ZI. The assessment of serum non-transferrin bound iron (NTBI) in chelation therapy and iron supplementation. Blood. 2000;95:2975–82.
Funding
This work was supported by São José Hospital Research Center, Universidade do Extremo Sul Catarinense, Brazilian Council for Scientific and Technological Development and Fundação de Amparo a Pesquisa do Estado de Santa Catarina.
Availability of data and materials
Data are available on request.
Authors’ contributions
CMF conceived the work, drafted the manuscript, and approved the final version. CR and FD-P conceived the work, revised the manuscript, and approved the final version. CDT designed the work and acquired data, revised the manuscript, and approved the final version. DCD and FV acquired data, revised the manuscript, and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The trial was approved by the São José Hospital Institutional Review Board. Written informed consent was obtained from all patients or their surrogates.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fraga, C.M., Tomasi, C.D., Damasio, D.d.C. et al. N-acetylcysteine plus deferoxamine for patients with prolonged hypotension does not decrease acute kidney injury incidence: a double blind, randomized, placebo-controlled trial. Crit Care 20, 331 (2016). https://doi.org/10.1186/s13054-016-1504-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-016-1504-1