Abstract
Background
Acute kidney injury (AKI) in patients with multisystem inflammatory syndrome (MIS), COVID-19 related infection has been increasingly recognized with a paucity of data on AKI incidence, related mortality, and the requirement of renal replacement therapy in children with MIS (MIS-C).
Methods
This is a retrospective study evaluating the prevalence, severity, management and outcomes of AKI in a cohort of Egyptian children with MIS-children (MIS-C) post-COVID infection. Patients were included if they met the criteria for MIS-C based on CDC guidelines. All patients were evaluated for AKI diagnosis and staging according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria.
Results
Between March 2021 and June 2023, a total of 655 confirmed COVID-19 cases were admitted and then followed up in our hospital, of whom 138 (21%) were diagnosed with MIS-C. Fifty-one patients developed AKI associated with MIS-C post-COVID infection, 42 of whom were included in the analysis. Thirty-one patients had AKI in a formerly healthy kidney, of whom 51% (16 patients) were classified as KDIGO stage 3, 5 patients needed hemodialysis and 13 needed mechanical ventilation. Higher WBCs count, and serum ferritin on admission were associated with more severe AKI (KDIGO stage 3) (p = 0.04), while multivariate analysis showed high serum ferritin to be independent predictor of more severe AKI (p = 0.02). Two patients (2/31) died during hospital admission, while no residual renal impairment was reported at the time of discharge of patients with previously normal kidney functions.
Conclusion
More than one-third of patients with MIS-C develop AKI. Avoidance of nephrotoxic drugs, early recognition, and prompt management of AKI, including well-timed commencement of dialysis in MIS-C cases, is associated with favorable outcomes.
Similar content being viewed by others
Introduction
Since late 2019, a global pandemic known as coronavirus disease-2 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-19), has had a negative impact on people's wellbeing as well as all features of life. The pathophysiology of COVID-19, its effects, and associated disorders have become more obvious over time [1]. Multisystem inflammatory syndrome in children (MIS-C) was initially described in confirmed COVID-19 cases in late April 2020, and by December 2020, the Centers for Disease Control and Prevention (CDC) recorded 1288 cases [2, 3] and defined MIS-C as having persistent fever and inflammatory laboratory markers, as well as signs of severe disease requiring hospitalization and multiorgan involvement (e.g., cardiac, gastrointestinal, renal, hematologic, dermatologic, and neurologic). The affected individual must be less than 21 years old and have had exposure to a confirmed or suspected patient with COVID-19 [4]. The incidence of MIS-C is reported to be nearly 3.16 cases per 10,000 SARS-CoV-2-infected persons [5].
The proportion of pediatric patients with SARS-CoV-2 infection who develop acute kidney injury (AKI) is highly variable between reports [6, 7], with a prevalence in critically ill children approaching 44% [8]. A systematic review lately reported a pooled proportion of patients with MIS-C developing AKI of 20% (11 studies, 4947 patients) [9].
The pathophysiology of renal dysfunction in patients with SARS-CoV-2 infection is multifactorial, including dehydration, poor cardiac output, cytokine storm, direct cytopathic effect of the virus on renal tubular cells and use of nephrotoxic agents [10], whereas in patients presenting with MIS-C, renal hypoperfusion seems to be the major underlying factor for AKI [11, 12].
With insufficient data on AKI incidence, risk factors, effect on mortality, and the required care in children with MIS-C worldwide and the absence of such data from our locality, this retrospective study aimed to evaluate the incidence of AKI, renal pathology, mortality, and the need for kidney replacement therapy (KRT) in MIS-C-related COVID-19 infection for the first time in Egyptian children.
Materials and methods
A retrospective study of patients with AKI associating MIS-C, related COVID-19 infection admitted to Pediatric Nephrology and Pediatric Intensive Care Units of Mansoura University Children’s Hospital, Mansoura, Egypt. Data from patients admitted and then followed-up between March 2021 and June 2023 were extracted, including 655 cases of COVID infection, of which 51 were diagnosed with AKI associating MIS-C, related COVID-19 infection. All patient data were extracted from the electronic database registry of the hospital. The study protocol was approved by the local Ethics Committee of Mansoura Faculty of Medicine-Institutional Research Board (IRB) (R.23.06.2233). All patients/guardians provided informed consent at the time of hospital admission for possible use of their data in future research.
Study participants
Forty-two patients with AKI associating MIS-C, related COVID-19 infection were included in the analysis, 31 with AKI affecting normal kidney and 11 patients had AKI on top of previously diagnosed chronic kidney disease (CKD) (Fig. 1). Out of 138 cases of MIS-C; 47 fulfilled CDC criteria at time of hospital admission while 91 were diagnosed during hospital stay (isolation ward) then were transferred to PICU.
Inclusion criteria:
-
◦ Patients with confirmed diagnosis of SARS-CoV-2 by a positive reverse transcriptase-polymerase chain reaction (RT‒PCR) (with or without a positive SARS-CoV-2 antibody test) or positive SARS-CoV-2 serology test only (with negative or unknown RT‒PCR results) were included.
-
◦ The case definition for MIS-C, COVID-19, and related illness was based on the CDC criteria. A confirmed case meets both the clinical and laboratory criteria [13].
-
◦ Definition and staging of AKI (stages 1, 2 or 3) was performed based on the Kidney Disease Improving Global Outcomes (KDIGO)-2012 criteria [14].
-
◦ Patients with AKI developed on top of previously diagnosed CKD were included and analyzed separately.
Exclusion criteria:
-
◦ Patients who had a clinical presentation of MIS-C but did not meet the CDC case definition criteria were excluded.
Methods
Clinical evaluation
Data extraction included patient demographic features [age, weight, height, body mass index (BMI)] and presenting symptoms. Hospitalization information collected included the duration of hospital stay, level of care needed [pediatric intensive care unit (PICU)/inpatient isolation room], medications received and clinical support needs, including inotropic support, mechanical ventilation, KRT and days to recovery of renal function.
Biochemical data
Serum creatinine (at admission, peak, and before discharge), complete blood count (CBC), peak values of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), D-dimer, ferritin, and urinalysis.
Renal pathology
Percutaneous ultrasound-guided renal biopsy was performed in 3 cases due to persistently elevated serum creatinine despite improvement of general condition, respiratory distress, and inflammatory markers.
Follow-up
After discharge, all patients were followed in the outpatient clinic for 6 months (after 2 weeks, then monthly). Each follow-up visit included clinical examination, blood pressure monitoring, CBC, urine analysis and serum creatinine assessment.
Statistical analysis
Data were analyzed with SPSS Statistics (SPSS, IBM Company, Chicago, IL, USA) version 23. The normality of the data was first tested with the Shapiro–Wilk test. Continuous variables were presented as the mean ± SD for parametric data and were compared with Student̕̕ s t test or one-way ANOVA test, while for nonparametric data, median (minimum–maximum) was used and Mann‒Whitney or Kruskal‒Wallis tests. Qualitative data are presented as frequencies and percentages. The association between categorical variables was tested using the chi-square test. Kaplan‒Meier curves were plotted for the three stages of AKI to detect the time elapsed until AKI recovery. The P value to reject the null hypothesis and consider statistical significance is < 0.05.
Results
Participants and descriptive data
Over 24 months, a total of 655 COVID-19 confirmed cases were admitted to our hospital, of which 51 cases developed AKI associating MIS-C post-COVID infection (9 excluded due to incomplete data). The 42 AKI cases included: 11 cases with previously diagnosed CKD (6 cases with unknown primary etiology, 3 cases with steroid-resistant nephrotic syndrome and 2 cases with lupus nephritis) and 31 cases with previously normal kidney functions. For demographic characteristics, patient age, sex and anthropometric measures did not significantly vary between the study groups. Moreover, clinical data, including presenting symptoms, MIS-C associating AKI, vasopressor need and mechanical ventilation use, were not significantly different between the AKI affecting the previously normal kidney group and the CKD group, as summarized in Table 1. Patients with known CKD included 5 patients with CKD stage III, 1 patient with stage IV and 5 patients with stage V. One of those 11 patients died, and 8 continued hemodialysis after discharge.
Patients with AKI in previously normal kidney functions
Patients with AKI affecting previously normal kidneys were further categorized according to KDGIO staging into 3 groups. The basic demographic and clinical features of the 3 groups are compared in Table 2. Comorbidities included congenital heart disease (3 cases), B-thalassemia (2 cases), type 1 diabetes (3 cases) and juvenile idiopathic arthritis (1 case).
Table 3 demonstrates the laboratory values of stage 1, 2 and 3 AKI patients, CBC on admission and ESR, CRP, ferritin and D. dimer peak values. Admission creatinine levels were significantly higher in stage 3 (p = 0.001) than in stages 1 and 2. Higher WBCs count, and serum ferritin on admission were associated with more severe AKI (KDIGO stage 3) (p = 0.04), while multivariate analysis showed high serum ferritin to be independent predictor of more severe AKI (p = 0.02). Renal biopsy was performed in 3 cases: the first was a concomitantly discovered case of class III lupus nephritis, the second case was a newly discovered CKD stage II, and pathology revealed a chronic stage of diffuse crescentic glomerulonephritis. The third case was a 7-year-old female with AKI in a previously healthy kidney. Figure 2 shows light and electron microscopic findings of the case that revealed the early chronic stage of thrombotic microangiopathy “glomerular type”, focal acute tubular injury and faint IgM deposits.
A Light microscopy showing a broadened fibrillary mesangium without associated hypercellularity. Some capillaries exhibit a double contoured appearance (→). (PAS stain × 400). B and C) Electron microscopy showing that the double contoured appearance seen by light microscopy is due to expansion of the subendothelial space by an electron-lucent, fluffy finely granular material with mesangial interposition. (L: capillary lumen, S: subendothelial space divided into subcompartments)
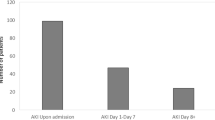
All patients received intravenous immunoglobulin (IVIG) at a dose of 2 g/kg, which was repeated after 48 h if fever persisted, cardiac affection, organ dysfunction deteriorated, or inflammatory markers worsened otherwise corticosteroids was considered. Only 2 patients received favipiravir (due to limited availability and oral route which was not appropriate for several cases with GIT involvement). Hemodialysis was initiated in 5 cases (due to volume overload, refractory metabolic acidosis or uremia), and the number of dialysis sessions ranged between 5–12 sessions. In Table 4, a significantly higher duration of hospital admission was observed in Stage II (29.3 ± 15.3 days; p = 0.018), with the longest duration of PICU stay also reported in stage 3 (20 days), but with no statistically significant difference in PICU stay reported between AKI stages. Days elapsed for resolution of AKI were significantly higher in stage 3 [20 (7–56) when compared with stage 1 [8 (4–19) and stage 2 [10 (7–63); (p = 0.034) (Fig. 3).
Two patients deceased during hospital admission. The first was diagnosed with dilated cardiomyopathy (familial) and died with extensive pneumonia, while the second patient had ARDS, pancytopenia, autoimmune encephalitis, acquired secondary bacterial infection and septic shock.
Discussion
At the beginning of the pandemic, children were considered to be at lower risk of COVID-19, with a lower incidence of infection and milder clinical course [15]. Later, small cohort studies and case reports highlighted kidney involvement in children with COVID-19 infection [16, 17]. There have also been few reports supporting a high risk of AKI occurrence in MIS-C, related COVID-19 infection [9] however, there has been no consensus regarding the accurate incidence, outcomes, and related mortality among this population. Therefore, our study was executed to address the prevalence of AKI in children with post-COVID MIS-C and to assess severity and outcome in different stages.
The reported incidence of AKI in children with COVID-19 infection is highly variable between studies. In the current study, 51 patients were diagnosed with AKI, which represented 37% of patients with MIS-C-related COVID-19 and 7.8% of total COVID-19 patients admitted to our hospital during the study period. Grewal et al., reported AKI in 24% of total COVID cases, with a differential incidence of 54% among MIS-C patients and only 9% in those with acute SARS-CoV-2 infection without MIS-C [12], and in a recent systematic review, AKI was reported in 20% of MIS-C patients [9]. This variability could be related to variances in AKI definitions applied, rates of hospitalization, concomitant comorbid factors, and incidence of MIS-C cases. A systematic review reported that the incidence of AKI was also higher in studies that used the KDIGO definition of AKI (24%) than in studies that used alternative definitions of AKI (18%), and there was a slightly higher proportion of children developing AKI in single-center studies (23%) than in multicenter studies (20%) [9]. The incidence of AKI in pediatric patients is variable, ranging from 5–37% in the PICU with variable requirements for KRT; therefore, patients with MIS-C have a comparable risk of AKI when compared to patients in the PICU due to other causes [18, 19].
The mechanism of AKI in SARS-CoV-2 patients is multifactorial, including dehydration, poor cardiac output, cytokine storm and microvascular thrombosis [20] as well as the direct cytopathic effect of the virus on renal tubular cells due to the presence of angiotensin-converting enzyme 2 receptors, which serve as an entrance door for SARS-CoV-2, and the use of nephrotoxic drugs. However, the mechanism implicated in AKI development in MIS-C patients is chiefly due to renal hypoperfusion because those patients had longer durations of ICU stay, were more susceptible to hypoxemia, particularly if they developed ARDS [21], or needed more inotropic support and had higher markers of cardiac dysfunction compared to acute SARS-CoV-2-AKI patients [22, 23].
Fever and dyspnea were the most common presenting features in our patients (36/42, 85.7%, each). In addition, more than 30% of patients with stage 3 AKI needed oxygen therapy, and 50% needed invasive mechanical ventilation, which may support the role of hypoxemia in AKI pathogenesis. This is consistent with the findings of Sabaghian and colleagues, who reported a statistically significant increase in the number of cases with respiratory system involvement in adults with stage 3 AKI [24].
Different comorbidities have been reported to be associated with the development and worsening of AKI in MIS and COVID-19 infection in both adults and children. Grewal et al. reported that nearly 60% of children in the AKI group had underlying comorbidities: pulmonary (18%), neurological (8%), malignancies (7%), sickle cell disease (5%), hypertension (3%), immunosuppression (2%), type 1 (2%), type 2 diabetes mellitus (2%), and cardiac conditions (1%) [12]. Adult studies reported that the most common comorbidities were diabetes, hypertension and hyperlipidemia that were documented in 40%, 61.4% and 57.1% of patients with COVID-19 and AKI, respectively [25], while a history of previously diagnosed CKD was reported in 22.2% of patients [23].
Kidney biopsy was performed in 3 cases in our cohort. Recognizing and properly diagnosing renal involvement in patients with COVID-19 could be challenging (due to the lack of direct proof of viral infection, e.g., viral particles) and requires a proper integration of clinical and pathological data [26]. A wide range of renal pathological findings have been described to associate COVID-19 infection in adults both in native and transplanted kidneys, such as collapsing glomerulopathy (CG) and other forms of focal segmental glomerulosclerosis, acute tubular necrosis (ATN), IgA nephropathy, thrombotic microangiopathy, crescentic glomerulonephritis, minimal change disease, membranous nephropathy, and anti-glomerular basement membrane disease [27,28,29,30,31,32,33]. SARS-CoV-2 antigens have been detected by immunohistochemistry in kidney tubules, and virus particles were detected in the renal tubular epithelium and podocytes by transmission electronic microscopy [34]. COVID-19-related immune thrombosis is due to macrophage activation and cytokine storms leading to elevated CRP, ferritin and D-dimer levels, which are correlated with worse outcomes [35]. Scarce renal pathological reports in children with COVID-19 infections are available including minimal change disease, C3 glomerulopathy [26], acute necrotizing glomerulonephritis [36], diffuse and segmental mesangial-proliferative glomerulonephritis and acute tubule-interstitial nephritis [37].
Stage 3 AKI patients reported significantly higher WBCs count, ESR, serum ferritin and creatinine on admission, while no significant difference was detected between AKI stages regarding age, associated comorbid diseases, need for inotropes and mechanical ventilation. Tastemel Ozturk et al. reported a significant relationship between older age and AKI in MIS-C patients in univariate analysis, which was lost in multivariate analysis [38]. The median age of patients with AKI was more likely to be younger than that of patients without AKI (9 years vs. 10.5 years; p = 0.08) [39]. Higher values of inflammatory biomarkers, such as WBCs, CRP, procalcitonin, D-dimer, ferritin and IL-6, were observed in MIS-C patients presenting with AKI. In the same studies associated with systolic dysfunction, the need for inotropes and lower levels of albumin and bicarbonates were monitored. Therefore, these data suggest a double component in the pathogenesis of AKI in MIS-C due to both an inflammatory pathway and prerenal injury [40,41,42].
Hemodialysis (conventional and hemodiafiltration) was initiated in 5 patients (AKI stage 3), and 5–12 sessions were performed per patient. Unfortunately, continuous renal replacement therapy (CRRT) was not available at our institution at that time, so intermittent dialysis was used. KRT in the setting of multiorgan disease syndrome should be initiated with expertise. KRT can be utilized non-selectively to clear inflammatory mediators via convection, adsorption and diffusion. CRRT corrects fluid overload and manages solute levels to provide hemodynamic stability in catabolic pediatric patients [43]. Immediate initiation of preemptive KRT in cases with progressive symptomatic respiratory insufficiency improves overall outcomes. The pediatric CRRT group advises the early initiation of KRT in critically ill COVID-19-related immune dysregulation syndrome patients as it has been shown to mitigate fluid overload, enhance cytokine clearance, improve oxygenation and create hemodynamic stability earlier, leading to better overall outcomes, but if CRRT is not available, intermittent hemodialysis is satisfactory [44].
The mean duration of PICU stay for patients with AKI with previously normal kidney function was 9.9 days, with the longest duration reported in AKI stage 3 (20 days). Grewal et al. reported that all patients in the MISC-AKI group needed admission to the PICU with a median duration of PICU stay in patients with AKI of 7.5 days [12], while Özen et al. (2023) reported a length of stay in the PICU of 8 days [45]. These differences may be related to differences in the severity of cases and standards for PICU admission and discharge.
Only 2 mortalities were reported in our cohort, representing 4.8% of 42 cases with AKI, 1.4% of 138 MISC-related COVID-19 cases and 0.3% of total COVID-19 cases admitted over 2 years. A report from North America reported a 7.7% mortality rate among 274 pediatric COVID patients with AKI [46], while a report from Saudi Arabia stated that AKI was significantly associated with mortality (42% vs. 0%), compared to patients with normal kidney function, and after adjustment for age, sex, and the presence of comorbidities, AKI was still drastically associated with mortality [7]. Bjornstad et al., a multicenter study throughout the United States, Eastern Europe, and Russia, reported a mortality rate of 6% among AKI COVID-19 pediatric patients and 5% among pediatric COVID-19 patients without AKI [16].
AKI is considered an independent risk factor for increased mortality in critically ill patients with any disease [47]. Kidney involvement has also been reported as an indicator of poor prognosis irrespective of initial COVID-19 severity [48], yielding avoidance of nephrotoxic drugs, early detection and prompt management of renal function abnormalities improve the prognosis, which can explain the low mortality rates in our cohort. Additionally, no residual renal impairment was reported in 29 children (with previously normal kidney function) at the time of discharge, and all had normal urine analysis and serum creatinine during the 6-month follow-up period. This is inconsistent with the findings of Kari et al., who reported that 9% of their study population had residual renal impairment at the time of discharge and that factors associated with residual renal impairment were decreased tissue perfusion, sepsis, worsening clinical condition, or comorbidities [7]. Tastemel Ozturk et al. reported AKI recovery at discharge to be 63.6% in COVID-19 survivors and 100% in MIS-C patients [38].
Strengths and limitations
This is the first study to present the epidemiological features and outcomes of AKI in Egyptian children with MIS-C-related COVID-19 infection. The limitations of the study include the limited number of subjects (owing to the percentage of MISC among COVID children), being a single center study, the retrospective nature of the study, the changes in virus virulence and emergence of new variants in this process due to the long period in which the patients were included, and the changes in the treatments applied and not including a non-AKI group for comparison.
Conclusion
AKI can occur in more than one-third of MIS-C cases admitted to the PICU, with higher WBC count, ESR, and serum ferritin on admission associated with more severe AKI. Avoidance of nephrotoxic drugs, early recognition, and prompt management of renal impairment in MIS-C cases, including well-timed initiation of dialysis, are associated with favorable prognosis.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- ARDS:
-
Acute respiratory distress syndrome
- CDC:
-
Centers for Disease Control and Prevention
- CKD:
-
Chronic kidney disease.
- COVID-19:
-
Coronavirus Disease-2
- CRRT:
-
Continuous renal replacement therapy
- HD:
-
Hemodialysis
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- MIS-C:
-
Multisystem inflammatory syndrome in children
- PCR:
-
Polymerase chain reaction.
- PICU:
-
Pediatric intensive care unit
- KRT:
-
Kidney replacement therapy
- SARS-CoV-19:
-
Severe acute respiratory syndrome coronavirus-2
References
Sohrabi C, Alsaf Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifdis C, Agha R. World Health organization declares global emergency: a review of the novel coronavirus (2019) (COVID-19). Int J Surg. 2020;76:71–6. https://doi.org/10.1016/j.ijsu.2020.02.034.
Royal College of Pediatrics and Child Health (2020). Pediatric multisystem inflammatory syndrome [PIMS) temporally associated with COVID-19 - guidance for clinicians. Available at: https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance.
Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases (U.S.) (2021). Health department-reported cases of multisystem inflammatory syndrome in children [MIS-C) in the United States. Available at: https://stacks.cdc.gov/view/cdc/106439.
Centers for Disease Control and Prevention: Multisystem Inflammatory Syndrome (MIS-C): Health department–reported cases of Multisystem Inflammatory Syndrome (MIS-C) in the United States. Available at https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance. Accessed 1 Oct 2023.
Payne AB, Gilani Z, Godfredcato S, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4(6):2116420. https://doi.org/10.1001/jamanetworkopen.2021.16420.
Stewart DJ, Hartley JC, Johnson M, Marks SD, du Pré P, Stojanovic J. Renal dysfunction in hospitalized children with COVID-19. Lancet Child Adolesc Health. 2020;4:e28–9. https://doi.org/10.1016/S2352-4642(20)30178-4.
Kari JA, Shalaby MA, Albanna AS, Alahmadi TS, Alherbish A, Alhasan KA. Acute kidney injury in children with COVID-19: a retrospective study. BMC Nephrol. 2021;22:1–8. https://doi.org/10.1186/s12882-021-02389-9.
Basu RK, Bjornstad EC, Gist KM, Starr M, Khandhar P, Chanchlani R, Krallman KA, Zappitelli M, Askenazi D, Goldstein SL, Investigators SPARC. Acute kidney injury in critically Ill children and young adults with suspected SARS-CoV-2 infection. Pediatr Res. 2022;91:1787–96. https://doi.org/10.1038/s41390-021-01667-4.
Tripathi AK, Pilania RK, Bhatt GC, Atlani M, Kumar A, Malik S. Acute kidney injury following multisystem inflammatory syndrome associated with SARS-CoV-2 infection in children: a systematic review and meta-analysis. Pediatr Nephrol. 2023;38(2):357–70. https://doi.org/10.1007/s00467-022-05701-3.11.
Deep A, Bansal M, Ricci Z. Acute kidney injury and special considerations during renal replacement therapy in children with Coronavirus disease-19: perspective from the critical care nephrology section of the European society of pediatric and neonatal intensive care. Blood Purif. 2021;50:150–60. https://doi.org/10.1159/000509677.
Lipton M, Mahajan RG, Kavanagh C, Shen C, Batal I, Dogra S, Jain NG, Lin F, Uy NS. Acute kidney injury in COVID-19-associated multisystem inflammatory syndrome in children (MIS-C). Kidney. 2021;360(2):611–8. https://doi.org/10.34067/KID.0005372020.
Grewal MK, Gregory MJ, Jain A, Mohammad D, Cashen K, Ang JY, Thomas RL, Valentini RP. Acute kidney injury in pediatric acute SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-C): is there a difference? Front Pediatr. 2021;9:692256. https://doi.org/10.3389/fped.2021.692256.
Information for Healthcare Providers About Multisystem Inflammatory Syndrome in Children (MIS-C) (2021). Available online at: https://www.cdcgov/mis-c/hcp/index.html. Acessed 7 Oct 2023.
Selewski DT, Cornell TT, Heung M, Troost JP, Ehrmann BJ, Lombel RM, Blatt NB, Luckritz K, Hieber S, Gajarski R, Kershaw DB, Shanley TP, Gipson DS. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med. 2014;40(10):1481–8. https://doi.org/10.1007/s00134-014-3391-8.
Dawood FS, Porucznik CA, Veguilla V, et al. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City New York. JAMA Pediatr. 2022;176(1):59–67. https://doi.org/10.1001/jamapediatrics.2021.4217.
Bjornstad EC, Krallman KA, Askenazi D, Zappitelli M, Goldstein SL, Basu RK. Preliminary assessment of acute kidney injury in critically Ill children associated with SARS-CoV-2 infection: a multicenter cross-sectional analysis. Clin J Am Soc Nephrol. 2021;16(3):446–8. https://doi.org/10.2215/CJN.11470720.
Wu HHL, Shenoy M, Kalra PA, Chinnadurai R. Intrinsic kidney pathology following COVID-19 infection in children and adolescents: a systematic review. Children (Basel). 2021;9(1):3. https://doi.org/10.3390/children9010003.
Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, Liang X, Fu P, Liu ZH, Mehta RL. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324–31. https://doi.org/10.2215/CJN.04360514.
Restrepo JM, Mondragon MV, Forero-Delgadillo JM, Lasso RE, Zemanate E, Bravo Y, Castillo GE, Tetay S, Cabal N, Calvache JA. Acute renal failure in children. Multicenter prospective cohort study in medium-complexity intensive care units from the Colombian southeast. PLoS One. 2020;15:e0235976. https://doi.org/10.1371/journal.pone.0235976.
Gabarre P, Dumas G, DuPont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensiv Care Med. 2020;46:1339–48. https://doi.org/10.1007/s00134-020-06153-9.
Joannidis M, Forni LG, Klein SJ, et al. Lung–kidney interactions in critically ill patients: consensus report of the acute disease quality initiative (ADQI) 21 workgroup. Intensiv Care Med. 2020;46:654–72. https://doi.org/10.1007/s00134-019-05869-7.
Deep A, Upadhyay G, du Pré P, et al. Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 pandemic: experience from PICUs across United Kingdom. Crit Care Med. 2020;48:1809–18. https://doi.org/10.1097/ccm.0000000000004662.
Diorio C, McNerney KO, Lambert M, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4:6051–63. https://doi.org/10.1182/bloodadvances.2020003471.
Sabaghian T, Kharazmi AB, Ansari A, et al. COVID-19 and acute kidney injury: a systematic review. Frontiers. 2022;4(9):705908. https://doi.org/10.3389/fmed.2022.705908.
Fabrizi F, Alfieri CM, Cerutti R, Lunghi G, Messa P. COVID-19 and Acute kidney injury: a systematic review and meta-analysis. Pathogens. 2020;9(12):1052. https://doi.org/10.3390/pathogens9121052.
Gambella A, Barreca A, Biancone L, Roccatello D, Peruzzi L, Besso L, Licata C, Attanasio A, Papotti M, Cassoni P. Spectrum of kidney injury following COVID-19 disease: renal biopsy findings in a single italian pathology service. Biomolecule. 2022;12(2):298. https://doi.org/10.3390/biom12020298.
Kesiena O, Papadopoulos P, Amakye D, Hama E, Mackay R. COVID-19 associated collapsing glomerulopathy presenting as acute kidney injury on chronic kidney disease: a case report and review of the literature. CEN Case Rep. 2022;11(2):273–7. https://doi.org/10.1007/s13730-021-00667-x.
Kudose S, Santoriello D, Bomback AS, et al. Longitudinal outcomes of COVID-19-associated collapsing glomerulopathy and other podocytopathies. J. Am. Soc Nephrol. 2021;32:2958–69. https://doi.org/10.1681/asn.2021070931.
Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR. COVID-19-associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2020(2):493–7. https://doi.org/10.1016/j.xkme.2020.05.005.
May RM, Cassol C, Hannoudi A, et al. A multicenter retrospective cohort study defines the spectrum of kidney pathology in coronavirus 2019 disease (COVID-19). Kidney Int. 2021;2021(100):1303–15. https://doi.org/10.1016/j.kint.2021.07.015.
Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Nickolas T, Kisselev S, Gharavi A, Canetta P. Acute kidney injurydue to collapsing glomerulopathy following COVID-19 Infection. Kidney Int Rep. 2020;2020(5):940–5. https://doi.org/10.1016/j.ekir.2020.04.017.
Noble R, Tan MY, McCulloch T, Shantier M, Byrne C, Hall M, Jesky M. Collapsing glomerulopathy affecting native and transplant kidneys in individuals with COVID-19. Nephron. 2020;144:589–94. https://doi.org/10.1159/000509938.
Wu H, Larsen CP, Hernandez-Arroyo CF, et al. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol. 2020;31:1688–95. https://doi.org/10.1681/ASN.2020050558.
Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12(1):2506. https://doi.org/10.1038/s41467-021-22781-1.
Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–8. https://doi.org/10.1515/cclm-2020-0369.
Basiratnia M, Derakhshan D, Yeganeh BS, Derakhshan A. Acute necrotizing glomerulonephritis associated with COVID-19infection: Report of two pediatric cases. Pediatr Nephrol. 2021;36:1019–23. https://doi.org/10.1007/s00467-021-04944-w.
Serafinelli J, Mastrangelo A, Morello W, et al. Kidney involvement and histological findings in two pediatric COVID-19 patients. Pediatr Nephrol. 2021;36:3789–93. https://doi.org/10.1007/s00467-021-05212-7.
Tastemel Ozturk T, Düzova A, Oygar PD, et al (2023). Acute kidney injury in children with moderate-severe COVID-19 and multisystem inflammatory syndrome in children: a referral center experience. Pediatr Nephrol. 7. https://doi.org/10.1007/s00467-023-06125-3.
Stewart DJ, Mudalige NL, Johnson M, Shroff R, du Pr P, Stojanovic J. Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) is not associated with progression to chronic kidney disease. Arch Dis Child. 2022;107:e21. https://doi.org/10.1136/archdischild-2021-322866.
Raina R, Mawby I, Chakraborty R, Sethi SK, Mathur K, Mahesh S, Forbes M. Acute kidney injury in COVID-19 pediatric patients in North America: Analysis of the virtual pediatric systems data. PLoS ONE. 2022;17:e0266737. https://doi.org/10.1371/journal.pone.0266737.
Basalely A, Gurusinghe S, Schneider J, et al. Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19. Kidney Int. 2021;100(1):138–45. https://doi.org/10.1016/j.kint.2021.02.026.
Sethi SK, Rana A, Adnani H, McCulloch M, Alhasan K, Sultana A, Safadi R, Agrawal N, Raina R. Kidney involvement in multisystem inflammatory syndrome in children: a pediatric nephrologist’s perspective. Clin Kidney J. 2021;14:2000–11. https://doi.org/10.3390/children10101661.
Ankawi G, Neri M, Zhang J, Breglia A, Ricci Z, Ronco C. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: the promises and the pitfalls. Crit Care. 2018;22(1):262. https://doi.org/10.1186/s13054-018-2181-z.
Raina R, Chakraborty R, Sethi SK, Bunchman T. Kidney replacement therapy in COVID-19 induced kidney failure and septic shock: a pediatric continuous renal replacement therapy [PCRRT] position on emergency preparedness with resource allocation. Front Pediatr. 2020;8:413. https://doi.org/10.3389/fped.2020.00413.
Özen H, Aslan AD, Balaban B, Perk O, Uçmak H, Özcan S, Gurbanov A, Uyar E, Kahveci F, Gün E, Tehci AK, Emeksiz S, Kendirli T. Acute kidney injury in critically ill children with COVID-19 and MIS-C. Pediatr Nephrol. 2023;38(10):3475–82. https://doi.org/10.1007/s00467-023-05987-x.
Raina R, Chakraborty R, Mawby I, Agarwal N, Sethi S, Forbes M. Critical analysis of acute kidney injury in pediatric COVID-19 patients in the intensive care unit. Pediatr Nephrol. 2021;36:2627–38. https://doi.org/10.1007/s00467-021-05084-x.
Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–23. https://doi.org/10.1007/s00134-015-3934-7.
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–38. https://doi.org/10.1016/j.kint.2020.03.005.
Acknowledgements
“Not applicable”.
Permission note
All materials in the manuscript are original.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). There is no external funding for this manuscript.
Author information
Authors and Affiliations
Contributions
HE and MZ conceived the main study idea and design; HE was involved in clinical data collection and data analysis and wrote the first draft of manuscript; RE: shared in clinical data collection, data analysis and writing the manuscript. HE, MZ, AH, AA, AEH: provided medical care to all patients. FM: pathological analysis of renal biopsy specimens. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the local Ethics Committee of Mansoura Faculty of Medicine-Institutional Research Board (IRB) (R.23.06.2233).
All patients/guardians provided informed consent at the time of hospital admission for possible use of their data in future research.
Consent for publication
“Not applicable”.
Competing interests
The authors have no financial or nonfinancial conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El-Halaby, H., Eid, R., Elagamy, A. et al. A retrospective analysis of acute kidney injury in children with post-COVID-19 multisystem inflammatory syndrome: insights into promising outcomes. Ital J Pediatr 50, 23 (2024). https://doi.org/10.1186/s13052-024-01598-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-024-01598-w