Abstract
Background
Leukoencephalopathy with vanishing white matter (VWM) is an autosomal recessive neurological disease. The physiopathology of disease is still little understood, but it seems to involve impairment in maturation of astrocytes; as a consequence white matter is more prone to cellular stress. Disease is caused by mutations in five genes encoding subunits of the translation initiation factor eIF2B. We know five different types of VWM syndrome classified based different ages of onset (prenatal, infantile, childhood, juvenile and adult onset).
Case presentation
We report the case of a 4-month-old boy with early seizure onset, recurrent hypoglycemia and post mortem diagnosis of vanishing white matter disease (VMD). At the admission he presented suspected critical episodes, resolved after intravenous administration of benzodiazepines. The brain MRI showed total absence of myelination that suggested hypomyelination leukoencephalopathy. The whole exome sequencing (WES) revealed a variant of EIF2B2 gene (p. Val308Met) present in homozygosity. In this case report we also describe the clinical evolution of seizures, in fact the epileptic seizures had a polymorphic aspect, from several complex partial seizures secondarily generalized to status epilepticus.
Conclusion
Infantile and early childhood onset forms are associated with chronic progressive neurological signs, with episodes of rapid neurological worsening, and poor prognosis, with death in few months or years. Clinical presentation of epilepsy is poorly documented and do not include detailed information about the type, time of onset and severity of seizures. No therapeutic strategies for VWM disease have been reported.
Similar content being viewed by others
Background
Vanishing white matter disease (VWMD), also known as childhood ataxia with central hypomyelination (CACH disease), is an autosomal recessive leukoencephalopathy. It is caused by mutations in the genes EIF2B1–5 that are involved in initiation and regulation of protein synthesis. VWMD is characterized by a slow progressive deterioration of white matter often exacerbated by infectious episodes or minor head injuries. The typical neuroradiological pattern is characterized by hypo intensity of the white matter in T1 and hyperintensity in T2. Despite being probably the most frequent form of genetic leukoencephalopathy of childhood, onset in the first year of life is very uncommon and associated with rapid decline and poor prognosis in all cases. Here we report a clinical case of a 4 month old boy with early seizure onset and post mortem diagnosis of VWMD.
Case presentation
A 4-month-old boy born from third-degree consanguineous parents was referred to Pediatric Casualty Ward of Modena’s Hospital for suspected critical episodes, resolved after intravenous administration of benzodiazepines. The episodes were characterized by clones of the right side of the body, chewing movements and eyes blinking; they first appeared a month after a vaccination followed by fever and occurred many times during the day (up to 10), with short duration and self-resolution. A decrement of feeding was also reported as well as loss of previously acquired neuromotor skills with worsening head control and poor reactivity.
The baby was born at 36 weeks of gestation with regular birth history except for transient hypoglycemia; Apgar score 10–10; anthropometric parameters were in the normal range (weight 2950 g, 61° centile; length 49 cm, 67° centile, head circumference 33 cm, 36° centile). The family history was normal.
The neurological examination showed marked axial hypotonus, poor eye contact, social interaction and weak sucking. Vital signs, routine blood exams and electrocardiogram were normal. The body percentiles at the evaluation showed a weight (7160 g) below the third percentile with length (70 cm) and head circumference (34 cm) between 10°-25° percentile.
The first electroencephalogram (EEG) video registration (Fig. 1A) showed recurrent slow waves localized in right temporal hemispheres and asynchronous in the left temporo-occipital area, sometimes followed by suppression of background activity. During this registration, we tried administration of intravenous pyridoxine (100 mg) without electroclinical improvement. After the beginning of therapy with levetiracetam (57 mg/kg) we observed incomplete response with persistence of 1–2 daily seizures.
In the Fig. 1 is reported the evolution of the EEG anomalies. At four months of life (A) the electroencephalogram reveals recurrent slow waves localized in right temporal hemispheres and asynchronous in the left temporo-occipital area. The EEG at eight months of life (B) shows worsening of the background activity by sub continuous presence of focal anomalies, independent over the two hemispheres with recurrent electroclinical episodes characterized by theta-delta activity on the central temporal regions of the right hemisphere and, asynchronously, on the frontal and central regions of the left hemisphere. The EEG at ten months of life (C) reveal worsening of underlying activity with electrical disorder and sub-continuous irritant activity especially in in the right temporal zone
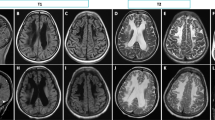
Brain Magnetic Resonance (MRI) was performed and showed thinned corpus callosum, white matter hyperintensity in T2 and hypo intensity in T1, total absence of myelination of the posterior arm of the internal capsule bilaterally (Fig. 2 A and B). The diagnostic orientation was a hypomyelination leukoencephalopathy likely of genetic or metabolic origin.
A and B showed T2 sequences -MRI at three months of life; C and D were the T2 sequences- MRI performed at ten months of life: in A and B white matter appears extensively hyperintense with absence of regular myelination. At ten months of life (C and D) T2-MRI shows increased hyperintensity of both cortical and deep white matter with significant dilatation of the ventricular system
Complete blood count, liver and renal function test, serum ammonia, and serum lactate were all normal. No alterations of plasma amino acids and urinary organic acids were found and the search for mutated sialotrasferrins was not indicative; metabolic investigations were also negative for oligosaccharidosis, mucopolysaccharidosis, neurotransmitter deficiency, B6 and pyridoxal-phosphate deficiency, primary hyperlactatemia, brain glucose transport deficiency and peroxisomal deficiency. Cardiac and abdominal ultrasound didn’t highlight abnormalities. CGH array did not document anomalies such as duplications or deletions.
During the following months the infant was hospitalized several times for the worsening of seizures, often triggered by infectious episodes. Several trials with phenytoin, phenobarbital e diazepam have been made, but the association of levetiracetam, clonazepam and ketogenic diet gave better results. ACTH has been administrated with only partial response.
In the following months the patient needed numerous hospitalizations for worsening of the epileptic seizures, treated with continuous infusion and boluses of intravenous midazolam. In this period in the epileptic seizures had a polymorphic aspect, we observed several complex partial seizures secondarily generalized with status epilepticus and need for intensive support. In the intercritical period we observed numerous episodes, characterized by clones of limbs, deviation of the eyes, drooling and masticatory movements lasting a few seconds with a frequency between five and ten episodes in an hour.
The clinical history was also characterized by frequent episodes of hypoglycemia during a ketogenic diet despite ketonemia values within the therapeutic limits (ketonemia between 2 and 5 mmol/L). The endocrine and metabolic tests performed were not indicative, immunodeficiency was excluded. The EEG evolution showed, at eight months of age, a marked worsening of the background activity with sub continuous presence of focal abnormalities like spike and slow high voltage waves, independent over the two hemispheres; we could observe recurrent electroclinical episodes characterized by theta-delta activity on the central temporal regions of the right hemisphere and, asynchronously, on the frontal and central regions of the left hemisphere (Fig. 1 B); in correspondence of these episodes the patient showed repetitive head movements, deviation of eyes towards left side, eyelids clones, nystagmus, uncoordinated diaphragm movements and right wrist writing movements with migrating clones from right to left side of the body in absence of hyper tone and clones or myoclonic events.
At ten month of life, the EEG with video-registration showed further worsening of underlying activity with electrical disorder and sub-continuous irritant activity of slow high voltage waves especially in in the right temporal zone (Fig. 1 C).
The patient was hospitalized one last time at one year of life for sub-continuous epileptic episodes associated with bradycardia and desaturation that led to degeneration of general clinical conditions and then to death.
During the observation we repeated MRI that showed a marked reduction of white matter not only in the cerebral hemispheres but also in midbrain with almost complete involvement of the bulb. It was associated to important dilatation of the supra and sub tentorial ventricular system (Fig. 3 C and D). The diagnostic suspect, based on clinic course and imaging, was VWMD, confirmed post-mortem by clinic exome. The analysis revealed a variant in homozygosity (p.Val308Met) of EIF2B2 gene. This mutation is pathognomonic of VWMD and the parents were carriers in heterozygosity. Furthermore, post mortem results were obtained from the investigations on the respiratory chains which documented the reduction of the activity of complexes I and IV on muscle. Exome analysis revealed no mutations in nuclear genes associated with mitochondrial diseases.
Discussion and conclusion
VWMD or childhood ataxia with central hypomyelination (CACH) syndrome is an autosomal recessive leukoencephalopathy. VWM is caused by mutations in the genes EIF2B1-5, encoding the subunits of eIF2B, a protein necessary for translation initiation and regulation of protein synthesis under different conditions, including cellular stress [1]. The prevalence of known living affected individuals is ~ 1.4:1,000,000 inhabitants, while the prevalence of individuals carrying two mutant alleles of a gene encoding a specific eIF2B subunit has been estimated to be 1:80,000–100,000 live births [1]. It is a chronic progressive disease with episodes of rapid and major neurological worsening caused by stress (fever, minor head trauma, acute fright) with incomplete recovery [2, 3].
The physiopathology of the disease is still little understood, but it seems to involve impairment in maturation of astrocytes; as a consequence the white matter is more prone to cellular stress. No effective therapy is known. Corticosteroids have proven to be sometimes useful in acute stages [4].
We know five different types of VWM syndrome classified based different age of onset (prenatal, infantile, childhood, juvenile and adult onset). The earlier is the age of onset, the higher is the risk of rapid evolution and death [5]. The childhood VWMD occurs primarily between age 2 to 6 years [6], while later/adult onset VWMD with either clinical manifestations or MRI abnormality alone is also recognized and usually implies a milder disease course [7]. The infantile VWMD variant is typically characterized by rapid neurological deterioration, ataxia and spasticity.
In a follow up study that involved 14 infantile and 26 childhood patients Zhou et al. [8] compared the natural history and the typical distribution of the damage in Childhood and Infantile VWMD. Compared with early childhood onset VWMD, infantile VWMD showed more extensive involvement of the subcortical WM in the frontal lobe, anterior limb of the internal capsule, midbrain, pons and dentate nuclei of the cerebellum. As a matter of fact, the second RMI performed to our patient showed marked worsening of white matter impairment extended to cerebral hemispheres, pons, midbrain and bulb.
MRI is important for differential diagnosis; indeed in patients presenting with rapid neurological deterioration it is important to exclude demyelinating encephalomyelitis and encephalitis. In patients with subacute or chronic neurological deterioration and an MRI showing diffuse cerebral white matter abnormalities, mitochondrial leukoencephalopathies are important disorders in the differential diagnosis. In mitochondrial defects, MRI typically shows well delineated cysts, the white-matter abnormalities tend to be less diffuse then in the VWM [9].
White matter abnormalities similar to VMD are described in metabolic disease such as L-2-hydroxyglutaric aciduria, non-ketotic hyperglycinemia, classical phenylketonuria, mucopolysaccharidoses, GM gangliosidoses, Zellweger disease, adrenomyeloneuropathy and Van der Knaap disease [10].
Infantile and childhood patients shared similarities in the incidence of epileptic seizure (35.7 vs. 38.5%) and episodic aggravation (92.9 vs. 84.6%). Developmental delay before disease onset was more common in infantile patients. The distribution of mutations among EIF2B1—5 was not significantly different between infantile and early childhood patients. [8]
Although correlation between genotype and phenotype is not described in literature, it has been observed that mutations in not conserved amino acids are associated with later onset, slow progression and longer survival, giving the impression that a specific genetic mutation might predict a specific clinical phenotype [11]. This observation is consistent with our experience, because the residual amino acid was highly conserved and we observed early onset and rapid worsening.
This report shows a rare case of VWMD syndrome with onset at four months of age. As mentioned before, case reports of infantile forms of VWMD are very infrequent. The diagnosis was confirmed by post-mortem diagnostics through clinical exome analysis documenting mutation in homozygosis of the EIF2B2 gene (p.Val308Met). The analysis in parents revealed the presence of this mutation in heterozygosis. This variant is very rare in the general population (0.000008122) and described by Zhang et al. [12] in 2015 in heterozygosis with another mutation, while Rezaei et al. [13] described the same mutation in homozygosis.
In a recent proteomics study focusing on remyelination in adult mice brains in response to cuprizone-induced demyelination, Gat Viks et al. found that dysregulation of mitochondrial functions, altered proteasomal activity and impaired balance between protein synthesis and degradation play a role in VWM pathology [14]. Since post-mortem brains of several VWMD patients contain oligodendrocytes with “foamy” cytoplasm due to increased number of abnormal mitochondrial membranes [15], we aimed to find a biochemical link between eIF2B mutations and mitochondrial function. A study by Raini et al. in 2016 [16] on mouse embryonic fibroblasts (MEFs) showed decreased activity of complex I and complex IV by 30% and 40%, respectively, in mutated MEFs compared to wild type. A similar result was found in our patient’s muscular biopsy.
Recent studies have shown a rare association between VWMD and hyperinsulinemia hypoglycemia. Bursle et al. reported three cases of infantile onset VWMD with hypoglycemia [17]. In these patients hypoglycemia became apparent at 6 and 8 months of life, although in one patient transient was also documented neonatal hypoglycemia.
The pathophysiology of hyperinsulinism in VWMD is not clear; it may involve dysregulation of transcription of genes related to insulin secretion. We have described a rare case of infantile VWMD with rapid evolution and association with several episodes of hypoglycemia. In literature are reported few cases of this association, unfortunately these patients have invariably a bad prognosis.
In Table 1 we illustrated all cases of infantile VWMD reported in literature and we observed that the most frequent forms of early onset VWMD are linked to mutation of EIF2B5. Clinical phenotype is variable and the most common manifestations are worsening and progressive psychomotor deterioration, hypotonia, epilepsy and feeding difficulties. Epilepsy is common in these patients but forms of refractory epilepsy are not frequently reported in early onset VWMD. The case described by Rezaei et al. is also characterized by early-onset seizures refractory to antiepileptic drugs. In this case a good control was obtained with the association of phenobarbital, topiramate, lamotrigine, and oxcarbazepine [13].
Although refractory generalized tonic- myoclonic seizures were previously described [23, 24, 27], clinical presentation of epilepsy is poorly documented and do not include detailed information about the type, time of onset and severity of seizures. Most of patients reported in Table 1 present conditions of stress as a trigger of rapid and major neurological worsening [18, 20, 21, 23, 25].
In conclusion we described a case of early onset VWMD rapid evolution and association with refractory epilepsy and several episodes of hypoglycemia. This report shows also the clinical evolution of seizures, the onset and role of stress condition (fever, head trauma, viral infection) in neurological worsening. The treatment is based on antiepileptic drugs and support care. Clinicians play a key role in the management of these patients, because no therapeutic strategies for VWMD disease are reported.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- VWMD:
-
Vanishing white matter disease
- eIF2B:
-
Eukaryotic initiation factor 2B
- WES:
-
Whole exome sequencing
- EEG:
-
Electroencephalogram
- MRI:
-
Magnetic Resonance imaging
- CACH:
-
Childhood ataxia with central hypomyelination
- VWM:
-
Vanishing white matter
- WM:
-
White matter
- MEFs:
-
Mouse embryonic fibroblasts
References
Fogli A, Schiffmann R, Bertini E, Ughetto S, Combes P, Eymard-Pierre E, et al. The effect of genotype on the natural history of eIF2B-related leukodystrophies. Neurology. 2004;62(9):1509–17.
Van der Knaap MS, Barth PG, Gabreëls FJ, Franzoni E, Begeer JH, Stroink H, et al. A new leukoencephalopathy with vanishing white matter. Neurology. 1997;48(4):845–55.
Vermeulen G, Seidl R, Mercimek-Mahmutoglu S, Rotteveel JJ, Scheper GC, van der Knaap MS. Fright is a provoking factor in vanishing white matter disease. Ann Neurol. 2005;57(4):560–3.
Labauge P, Fogli A, Niel F, Rodriguez D, Boespflug-Tanguy O. CACH/VWM syndrome and leucodystrophies related to EIF2B mutations. Rev Neurol (Paris). 2007;163(8–9):793–9.
Yavuz H. A review of infantile vanishing white matter disease and a new mutation. Acta Neurol Taiwan. 2017;26(4):167–76.
Schiffmann R, Moller JR, Trapp BD, Shih HH, Farrer RG, Katz DA, et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol. 1994;35(3):331–40.
van der Knaap MS, Kamphorst W, Barth PG, Kraaijeveld CL, Gut E, Valk J. Phenotypic variation in leukoencephalopathy with vanishing white matter. Neurology. 1998;51(2):540–54.
Zhou L, Zhang H, Chen N, Zhang Z, Liu M, Dai L, et al. Similarities and differences between infantile and early childhood onset vanishing white matter disease. J Neurol. 2018;265(6):1410–8.
van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol. 2006;5(5):413–23.
Patay Z. Diffusion-weighted MR imaging in leukodystrophies. Eur Radiol. 2005;15(11):2284–303.
van der Lei HD, van Berkel CG, van Wieringen WN, Brenner C, Feigenbaum A, Mercimek-Mahmutoglu S, et al. Genotype-phenotype correlation in vanishing white matter disease. Neurology. 2010;75(17):1555–9.
Zhang H, Dai L, Chen N, Zang L, Leng X, Du L, et al. Fifteen novel EIF2B1-5 mutations identified in Chinese children with leukoencephalopathy with vanishing white matter and a long term follow-up. PLoS One. 2015;10(3):e0118001.
Rezaei N, Nikbakht S, Ashrafi M, Rezaei Z, Mahdieh N, Alizadeh H, et al. Seizure as the early and main manifestation of infantile vanishing white matter disease: a case report. Iran J Pediatr. 2018;28(2):e65620.
Gat-Viks I, Geiger T, Barbi M, Raini G, Elroy-Stein O. Proteomics-level analysis of myelin formation and regeneration in a mouse model for Vanishing White Matter disease. J Neurochem. 2015;134:513–26.
Wollmann R, Gilbert E, Le TQ, Bradley CA, Crutchfield K, Schiffmann R. Foamy cells with oligodendroglial phenotype in childhood ataxia with diffuse central nervous system hypomyelination syndrome. Acta Neuropathol. 2000;100(6):635–46.
Raini G, Sharet R, Herrero M, Atzmon A, Shenoy A, Geiger T, et al. Mutant eIF2B leads to impaired mitochondrial oxidative phosphorylation in vanishing white matter disease. J Neurochem. 2017;141(5):694–707.
Bursle C, Yiu EM, Yeung A, Freeman JL, Stutterd C, Leventer RJ, et al. Hyperinsulinaemic hypoglycaemia: a rare association of vanishing white matter disease. JIMD Rep. 2019;51(1):11–6.
Francalanci P, Eymard-Pierre E, Dionisi-Vici C, Boldrini R, Piemonte F, Virgili R, et al. Fatal infantile leukodystrophy: a severe variant of CACH/VWM syndrome, allelic to chromosome 3q27. Neurology. 2001;57(2):265–70.
Rosemberg S, Leite Cda C, Arita FN, Kliemann SE, Lacerda MT. Leukoencephalopathy with vanishing white matter: report of four cases from three unrelated Brazilian families. Brain Dev. 2002;24(4):250–6.
Sijens PE, Boon M, Meiners LC, Brouwer OF, Oudkerk M. 1H chemical shift imaging, MRI, and diffusion-weighted imaging in vanishing white matter disease. Eur Radiol. 2005;15(11):2377–9.
Sharma S, Ajij M, Singh V, Aneja S. Vanishing white matter disease with mutations in EIF2B5 Gene. Indian J Pediatr. 2014;82(1):93–5.
Hata Y, Kinoshita K, Miya K, Hirono K, Ichida F, Yoshida K, et al. An autopsy case of infantile-onset vanishing white matter disease related to an EIF2B2 mutation (V85E) in a hemizygous region. Int J Clin Exp Pathol. 2014;7(6):3355–62.
Takano K, Tsuyusaki Y, Sato M, Takagi M, Anzai R, Okuda M, et al. A Japanese girl with an early-infantile onset vanishing white matter disease resembling Cree leukoencephalopathy. Brain Dev. 2015;37(6):638.
Gungor O, Ozkaya AK, Hirfanoglu T, Dilber C, Aydin K. A rare mutation in EIF2B4 gene in an epileptic child with vanishing white matter disease: a case report. Genet Couns. 2015;26(1):41–6.
Esmer C, Blanco Hernández G, Saavedra Alanís V, Reyes Vaca JG, Bravo Oro A. Association between homozygous c.318A>GT mutation in exon 2 of the EIF2B5 gene and the infantile form of vanishing white matter leukoencephalopathy. Bol Med Hosp Infant Mex. 2017;74(5):364–9.
Song H, Haeri S, Vogel H, van der Knaap M, Van Haren K. Postmortem whole exome sequencing identifies novel EIF2B3 mutation with prenatal phenotype in 2 siblings. J Child Neurol. 2017;32(10):867–70.
Porciuncula R, Spada PKWDS, Goulart KOB. Leukoencephalopathy with evanescent white matter: a case report. Rev Paul Pediatr. 2018;36(4):515–8.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
GC, EC and LI contributed to the conception and design. IS, IF, ES, PB and AT contributed to the acquisition and interpretation of data. GC and LI revised and edited the manuscript. All authors drafted the article and approved its final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Parental written informed consent was obtained. Ethical Committee is not a applicable for case report.
Consent for publication
Written consent for the publication of data and images was obtained from the parents of the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Filareto, I., Cinelli, G., Scalabrini, I. et al. EIF2B2 gene mutation causing early onset vanishing white matter disease: a case report. Ital J Pediatr 48, 128 (2022). https://doi.org/10.1186/s13052-022-01325-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-022-01325-3