Abstract
Background
Familial Mediterranean Fever is an autoinflammatory disease typically expressed with recurrent attacks of fever, serositis, aphthous stomatitis, rash. Only a few reports describe the association with hepatic involvement.
Case presentation
We describe the clinical case of a child affected, since the age of 1 year, by recurrent fever, aphthous stomatitis, rash, arthralgia, associated with abdominal pain, vomiting, lymphadenopathy. The diagnosis of Familial Mediterranean Fever was confirmed by the genetic study of MEFV gene; the homozygous mutation M694 V in exon was documented. A partial control of attacks was obtained with colchicine. The child continued to manifest only recurrent episodes of abdominal pain without fever, however serum amyloid A persisted high, in association with enhanced levels of CRP, AST and ALT (1.5 x n.v.). The dosage of colchicine was increased step by step and the patient achieved a better control of symptoms and biochemical parameters. However, the patient frequently needed an increase in the dose of colchicine, suggesting the possible usefulness of anti-interleukin-1 beta treatment.
Conclusions
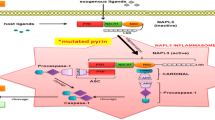
The unusual presentation of Familial Mediterranean Fever with liver disease suggests the role of inflammasome in hepatic inflammation. Colchicine controls systemic inflammation in most of the patients; however, subclinical inflammation can persist in some of them and can manifest with increased levels of CRP, ESR, serum amyloid A also in attack-free intervals.
Similar content being viewed by others
Background
Familial Mediterranean Fever (FMF) is an autoinflammatory disease typically expressed with recurrent attacks of fever, serositis leading to abdominal, thoracic or articular pain, aphthous stomatitis, erysipelas-like erythema [1]. Only a few reports in the international literature describe the association with hepatic involvement, documented by ultrasound and increased levels of AST, ALT, gamma-GT, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR). In these cases, an accurate differential diagnosis is needed to exclude other immune-mediated or infectious diseases mimicking a liver disease [2, 3].
Case presentation
We report on a 10.6-year-old child affected, since the age of 1 year, by recurrent fever, aphthous stomatitis, rash, arthralgia, associated with abdominal pain, vomiting, lymphadenopathy. Recurrence was every 15–30 days. Serum amyloid A (SAA) levels were 33 mg/l, CRP was 24.8 mg/dl, ESR was 86, AST and ALT were 1.5 x n.v.. Though, he showed a height gain corresponding to age (stature: 132.2 cm: - 1.55 SDS; weight: 27 kg: - 1.72 SDS), he did not showed obesity with a Body Mass Index of 15.5 (− 1.3 SDS).
The diagnosis of FMF was considered for the clinical presentation of the child [1] and confirmed at the age of 6 years by the genetic study of MEFV gene. The patient showed a homozygous mutation of exon 10: M694 V. Therefore, he started the treatment with colchicine at the dosage of 0.03 mg/kg/day. The grandfather died for renal insufficiency. The parents did not refer recurrent fever and/or other symptoms associated with FMF, and they did not agree to be investigated genetically for FMF.
A partial control of attacks was gained by colchicine (however, recurrent episodes of abdominal pain, without fever persisted) and increased levels of SAA persisted. For these reasons, the dosage of colchicine was gradually increased to 0.05 mg/kg/day, following the regular weight gain during growth, with a good control of biochemical parameters. SAA showed high levels (33–223.8 mg/l: n.v.: < 6.4) in some phases of the follow up, in correlation with the dosage of colchicine. However, the normalization was achieved after the progressive increase of the dose.
Abdominal ultrasound documented lymphadenopathy and an echography pattern of “starry sky liver” (Fig. 1) consensual with the phases of the disease characterized by a significant increase of SAA and a mild increase of AST, ALT (1.5 x n.v.). A better control of the disease with increased doses of colchicine, however, allowed to normalize SAA, AST, ALT levels and liver ultrasound pattern. These data confirm that liver involvement was not secondary to colchicine toxicity. Furthermore, the patient maintained a low BMI, excluding obesity as a cause of hepatopathy. He did not assume other drugs further than colchicine that could contribute to the increase of transaminases or induce hepatopathy.
Currently, SAA is 1.77 mg/l; CRP: 0.18; AST: 24; ALT: 28; urinalysis is in the normal range without microalbuminuria (Table 1).
Discussion and conclusions
We described this case for the unusual presentation of FMF with fever, rash, aphthous stomatitis, liver disease, not associated to other conditions, as infections, drugs intolerance [2], autoimmune disease, celiac disease [4]. The described case underscores the chance that MEFV mutations provide hepatic inflammation, as seen in this child, by the inflammasome involvement.
The reported case of a male child with FMF, presenting recurrent episodes of hepatitis (spontaneously resolved) during fever attacks or disease phases with a low control by colchicine, highlight the role of an appropriate colchicine dosage in the control of inflammation. In this view, the inflammatory role of the disease in the liver involvement is confirmed by the consensual increase of SAA, AST, ALT. Therapeutic trial with increased doses of colchicine was successful, confuting the hypothesis of the possible role of colchicine toxicity in this case.
Cryptogenic liver disease was described in patients with FMF only in a few studies [5, 6]. However, the cases reported in the international literature showed more frequently the M694 V mutation, especially in homozygous state.
Some studies reported the safety profile of IL-1 blockers from real life [7]. We discussed with the parents and the patient the opportunity to shift treatment to canakinumab, a fully human anti- interleukin (IL)-1 beta monoclonal antibody, to better control the inflammatory state of the patient and the symptoms.
Canakinumab has not been started yet, because at the last visit SAA levels were in the normal range and the patient was asymptomatic. However, the opportunity to shift the treatment to canakinumab for this patient could be the optimal choice to minimize the inflammatory state and to control the risk of amyloidosis, improving long-term prognosis and quality of life.
Availability of data and materials
Materials and data of the patient are included in the medical records of the patient.
Abbreviations
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- FMF:
-
Familial Mediterranean Fever
- IL-1:
-
Interleukin-1
- SAA:
-
Serum amyloid A
References
Papa R, Doglio M, Lachmann HJ, Ozen S, Frenkel J, Simon A, Neven B, Kuemmerle-Deschner J, Ozgodan H, Caorsi R, Federici S, Finetti M, Trachana M, Brunner J, Bezrodnik L, Pinedo Gago MC, Maggio MC, Tsitsami E, Al Suwairi W, Espada G, Shcherbina A, Aksu G, Ruperto N, Martini A, Ceccherini I, Gattorno M. Paediatric Rheumatology International Trials Organisation (PRINTO) and the Eurofever Project. A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry. Orphanet J Rare Dis. 2017;12(1):167. https://doi.org/10.1186/s13023-017-0720-3.
Maggio MC, Liotta A, Cardella F, Corsello G. Stevens-Johnson syndrome and cholestatic hepatitis induced by acute Epstein-Barr virus infection. Eur J Gastroenterol Hepatol. 2011 Mar;23(3):289. https://doi.org/10.1097/MEG.0b013e32832b8e10.
Tweezer-Zaks N, Doron-Libner A, Weiss P, Ben-Horin S, Barshack I, Lidar M, Livneh A. Familial Mediterranean fever and cryptogenic cirrhosis. Medicine (Baltimore). 2007;86(6):355–62.
Migita K, Abiru S, Tanaka M, Ito M, Miyashita T, Maeda Y, Koga T, Nakamura M, Komori A, Yatsuhashi H, Ida H, Eguchi K, Hirayama K, Yasunami M, Ishibashi H. Acute hepatitis in a patient with familial Mediterranean fever. Liver Int. 2008;28(1):140–2 PeHUB 2007 Oct 25.
Tzifi F, Hawkins P, Atsali E, Kotzia D, Attilakos A. Acute hepatitis in a child heterozygous for the I259V MEFV gene variant. Prague Med Rep. 2014;115(3–4):128–33.
Unal F, Cakir M, Baran M, Arıkan C, Yuksekkaya HA, Aydoğdu S. Liver involvement in children with familial Mediterranean fever. Dig Liver Dis. 2012 Aug;44(8):689–93. https://doi.org/10.1016/j.dld.2012.01.003 Epub 2012 Feb 12.
Sota J, Vitale A, Insalaco A, Sfriso P, Lopalco G, Emmi G, Cattalini M, Manna R, Cimaz R, Priori R, Talarico R, de Marchi G, Frassi M, Gallizzi R, Soriano A, Alessio M, Cammelli D, Maggio MC, Gentileschi S, Marcolongo R, La Torre F, Fabiani C, Colafrancesco S, Ricci F, Galozzi P, Viapiana O, Verrecchia E, Pardeo M, Cerrito L, Cavallaro E, Olivieri AN, Paolazzi G, Vitiello G, Maier A, Silvestri E, Stagnaro C, Valesini G, Mosca M, de Vita S, Tincani A, Lapadula G, Frediani B, De Benedetti F, Iannone F, Punzi L, Salvarani C, Galeazzi M, Angotti R, Messina M, Tosi GM, Rigante D, Cantarini L. “Working group” of systemic autoinflammatory diseases of SIR (Italian Society of Rheumatology). Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clin Rheumatol. 2018;37(8):2233–40. https://doi.org/10.1007/s10067-018-4119-x Epub 2018 May 17.
Acknowledgements
Not applicable.
Funding
No funding is declared.
Author information
Authors and Affiliations
Contributions
MCM prepared the case presentation, followed the patient and wrote the paper; MC performed the abdominal ultrasound study of the patient; GC revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Consent for publication was obtained from the parents of the child. The consent is included in the medical records of the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Maggio, M.C., Castiglia, M. & Corsello, G. Familial Mediterranean Fever: an unusual cause of liver disease. Ital J Pediatr 45, 121 (2019). https://doi.org/10.1186/s13052-019-0712-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-019-0712-0