Abstract
The prevalence of pediatric food allergy and anaphylaxis has increased in the last decades, especially in westernized countries where this emerging phenomenon was marked as a “second wave” of the allergic epidemic. Over recent years great advances have been achieved in the field of in vitro allergy testing and component-resolved diagnosis has increasingly entered clinical practice. Testing for allergen components can contribute to a more precise diagnosis by discriminating primary from cross-reactive sensitizations and assessing the risk of severe allergic reactions.
The basic concept of the management of food allergy in children is also changing. Avoidance of the offending food is still the mainstay for disease management, especially in primary health care settings, but it severely affects the patients’ quality of life without reducing the risk of accidental allergic reactions. There is a growing body of evidence to show that specific oral tolerance induction can represent a promising treatment option for food allergic patients. In parallel, education of food allergic patients and their caregivers as well as physicians about anaphylaxis and its treatment is becoming recognized a fundamental need. International guidelines have recently integrated these new evidences and their broad application all over Europe represents the new challenge for food allergy specialists.
Similar content being viewed by others
Introduction
The prevalence of pediatric food allergy (FA) and anaphylaxis has increased in the last decades, with westernized countries experiencing the highest rates. This emerging phenomenon marked as a “second wave” of the allergic epidemic has raised concern among the scientific community, which is currently investigating on potential risk factors [1-5]. Pediatric allergists are also experiencing remarkably changes in the pattern of allergic sensitization and disease manifestations, with a wider range of allergenic foods and increase in non-IgE-mediated gastrointestinal disorders [6].
The cornerstone in the diagnostic workup of FA is the oral food challenge (OFC) which is time and cost-consuming and involves the risk of adverse allergic reactions. To this purpose, there is growing interest concerning in vitro allergy diagnostic tests to reduce the need for OFC [7].
FA treatment has also emerged as a developing research field over the past decades. Indeed, the current management of FA comprises a strict avoidance of triggering foods, which has significant negative impact on nutritional, psychological and economic status and poses the risk of developing allergic reactions after accidental exposure [8].
This review examines the existing relevant literature focusing on new diagnostic and therapeutic strategies for FA in children.
Search strategy
References were identified by searches of MEDLINE, PubMed and online Cochrane databases. The terms searched were food allergy and all of the following, separately and in combination: diagnosis/treatment/ management/avoidance/anaphylaxis/component-resolved diagnosis/molecular allergy/oral tolerance/oral immunotherapy/oral tolerance induction. We only searched for English-language original studies, reviews and commentaries conducted on children aged 0-18 years. Manuscripts published until November 2014 were included.
Diagnostic procedures are improving through molecular approaches
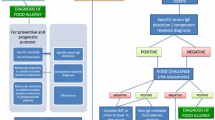
According to the current guidelines the basic approach to diagnosis of FA firstly includes a detailed clinical history and physical examination, which should drive the choice of the most appropriate allergy diagnostic tests [8,9]. Skin prick test (SPT) and serum-specific Immunoglobulin E (sIgE) for food allergens are the first-line tests to assess an IgE-sensitization, but their low positive predictive value makes the elimination diet and OFC necessary to confirm the diagnosis [10]. OFC, particularly the double-blind placebo-controlled food challenge, is still considered the reference standard test for diagnosis of IgE-mediated and non-IgE-mediated FA [11]. Performing OFC can better define the real prevalence of FA and improve patients’ quality of life by preventing unnecessary elimination diet [8]. However, OFCs are costly and time-consuming, require a specialized setting and team, and involve the risk of developing severe allergic reactions, which can be stressful for both patients and families [12,13].
Several studies have proposed threshold-levels of SPT and sIgE to predict a positive outcome in OFC [14,15]. Such cut-off values are influenced by the characteristics of both population examined and methodologies used and may not be generalizable to other populations, as recently reviewed by Peters et al. for SPT to egg and peanut [16]. A recent Dutch study showed that a positive outcome in OFC can be predicted by using a multivariate model risk score, which considers the provocative food, the time between allergen ingestion and development of symptoms and sIgE level [17]. Over recent years great advances have been achieved in the search for safe and reliable in vitro tests to reduce the need for OFC. To this purpose, the measurement of sIgE to allergenic molecular components from allergenic sources, namely component-resolved diagnosis (CRD), has increasingly entered clinical practice [18]. CRD can contribute to identify triggering allergens by discriminating primary from cross-reactive sensitization in poly-sensitized patients. Moreover CRD can enable the risk assessment of severe allergic reactions leading to an improved patient management [18].
-
Peanut and tree nut allergy - The major peanut allergen components Ara h 1, Ara h 2 and Ara h 3 are storage protein [19]. Ara h 8 is a member of the pathogenesis-related protein family (PR-10), which is relevant to patients with birch pollen allergy [20]. Ara h 9 is a nonspecific lipid transfer protein (nsLTP) and has recently been reported to be an important allergen in the Mediterranean area [21]. Ara h 2 has been highlighted as marker of primary sensitization, persistent allergy and severe reactions [22]. In a study of 181 French children with suspected peanut allergy, Ara h 6 and Ara h 2 were the best predictors of peanut allergy [23]. In a multicentre prospective study of 210 German children with suspected peanut or hazelnut allergy, sIgE to Ara h 2 and Cor a 14 resulted more reliable in predicting outcomes in OFC than sIgE to peanut or hazelnut extracts [24]. Similarly, in a recent Dutch study including 161 hazelnut-sensitized adult and children, sIgE to Cor a 9 and Cor a 14 were strongly associated with OFC-proven hazelnut allergy [25]. Among 123 Spanish children with suspected peanut allergy, the frequencies of sensitization to Ara h 1, Ara h 2, and Ara h 3 were 60.0%, 72.7% and 43.6% respectively, with significantly higher sIgE levels in the allergic group [26]. Among 40 Thai children with peanut sensitization, sIgE to Ara h 2 was a marker of anaphylactic reactions, whereas sIgE to Ara h 9 was unrelated to severe reactions [27]. Among 57 Japanese peanut-sensitized children, sIgE to Ara h 2 (cut-off 0.35 kU/l) could discriminate allergic from tolerant children with a sensitivity of 88% and a specificity of 84% [28].
-
Cow’s milk allergy - Several studies have tried to determine the serum level cut-off of sIgE to cow’s milk useful to identify sensitized children who are at risk of developing allergic reactions to cow’s milk [14]. Among 123 Brazilian children with cow’s milk allergy (CMA), testing sIgE to whole cow’s milk extract (cut-off 3.06 kU/l) was more useful to diagnose CMA than testing sIgE to α-lactalbumin, β-lactoglobulin or casein [29]. Finally, SPT with milk protein may be more reliable than sIgE level in predicting outcomes in OFC with baked milk products [30].
-
Egg allergy - SPT and sIgE to egg white have been shown to be a poor predictor of clinical phenotypes of egg allergy (EA). Among 154 Spanish egg-sensitized infants with CMA and/or atopic dermatitis without previous egg consumption, an egg white SPT wheal reaction of 8 mm and/or sIgE >8.36 KU/l predicted a positive outcome in OFC of around 94% [31]. Most children with EA seem to tolerate baked egg, but tolerance cannot be predicted with conventional allergy testing. In 186 English children with suspected EA who underwent an OFC, SPT to egg extract was slightly better in predicting a positive outcome than SPT to raw egg [32]. Egg white and yolk contain more than 20 different glycoproteins and measurement of sIgE to egg white subcomponents is considered as a new method for diagnosis [33]. Recently, it has been shown that sIgE to egg component Gal d 1 (i.e. ovomucoid) was more accurate in predicting raw EA compared with sIgE to egg white [34-36]. Moreover Gal d 1 negative children showed high frequency of tolerance to boiled egg [37]. In a prospective study of 143 children with EA, SPT to muffin < 2 mm had a high negative predictive value for baked egg OFC, whereas ovomucoid SPT 11 mm was very likely to predict a reaction to baked egg [38]. In 85 Spanish children sIgG4 to ovoalbumin resulted an independent predictor of tolerance development to uncooked egg [39].
-
Wheat allergy - sIgE to wheat has poor diagnostic predictability for wheat allergy. A prospective study reported that wheat sIgE levels required to identify subjects with >95% probability of reacting to a wheat challenge were 80 kUA/L [14]. The association of sensitization to ω-5-gliadin with wheat-dependent exercise-induced anaphylaxis (EIA) is one of the best documented forms of wheat allergy [40]. A recent study of 108 Finnish children with suspected wheat allergy, who underwent open or double-blinded, placebo-controlled oral wheat challenges identified the “dimeric alpha-amylase inhibitors 0.19” as a relevant allergen in clinically reactive participants [41]. Cases of wheat-dependent EIA with positivity to nsLTP in absence of ω-5-gliadin sensitization have been reported [42]; thus, patients with a history consistent with cofactor-enhanced food allergic anaphylaxis should be tested for sIgE to nsLTP (e.g. Tri a 14) and to ω-5-gliadin [43].
-
Fish and seafood allergy - The major codfish allergen component, Gad c 1 belongs to a group of Calcium-transporting muscle proteins known as parvalbumins. The variable degrees of clinical cross-reactivity in patients with fish allergy could be explained by the various degrees of amino acid homologies ranging from 60% to 80% shared by parvalbumins [44]. For shellfish allergy, tropomyosin is the major allergen responsible for cross-reactions among different species of the shellfish group. Molecular comparison of tropomyosin from different crustacean species revealed a very high homology of up to 98% [44]. It is well known that crustacean and mollusc allergens do not cross-react with fish allergens. On the other hand, patients with shellfish allergy are frequently reported to have allergic reactions to non-crustacean source, such as house dust mites and cockroaches. This cross-reactivity is probably due to the high amino acid homology of tropomyosins shared by these organisms [45]. Despite CRD revealed to be a useful tool to highlight in vitro-immunologic cross-reactivity in seafood allergy, its use in predicting clinical cross-reactivity is still to be improved.
-
Fruit and vegetable allergy - Pru p 3 is an nsLTP and considered a major peach allergen component. In 57 Spanish children suffering from allergic reactions after eating or having contact with peach, sIgE to Pru p 3 was detected in 96% of participants but OFC with peach pulp showed that more than 90% tolerated peeled peach [46]. Allergic reactions to fruits and vegetables can either result from primary sensitization to food or to inhalant allergens. In the latter case triggering foods share similar components with inhalant allergens causing cross-reactions. A study performed on 15 subjects from Belgium with birch pollen allergy and suspected soy allergy, showed that secondary soy allergy may be responsible for chronic allergic symptoms (e.g. chronic severe generalized itching, recurrent urticaria, chronic diarrhea and generalized atopic dermatitis), besides typical immediate manifestations (from oral allergic syndrome up to anaphylaxis). In patients with birch pollen allergy SPT with soy flour and sIgE to soy component Gly m 4 were proposed as valuable tools for the diagnosis of secondary soy allergy [47]. Usually, cross reactivity is attributable to heat-labile allergens (i.e., PR-10 and profilins) and it is associated with mild oral reactions. On the contrary heat and proteolysis-resistant allergens (such as storage proteins and nsLTP) that primary sensitize through the oral route are associated with local and systemic reactions [48].
In a cohort of 30 children with IgE-mediated lentil allergy, initial lentil sIgE level < 4.9 kU/l correlated with significantly higher likelihood (68.4% vs. 18.2%) of outgrowing the lentil allergy than initial sIgE level ≥ 4.9 kU/l (p = 0.008). This findings suggests that sIgE levels may be important for predicting clinical reactivity and persistence of lentil allergy [49]. Reports of allergy to lupin are increasing with the diffusion of the lupin flour consumption in bakery due to primary sensitization or cross-reactions with other legumes. In a group of 12 Italian children allergic to peanut, b-conglutin has been identified as the major lupin allergen causing in vitro and in vivo cross-reactivity with peanut components [50].
Despite the CRD shows promise in reducing the need for OFC, there are circumstances in which OFC remains the only reliable method to ascertain the diagnosis of FA, such as cases of suspected food-dependent EIA with discordant medical history and sIgE results [51], or cases of suspected food protein-induced enterocolitis syndrome (FPIES), a non-IgE mediated FA in which in vivo and in vitro allergy tests show poor diagnostic accuracy. An OFC may not be necessary to diagnose FPIES only in very indicative cases (i.e. two or more acute episodes triggered by the same food in a six month period, with prompt resolution after avoidance of the causative food) [9].
Food avoidance
Avoidance of the offending food and emergency treatment of adverse reactions are currently the mainstays for the management of IgE-mediated and non-IgE-mediated FA [8]. A dietary programme for FA should always include education on how to avoid specific allergens as well as comprehensive nutrition assessment on how to appropriately substitute foods in order to obtain adequate energy intake and nutrients for age [52]. Indeed, food avoidance poses the risk of malnutrition and nutrient deficiency in growing allergic children [53]. There is evidence that children with FA present a decreased weight-for-age and height-for-age compared to healthy subjects. Growth differences in children with FA are evident despite an appropriate caloric intake and correlate with the number of foods avoided and duration of the diet [54,55]. A recent US cross-sectional study confirmed a poor growth in children with FA (IgE- and non-IgE-mediated) supplemented with amino-acid formula, despite an adequate daily energy intake [56]. The mechanisms responsible for growth differences in children with FA are still unclear. Risk factors which may lead to growth deficit in such category of children seem to be the early onset of the disease, multiple food allergies, elimination of foods with high nutritional value (e.g. cow’s milk and egg) and subclinical intestinal inflammation with increased intestinal permeability [53]. In a recent prospective study conducted in 131 food allergic children on a restricted diet for cow’s milk and egg, about one-third of asymptomatic participants showed elevated intestinal permeability [57].
Another aspect that must be considered is that dietary elimination significantly affects the quality of life of allergic children and their families due to social restriction and risk of accidental reactions [58-60]. A Canadian study reported an annual incidence rate of 12.5% of accidental exposure to peanut in allergic children, with higher risk for adolescents and cases with recent diagnosis [61]. Questionnaires-based surveys on food allergic patients ‘quality of life addressed at different age groups, showed that food avoidance correlate with anxiety about an accidental adverse reaction [62,63]. Social occasions, trips and even only playing at friends’ houses are considered dangerous settings by parents of allergic children [64]. Shopping is considered a difficult activity because of the need to identify ingredients on food labels [52]. A restricted diet can even results in an economic burden for the family budget, especially in infants requiring amino-acid formula supplementation [65].
Finally, it must be considered that FA tends to resolve in most cases during the first years of life. Therefore the required period of strict elimination diet is not a priori established and periodical re-evaluations by the allergist are fundamental to assess the changing nutritional needs and eventually resolution of the disease.
Specific oral tolerance induction: a promising alternative treatment
Over the last two decades, alternative treatment strategies have been investigated for FA, mainly targeting foods that commonly trigger IgE-mediated FA in children (i.e. cow’s milk, egg and peanut) [66]. As FA develops as a result of failure or loss of oral tolerance to food allergens, one of the most promising therapeutic approach pursued is oral immunotherapy or specific oral tolerance induction (SOTI) [67]. SOTI consists in oral assumption of increasing doses of the relevant allergen performed in controlled setting; this build-up phase is followed by a daily regular assumption of the tolerated dose which typically occurs at home. The aim is to induce an immune modulation in order to achieve a permanent oral tolerance [68].
There is a growing body of literature to show that SOTI is effective in increasing the threshold of reactivity for the most common triggering foods. This process, also referred as “desensitization”, can confer protection against accidental allergic reactions and can contribute to improve nutritional status and quality of life [69-79]. In a recent controlled trial conducted by Dello Iacono et al., 20 children with severe hen’s EA were equally randomized to receive SOTI with raw hen’s egg emulsion or egg-free diet for 6 months. At the end of the study period 90% of the SOTI group achieved partial tolerance, whereas sensitivity to raw hen's egg remained unchanged in 90% of controls [70]. Longo et al. reported that SOTI was effective in desensitizing a significant percentage of children with very severe CMA and high levels of sIgE [71]. Staden et al. investigated the efficacy of SOTI in comparison with the elimination diet in children with CMA or hen’s EA. At the follow-up challenge 36% in the SOTI group showed permanent tolerance, 12% were tolerant with regular intake and 16% were partial tolerant, whereas only 35% developed tolerance in the control group [72].
An open point of discussion is whether SOTI induces persistent tolerance or transient desensitization which may need regular allergen intake to be maintained [80]. Recent evidence suggests that SOTI to milk, egg and peanut can result in sustained unresponsiveness to those foods after discontinuing the maintenance dose, but more research into the long-term outcomes of SOTI is needed [76-79]. The immunological mechanisms underlying SOTI are currently unknown and their understanding could address the issue regarding long-term response to this treatment [68]. Fuentes-Aparicio et al. reported that acquisition of tolerance after SOTI in hen’s egg-allergic children is accompanied by a decrease in effector-memory CD4+ T-cell population and an increase in a hypo-proliferative subset of CD4+ T-cell population. This subset could represent a marker of tolerance induction [81]. Additionally, Vila et al. showed that the development of clinical tolerance to egg through SOTI is associated with a decrease in the mean sIgE level and in the antigen-specific basophil responsiveness [82].
Despite SOTI has demonstrated efficacy for clinical desensitization, one of the major concern is its safety. Adverse reactions occurring during SOTI are usually mild to moderate, predominantly oropharyngeal and easy to manage, but systemic severe events have been reported [83]. Therefore SOTI has still to be considered an experimental treatment belonging to the contest of clinical trials and further research is needed to establish its long-term efficacy, safety and cost-effectiveness [67]. An emerging strategy and possible alternative to SOTI for the treatment of IgE-mediated CMA and EA is the use of extensively heated forms of these allergens, such as in baked products. Recent studies have shown that the majority of children with CMA and EA tolerated baked milk or egg during an OFC and safely incorporated these products into their diet [34,84]. Tolerance of baked milk or egg is considered a marker of transient IgE-mediated FA [85].
Anaphylaxis treatment
Prompt administration of intramuscular epinephrine is the first-line therapy for food-induced anaphylaxis [8]. Maintaining access to an adrenaline auto-injectors (AAI) as well as using an AAI are essential steps for an effective management of IgE-mediated FA [86]. First, it has to be decided who should be prescribed an AAI. However there are still no generally valid indication criteria for prescribing an AAI [87]. Johnson et al. showed the prescription practice of an AAI were very variable among pediatricians. The most influencing factors included peanut or tree nut allergy and parental anxiety [88]. So, as long as there are no improved anaphylaxis guidelines, the indication should be given individually considering the medical history and family’s needs [89]. The next step is defining when epinephrine should be used. The best outcome is provided when epinephrine is given as soon as early symptoms of anaphylaxis occur [87]. Hence, the focus should lie on early recognition of symptoms to promptly use the AAI. To this purpose Jacobs et al. conducted a survey to investigate the recognition and treatment of first initial food allergic reactions. They detected that only one-third of the initial reactions with symptoms likely to represent anaphylaxis were recognized and treated with epinephrine. Remarkably, only half of these patients treated with epinephrine were prescribed an AAI [90].
H1-antihistamines have long been used for mild, isolated, non-progressive cutaneous reactions to help relieving pruritus, hives, angioedema and conjunctivitis. However, H1- and H2-receptor antagonists cannot be used as substitute for epinephrine, but only as adjunctive medications during anaphylaxis [91]. In a recent EAACI systematic review on FA management, it has been reported a weak evidence (level of evidence III, grade C) to support the benefits of H1 antihistamines for children and adults with acute non-life-threatening symptoms of FA [8]. However no evidence for efficacy of antihistamines in the treatment of more severe symptoms was reported. Furthermore, the prophylactic administration of antihistamines can early mask symptoms of anaphylaxis and lead to delayed treatment of dangerous reactions with epinephrine. Inhaled beta-2 adrenergic agonists have been used for the relief of cough and wheezing in addition to epinephrine, but are not a substitute for epinephrine [91]. Oral or intravascular corticosteroids are often administered during an acute reaction to prevent protracted or biphasic episodes of anaphylaxis. Other interventions include supplemental oxygen and fluids which may be considered after the administration of epinephrine depending on the patient’s symptoms [92]. A recent Cochrane review was unable to identify relevant studies with evidence for the use of corticosteroids in anaphylaxis [93].
Conclusion
The management of FA in children is improving through the acquisition of new knowledge in the field of diagnosis and treatment. CRD and SOTI are being intensively investigated. Their implementation in routine clinical practice can offer new perspectives in the management of patients with FA. In parallel, education of physicians and food allergic patients about anaphylaxis and its treatment is becoming recognized as an unmet need. The recent integration of all this new knowledge in international guidelines was a great achievement. The broad implementation of these guidelines all over Europe is now the new challenge for specialists, patients and other stakeholders of FA.
Abbreviations
- AAI:
-
Adrenalin auto-injector
- CMA:
-
Cow’s milk allergy
- CRD:
-
Component resolved diagnosis
- EA:
-
Egg allergy
- EIA:
-
Exercise-induced anaphylaxis
- FA:
-
Food allergy
- FPIES:
-
Food protein-induced enterocolitis syndrome
- nsLTP:
-
Nonspecific lipid transfer protein
- OFC:
-
Oral food challenge
- PR-10:
-
Pathogenesis-related protein family
- sIgE:
-
Specific IgE
- SPT:
-
Skin Prick test
- SOTI:
-
Specific oral tolerance induction.
References
Osborne NJ1, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–76.
Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The Prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17.
Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States 1997-2011. NCHS Data Brief. 2013;121:1–8.
Chen J, Hu Y, Allen KJ, Ho MH, Li H. The prevalence of food allergy in infants in Chongqing, China. Pediatr Allergy Immunol. 2011;22:356–60.
Santos AF, Lack G. Food allergy and anaphylaxis in pediatrics: update 2010-2012. Pediatr Allergy Immunol. 2012;23:698–706.
Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow’s milk: a large-scale, prospective population-based study. J Allergy Clin Immunol. 2011;127:647–53.
Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307.
Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008–25.
Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:1–58. Suppl 6.
Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7.
Winberg A, Nordström L, Strinnholm Å, Nylander A, Jonsäll A, Rönmark E, et al. New validated recipes for double-blind placebo-controlled low-dose food challenges. Pediatr Allergy Immunol. 2013;24:282–7.
Calvani M, Berti I, Fiocchi A, Galli E, Giorgio V, Martelli A, et al. Oral food challenge: safety, adherence to guidelines and predictive value of skin prick testing. Pediatr Allergy Immunol. 2012;23:755–61.
Knibb RC1, Ibrahim NF, Stiefel G, Petley R, Cummings AJ, King RM, et al. The psychological impact of diagnostic food challenges to confirm the resolution of peanut or tree nut allergy. Clin Exp Allergy. 2012;42:451–9.
Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–6.
Peters RL, Allen KJ, Dharmage SC, Tang ML, Koplin JJ, Ponsonby AL, et al. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132:874–80.
Peters RL, Gurrin LC, Allen KJ. The predictive value of skin prick testing for challenge-proven food allergy: a systematic review. Pediatr Allergy Immunol. 2012;23:347–52.
Zomer-Kooijker K, Slieker MG, Kentie PA, van der Ent CK, Meijer Y. A prediction rule for food challenge outcome in children. Pediatr Allergy Immunol. 2012;23:353–9.
Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO - ARIA - GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;3:6–17.
Burks W, Sampson HA, Bannon GA. Peanut allergens. Allergy. 1998;53:725–30.
Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, Wensing M, Becker W-M, et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004;114:1410–7.
Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, et al. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009;124:771–8.
Lieberman JA, Glaumann S, Batelson S, Borres MP, Sampson HA, Nilsson C. The utility of peanut components in the diagnosis of IgE-mediated peanut allergy among distinct populations. J Allergy Clin Immunol Pract. 2013;1:75–82.
Agabriel C, Ghazouani O, Birnbaum J, Valérie L, Porri F, Gouitaa M, et al. Ara h 2 and Ara h 6 sensitization predict peanut allergy in Mediterranean pediatric patients. Pediatr Allergy Immunol, in press.
Beyer K, Grabenhenrich L, Beder A, Kalb B, Ziegert M, Finger A, et al. Predictive values of component-specific IgE for the outcome of peanut and hazelnut food challenges in children. Allergy. 2015;70:90–8.
Masthoff LJ, Mattsson L, Zuidmeer-Jongejan L, Lidholm J, Andersson K, Akkerdaas JH, et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J Allergy Clin Immunol. 2013;132:393–9.
Pedrosa M, Boyano-Martínez T, García-Ara MC, Caballero T, Quirce S. Peanut seed storage proteins are responsible for clinical reactivity in Spanish peanut-allergic children. Pediatr Allergy Immunol. 2012;23:654–9.
Suratannon N, Ngamphaiboon J, Wongpiyabovorn J, Puripokai P, Chatchatee P. Component-resolved diagnostics for the evaluation of peanut allergy in a low-prevalence area. Pediatr Allergy Immunol. 2013;24:665–70.
Ebisawa M, Movérare R, Sato S, Maruyama N, Borres MP, Komata T. Measurement of Ara h 1-, 2-, and 3-specific IgE antibodies is useful in diagnosis of peanut allergy in Japanese children. Pediatr Allergy Immunol. 2012;23:573–81.
Castro AP, Pastorino AC, Gushken AKF, Kokron CM, Filho UD, Jacob CMA. Establishing a cut-off for the serum levels of specific IgE to milk and its components for cow’s milk allergy: results from a specific population. Allergol Immunopathol (Madr), in press.
Bartnikas LM, Sheehan WJ, Hoffman EB, Permaul P, Dioun AF, Friedlander J, et al. Predicting food challenge outcomes for baked milk: role of specific IgE and skin prick testing. Ann Allergy Asthma Immunol. 2012;109:309–13.
Alvaro M, García-Paba MB, Giner MT, Piquer M, Domínguez O, Lozano J, et al. Tolerance to egg proteins in egg-sensitized infants without previous consumption. Allergy. 2014;69:1350–6.
Turner PJ, Mehr S, Joshi P, Tan J, Wong M, Kakakios A, et al. Safety of food challenges to extensively heated egg in egg-allergic children: a prospective cohort study. Pediatr Allergy Immunol. 2013;24:450–5.
Dang TD, Mills CEN, Allen KJ. Determination of the clinical egg allergy phenotypes using component-resolved diagnostics. Pediatr Allergy Immunol, in press.
Lemon-Mulé H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122:977–83.
Ando H, Movérare R, Kondo Y, Tsuge I, Tanaka A, Borres MP, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008;122:583–8.
Haneda Y, Kando N, Yasui M, Kobayashi T, Maeda T, Hino A, et al. Ovomucoids IgE is a better marker than egg white-specific IgE to diagnose boiled egg allergy. J Allergy Clin Immunol. 2012;129:1681–2.
Alessandri C, Zennaro D, Scala E, Ferrara R, Bernardi ML, Santoro M, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen’s egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2012;42:441–50.
Tan JW-L, Campbell DE, Turner PJ, Kakakios A, Wong M, Mehr S, et al. Baked egg food challenges - clinical utility of skin test to baked egg and ovomucoid in children with egg allergy. Clin Exp Allergy. 2013;43:1189–95.
Vazquez-Ortiz M, Pascal M, Jiménez-Feijoo R, Lozano J, Giner MT, Alsina L, et al. Ovalbumin-specific IgE/IgG4 ratio might improve the prediction of cooked and uncooked egg tolerance development in egg-allergic children. Clin Exp Allergy. 2014;44:579–88.
Palosuo K, Alenius H, Varjonen E, Koivuluhta M, Mikkola J, Keskinen H, et al. A novel wheat gliadin as a cause of exercise-induced anaphylaxis. J Allergy Clin Immunol. 1999;103:912–7.
Mäkelä MJ, Eriksson C, Kotaniemi-Syrjänen A, Palosuo K, Marsh J, Borres M, et al. Wheat allergy in children - new tools for diagnostics. Clin Exp Allergy. 2014;44:1420–30.
Pastorello EA, Farioli L, Stafylaraki C, Scibilia J, Mirone C, Pravettoni V, et al. Wheat-dependent exercise-induced anaphylaxis caused by a lipid transfer protein and not by ω-5 gliadin. Ann Allergy Asthma Immunol. 2014;112:386–7.
Luengo O, Cardona V. Component resolved diagnosis: when should it be used? Clin Transl Allergy. 2014;4:28.
Tsabouri S, Triga M, Makris M, Kalogeromitros D, Church MK, Priftis KN. Fish and shellfish allergy in children: review of a persistent food allergy. Pediatr Allergy Immunol. 2012;23:608–15.
Ayuso R, Reese G, Leong-Kee S, Plante M, Lehrer SB. Molecular basis of arthropod cross-reactivity: ige-binding cross-reactive epitopes of shrimp, house dust mite and cockroach Tropomyosins. Int Arch Allergy Immunol. 2002;129:38–48.
Boyano-Martínez T, Pedrosa M, Belver T, Quirce S, García-Ara C. Peach allergy in Spanish children: tolerance to the pulp and molecular sensitization profile. Pediatr Allergy Immunol. 2013;24:168–72.
De Swert LFA, Gadisseur R, Sjölander S, Raes M, Leus J, Van Hoeyveld E. Secondary soy allergy in children with birch pollen allergy may cause both chronic and acute symptoms. Pediatr Allergy Immunol. 2012;23:117–23.
Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010;40:1442–60.
Yavuz ST, Sahiner UM, Buyuktiryaki B, Tuncer A, Yilmaz EA, Cavkaytar O, et al. Role of specific IgE in predicting the clinical course of lentil allergy in children. Pediatr Allergy Immunol. 2013;24:382–8.
Ballabio C, Peñas E, Uberti F, Fiocchi A, Duranti M, Magni C, et al. Characterization of the sensitization profile to lupin in peanut-allergic children and assessment of cross-reactivity risk. Pediatr Allergy Immunol. 2013;24:270–5.
Povesi Dascola C, Caffarelli C. Exercise-induced anaphylaxis: a clinical view. Ital J Pediatr. 2012;14:38–43.
Groetch M, Nowak-Wegrzyn A. Practical approach to nutrition and dietary intervention in pediatric food allergy. Pediatr Allergy Immunol. 2013;24:212–21.
Giovannini M, D'Auria E, Caffarelli C, Verduci E, Barberi S, Indinnimeo L, et al. Nutritional management and follow up of infants and children with food allergy: italian society of pediatric nutrition/Italian society of pediatric allergy and immunology task force position statement. Ital J Pediatr. 2014;3:40–1.
Christie L, Hine RJ, Parker JG, Burks W. Food allergies in children affect nutrient intake and growth. J Am Diet Assoc. 2002;102:1648–51.
Flammarion S, Santos C, Guimber D, Jouannic L, Thumerelle C, Gottrand F, et al. Diet and nutritional status of children with food allergies. Pediatr Allergy Immunol. 2011;22:161–5.
Robbins KA, Guerrerio AL, Hauck SA, Henry BJ, Keet CA, Brereton NH, et al. Growth and nutrition in children with food allergy requiring amino acid-based nutritional formulas. J Allergy Clin Immunol. 2014;134:1463–6.
Järvinen KM, Konstantinou GN, Pilapil M, Arrieta M-C, Noone S, Sampson HA, et al. Intestinal permeability in children with food allergy on specific elimination diets. Pediatr Allergy Immunol. 2013;24:589–95.
Sicherer SH, Noone SA, Muñoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol. 2001;87:461–4.
Knibb C, Hourihane JO’B. The psychosocial impact of an activity holiday for young children with severe food allergy: a longitudinal study. Pediatr Allergy Immunol. 2013;24:368–75.
Mikkelsen A, Borres MP, Bjorkelund C, Lissner L, Oxelmark L. The Food hypersensitivity famiLy ImPact (FLIP) questionnaire - development and first results. Pediatr Allergy Immunol. 2013;24:574–81.
Nguyen-Luu NU, Ben-Shoshan M, Alizadehfar R, Joseph L, Harada L, Allen M, et al. Inadvertent exposures in children with peanut allergy. Pediatr Allergy Immunol. 2012;23:134–40.
Le T-M, Zijlstra WT, van Opstal EY, Knol MJ, L’Hoir MP, Knulst AC, et al. Food avoidance in children with adverse food reactions: Influence of anxiety and clinical parameters. Pediatr Allergy Immunol. 2013;24:650–5.
Flokstra-de Blok BMJ, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, O’Brien Hourihane J, et al. Development and validation of the self-administered food allergy quality of life questionnaire for adolescents. J Allergy Clin Immunol. 2008;122:139–44.
Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65:933–45.
Petrus NCM, Hulshof L, Rutjes NWP, de Vreede I, van Aalderen WMC, Sprikkelman AB. Response to: cost-effectiveness of using an extensively hydrolysed formula compared to an amino acid formula as first-line treatment for cow milk allergy in the UK. Pediatr Allergy Immunol. 2012;23:686.
Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133(2):318–23.
Eigenmann PA, Beyer K, Wesley Burks A, Lack G, Liacouras CA, Hourihane JO, et al. New visions for food allergy: an iPAC summary and future trends. Pediatr Allergy Immunol. 2008;19 Suppl 19:26–39.
Tang MLK, Martino DJ. Oral immunotherapy and tolerance induction in childhood. Pediatr Allergy Immunol. 2013;24:512–20.
Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE-mediated hen’s egg allergy: a new protocol with raw hen’s egg. Pediatr Allergy Immunol. 2013;24:75–83.
Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli Sopo S. Specific oral tolerance induction with raw hen’s egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol. 2013;24:66–74.
Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol. 2008;121:343–7.
Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–9.
Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122(6):1154–60.
Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–60.
Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009;64:1218–20.
Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–43.
Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129(2):448–55. 455.
Keet CA, Seopaul S, Knorr S, Narisety S, Skripak J, Wood RA. Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2013;132(3):737–9.
Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–75.
Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005;60:1320–2.
Fuentes-Aparicio V, Alonso-Lebrero E, Zapatero L, Infante S, Lorente R, Angeles Muñoz-Fernández M, et al. Oral immunotherapy in hen’s egg-allergic children increases a hypo-proliferative subset of CD4+ T cells that could constitute a marker of tolerance achievement. Pediatr Allergy Immunol. 2012;23:648–53.
Vila L, Moreno A, Gamboa PM, Martínez-Aranguren R, Sanz ML. Decrease in antigen-specific CD63 basophil expression is associated with the development of tolerance to egg by SOTI in children. Pediatr Allergy Immunol. 2013;24:463–8.
Barbi E, Longo G, Berti I, Neri E, Saccari A, Rubert L, et al. Adverse effects during specific oral tolerance induction: in-hospital “rush” phase. Eur Ann Allergy Clin Immunol. 2012;44(1):18–25.
Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122:342–7.
Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J Allergy Clin Immunol. 2011;128:125–31. e2.
Marrs T, Lack G. Why do few food-allergic adolescents treat anaphylaxis with adrenaline? Reviewing a pressing issue. Pediatr Allergy Immunol. 2013;24:222–9.
Niggemann B, Beyer K. Adrenaline autoinjectors in food allergy: in for a cent, in for a Euro? Pediatr Allergy Immunol. 2012;23:506–8.
Johnson MJ, Foote KD, Moyses HE, Roberts G. Practices in the prescription of adrenaline autoinjectors. Pediatr Allergy Immunol. 2012;23:125–8.
Dreborg S. When should adrenaline be given and by whom? Pediatr Allergy Immunol. 2013;24:97–8.
Jacobs TS, Greenhawt MJ, Hauswirth D, Mitchell L, Green TD. A survey study of index food-related allergic reactions and anaphylaxis management. Pediatr Allergy Immunol. 2012;23:582–9.
Simons FE, Ardusso LR, Bilo MB, El-Gamal YM, Ledford DK, Ring J, et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37.
Douglas DM, Sukenick E, Andrade WP, Brown JS. Biphasic systemic anaphylaxis: an inpatient and outpatient study. J Allergy Clin Immunol. 1994;93:977–85.
Choo KJ, Simons E, Sheikh A. Glucocorticoids for the treatment of anaphylaxis: cochrane systematic review. Allergy. 2010;65:1205–11.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PC, FC, AP and DP reviewed the relevant articles on the literature under the supervision of DGP and drew the first draft; CH revised the English version of the manuscript; CH and DGP critical revised the final draft for important intellectual content. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Comberiati, P., Cipriani, F., Schwarz, A. et al. Diagnosis and treatment of pediatric food allergy: an update. Ital J Pediatr 41, 13 (2015). https://doi.org/10.1186/s13052-014-0108-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-014-0108-0