Abstract
Background
Current guidelines from Scandinavian Neuro Committee mandate a 24-hour observation for head trauma patients on anticoagulants, even with normal initial head CT scans, as a means not to miss delayed intracranial hemorrhages. This study aimed to assess the prevalence, and time to diagnosis, of clinically relevant delayed intracranial hemorrhage in head trauma patients treated with oral anticoagulants.
Method
Utilizing comprehensive two-year data from Region Skåne’s emergency departments, which serve a population of 1.3 million inhabitants, this study focused on adult head trauma patients prescribed oral anticoagulants. We identified those with intracranial hemorrhage within 30 days, defining delayed intracranial hemorrhage as a bleeding not apparent on their initial CT head scan. These cases were further defined as clinically relevant if associated with mortality, any intensive care unit admission, or neurosurgery.
Results
Out of the included 2,362 head injury cases (median age 84, 56% on a direct acting oral anticoagulant), five developed delayed intracranial hemorrhages. None of these five cases underwent neurosurgery nor were admitted to an intensive care unit. Only two cases (0.08%, 95% confidence interval [0.01–0.3%]) were classified as clinically relevant, involving subdural hematomas in patients aged 82 and 87 years, who both subsequently died. The diagnosis of these delayed intracranial hemorrhages was made at 4 and 7 days following initial presentation to the emergency department.
Conclusion
In patients with head trauma, on oral anticoagulation, the incidence of clinically relevant delayed intracranial hemorrhage was found to be less than one in a thousand, with detection occurring four days or later after initial presentation. This challenges the effectiveness of the 24-hour observation period recommended by the Scandinavian Neurotrauma Committee guidelines, suggesting a need to reassess these guidelines to optimise care and resource allocation.
Trial registration
This is a retrospective cohort study, does not include any intervention, and has therefore not been registered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Head trauma is common and often leads to emergency department (ED) visits, ranging from mild concussions to severe, life-threatening injuries with intracranial hemorrhage (ICH), necessitating early detection and treatment. The initial symptoms of ICH may be subtle and resemble those of a concussion, which makes management in the ED challenging. Even when an initial computed tomography (CT) scan shows no ICH, patients may still develop a delayed ICH (d-ICH), specifically those on oral anticoagulants (OAC), presenting additional challenges. Management strategies to prevent overlooking d-ICH include repeat CT scanning, prolonged observation, and individual risk assessments.
In Sweden there are five commercially available OAC drugs, one being the vitamin K antagonist warfarin and the other four are direct oral anticoagulants (DOAC): dabigatran (direct thrombin inhibitor), rivaroxaban (factor Xa inhibitor), edoxaban (factor Xa inhibitor) and apixaban (factor Xa inhibitor). Oral drugs that inhibit thrombocyte aggregation, such as aspirin, non-steroidal anti-inflammatory drugs, and adenosine diphosphate receptor inhibitors, are not included in the term OAC.
The use of OAC treatment, particularly DOACs, is on a steady rise in recent years, especially among the elderly [1,2,3]. At the same time, studies have shown an increasing incidence of head injuries among those older than 65 years [4]. A third of these patients experience a fall at least once per year, with 10% of these falls resulting in a head injury [5].
Hence, head injuries in patients with OAC therapy are common and expected to increase in the coming years, placing a greater strain on our emergency departments and hospitals. Consequently, the development and updating of evidence-based clinical guidelines becomes crucial to effectively manage these patients with increased risks of traumatic ICH and d-ICH.
Most current guidelines for head injuries – including the New Orleans Criteria [6], Canadian CT Head Rule [7], EAST’s practice management guidelines [8], French guidelines for mild Traumatic Brain Injury [9], the Austrian interdisciplinary consensus statement [10] and the Scandinavian Neurotrauma Committee Guidelines (SNC) [11], mandate a head CT scan for patients on anticoagulation. Recently updated NICE guidelines [12] state that a head CT is to be considered, but not mandatory, for patients treated with OAC. In addition to an initial head CT, the Austrian consensus document and SNC guidelines both advise a 24-hour observation period for these patients, even if their initial CT scan is interpreted as normal, as a means not to miss cases of d-ICH [10, 11].
The recommendation to observe all head trauma patients on OAC treatment requires significant ED and in-hospital resources and may lead to adverse outcomes, particularly among elderly patients [13,14,15,16]. In addition, most d-ICH cases do not necessitate management changes [17], and it may be posited that only clinically relevant d-ICH, requiring neurosurgical intervention, intensive care unit (ICU) admission, or leading to death, warrant detection. The aim of this study was to analyze the incidence, and time to diagnosis, of clinically relevant d-ICH in a Scandinavian ED setting.
Method
A retrospective cohort study was conducted in Region Skåne, Sweden, where the hospital system serves a population of 1.3 million inhabitants. This encompasses eight emergency departments: three general EDs, three community EDs, and two academic center EDs, one of which is a neurosurgical center.
Data on adult ED visits during 2017 and 2018 were collected from local, regional, and national healthcare registers, including information from the hospital system’s medical records and national data from the National Board of Health and Welfare and the National Pharmacy Register.
Data was retrieved for adult patients, aged 18 years or older, presenting to an ED with head trauma registered as their chief complaint by triage staff in the ED information system. Patients were included in the study as an OAC treated patient if they had redeemed a prescription of any OAC at a Swedish pharmacy within 90 days prior to their ED visit.
Radiology reports for all CT scans capable of detecting ICH within 30 days of initial ED presentation were retrieved. This included standard non-contrast head/brain CT as well as CTs focused on neurocranium, facial bones, orbits, and cerebral angiography. Reports were categorised binarily as positive or negative for ICH after both manual and automatic review. To avoid ambiguity between d-ICH and delayed diagnostics we excluded ED visits where there was no CT performed or if the first CT scan was performed after 24 h of ED arrival.
Neurosurgical interventions, recorded nationally per the Swedish system “Classification of Healthcare Interventions (KVÅ)”, were identified using a compiled list of ICH-related intervention codes (see Appendix, Table 1). Registration of any of these codes indicated that the patient had undergone neurosurgery and included all types of craniectomy, duraplasty, intracranial pressure monitoring and hematoma evacuation as well as intracranial vascular and ventricular surgery.
Delayed ICH was defined as the finding of ICH within 30 days of head trauma, which was not identified on the first CT scan performed within 24 h of ED arrival, and without known repeat head trauma occurring since the preceding CT scan.
Hospital records and ambulance documentation were reviewed for suspected d-ICH cases. Cases were classified as not being d-ICH if there was documentation of repeat head trauma since the initial CT scan. Admission to an intensive care unit was verified through local regional hospital records.
Nationally registered mortality data was retrieved using national identity numbers. This data included cause of death and was used to identify whether patients had died due to traumatic brain injury outside of Region Skåne hospital system, where we would be unable to access hospital records.
The primary outcome, classified as clinically relevant d-ICH, was defined as d-ICH associated with neurosurgery, death, or any ICU treatment within 30 days of the index ED visit.
Given the study’s basis on ED visits, individual patients could be included multiple times if subsequent ED visits met inclusion criteria.
Statistics
Patient characteristics were reported using descriptive statistics. Data was assessed for normal distribution using visual assessment of histograms. Nonnormally distributed data were reported as medians with 25–75%, exclusive, quartiles range (QR). Data was analysed using Microsoft Excel 365. The 95% confidence interval (CI) for proportions was calculated with the Clopper-Pearson exact method using the Epitools epidemiological calculator [18].
Results
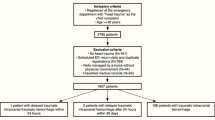
Data from 630,275 ED visits were retrieved, with 18,549 being visits due to a chief complaint of head trauma. Of these, a total of 2,362 head trauma ED visits were included in the study. See Fig. 1 for the inclusion process and main results, and Table 1 for patient demographics and observed outcomes. The median age was 84 (QR 77–89), and the majority of the patients were treated with DOAC (56.1%), with Apixaban being the most common DOAC (30.7% of total).
Within 30 days of ED presentation, 256 cases of ICH were diagnosed. Of these, 21 initially had a negative CT scan at their respective index ED visit, but a repeated CT within 30 days detected ICH. Subsequent review of hospital and ambulance records for these 21 patients revealed that 16 had suffered a repeat head trauma after the initial ED visit. Consequently, only five cases were classified as d-ICH (0.2%, 95% CI [0.07–0.5%]) by our definition. Of the five patients with d-ICH, four presented with subdural hematomas (SDH), and one had a minor intracerebral hemorrhage. None of the d-ICH cases were identified within the initial 24-hour period. Instead, repeated CT scans identified d-ICH on day 4, 8, 11, 21 and 28 post index ED visit, respectively. Detailed case information is provided in Table 2. Review of hospital notes for the five patients with d-ICH revealed no ICU admissions.
During the study period, 325 patients had more than one ED visit due to head trauma: 232 had two visits, 59 had three visits, 23 had four visits, and 11 had five or more visits. None of the d-ICH cases had more than one ED visit registered as head trauma.
Through national registry data, we found that six patients underwent a neurosurgical intervention within 30 days, all with evacuation of SDHs, and all had findings of ICH on their initial CT scan. None of the five d-ICH cases underwent a neurosurgical procedure.
We observed an all-cause 30-day mortality of 4.7% (95% CI [3.9–5.7%]). Two patients with d-ICH died within 30 days of their initial ED visit, at 7 and 14 days after the index ED visit, respectively. In our dataset, 1.2% of patients were inhabitants in a geographic region not covered by the Region Skåne hospital system, and three of those died within 30 days, all with ICH on their initial CT scan. Cross-referencing national mortality data with local hospital records revealed no further mortality cases with any type of ICH as a listed cause of death.
In summary, we identified two cases (0.08%, 95% CI [0.01–0.3%]) of clinically relevant d-ICH due to associated mortality, but none underwent neurosurgery nor admitted to ICU.
Discussion
In our study of 2,362 ED visits involving head trauma and OAC therapy, we identified five cases of d-ICH, accounting for 0.2% of the visits (95% CI [0.07–0.5%]). Of these, two cases (0.08%, [95% CI 0.01–0.3%]) were classified as clinically relevant, due to their association with mortality, yet neither required ICU admission or neurosurgery. None of the observed d-ICH cases were diagnosed within the 24-hour observation period recommended by SNC and the Austrian consensus document. On the contrary, the few cases of d-ICH in our study were diagnosed four or more days after their initial ED visit, aligning with Chenoweth et al.’s findings where two of three d-ICH cases were detected after 24 h [19]. This suggests that the 24-hour observation period may be inadequate in detecting d-ICH. Our findings challenge the utility of currently recommended 24-hour observation period by the Scandinavian Neurotrauma Committee (SNC) guideline and the Austrian expert consensus document. Our results lend support to other guidelines that do not mandate such observation [6,7,8,9, 12].
Mandatory observation for d-ICH in the ED and in-hospital units carries significant costs and adds a burden on already strained healthcare resources. In 2012, the cost in the USA was estimated at 1,000,000 USD to detect a single case of d-ICH, and this cost is likely much higher now [20]. Additionally, the typical patient demographic for OAC therapy – older and frail individuals – may be harmed by even short-term hospitalizations [13,14,15,16]. Furthermore, other concerns could be present that are of higher individual value than in-hospital observation for the rare event of d-ICH.
Previous studies assessing the risk of d-ICH in anticoagulated patients have utilized diverse methodologies and patient populations, making it difficult to determine its true prevalence. The latest meta-analysis by Puzio et al., conducted after the introduction of direct oral anticoagulant (DOAC) therapies, reported a 2.3% incidence of d-ICH in patients treated with OAC who suffered a blunt head injury [21]. However, several more recent studies, reflecting the increased use of DOACs compared to warfarin, generally suggest incidence rates of ≤ 1% [22,23,24,25,26]. The variance in incidence rates between studies could be attributed not only to differences in follow-up methodology but also to misdiagnosis. For instance, Verschoof et al. found that 66% of their d-ICH cases were actually erroneous, with subtle ICH noted retrospectively on initial CT scans [22]. The advent of advanced CT technology may reduce the incidence of d-ICH by enabling the detection of minimal ICH on initial scans [27].
Our study, which observed a d-ICH incidence of 0.2% (95% CI [0.07–0.5%]), was not designed to find all cases of d-ICH, being a retrospective cohort without structured follow-up or imaging. However, our findings are in line with recent studies that report a low incidence of d-ICH.
Many cases of d-ICH are minor and often managed conservatively. Clinically important d-ICH, which requires neurosurgery or causes death, appear to be very rare. Puzio et al.’s meta-analysis indicated a crude risk of death from d-ICH at 0.33% and d-ICH-related neurosurgical interventions at 0.13% [21]. Several newer studies have shown that important interventions, such as neurosurgery, is extremely rare, with many reporting no neurosurgical interventions across various patient populations [14, 16, 23,24,25,26]. This aligns with the results of our study, where we found no d-ICH related neurosurgical interventions (0.0%, 95% CI [0.0-0.03%]) or ICU admissions (0.0%, 95% CI [0.0-0.03%]), and a low mortality rate (0.08%, [95% CI 0.01–0.3%]).
Given the low incidence of clinically relevant d-ICH, and considering that very few cases seem appropriate for neurosurgical intervention, it has been proposed that mandatory observation for OAC treated patients should be omitted [28]. This perspective is mirrored in more recently reviewed guidelines, like those from NICE and French recommendations, which do not advocate for routine observation following a negative initial CT [9, 12]. This opinion, reinforced by recent evidence including findings from this study, supports reconsidering the necessity of mandatory observation and calls for a revision of the SNC guidelines.
Strengths and limitations
The study’s strengths include being the largest study, to our knowledge, that investigates clinically relevant outcomes in d-ICH among OAC-treated head trauma patients. It included a diverse and extensive cohort from two academic, three general, and three community EDs, which enhances the applicability of the results. Additionally, the use of Swedish nationwide registers ensures reliable follow-up data.
The study does have limitations. A primary concern is the reliance on prescription redemption to determine OAC use, which might not always reflect the actual ongoing treatment at the time of injury. This approach could include patients who had ceased OAC use for various reasons, such as compliance issues or the completion of a prescribed treatment course. This factor might lead to an underestimation of the actual rate of clinically relevant d-ICH. However, it is unlikely that the inclusion of such cases, which are probably few, significantly alters the overall interpretation of the study’s results.
Our study focused on patients with ‘head injury’ as the chief complaint registered by triage staff. This will lead to missing patients with head injuries registered under another major complaint, such as a hip fracture, syncope, or multi trauma, resulting in their non-inclusion in study. However, we believe this does not significantly impact our conclusions. Patients with other major issues, e.g. a hip fracture, would require in-hospital treatment and observation anyway.
This study focused on use of OAC treatment and did not evaluate use of thrombocyte inhibitors, neither on its own nor in combination with OAC. Findings should not be applied for patients treated with thrombocyte inhibitors.
We did not evaluate the mechanism of injury leading to head trauma. Investigating trauma energy as a predictor for clinically relevant d-ICH could potentially be valuable for further individual risk assessment. However, the rarity of d-ICH necessitates substantially larger studies to achieve adequate statistical power. In our data, d-ICH was identified only in cases of ground level falls, which could indicate that low-energy trauma increases the risk of developing d-ICH.
A minor limitation of our study is that 1.2% of the patients were not residents of the geographical area served by our hospital system, which could potentially affect the completeness of our follow-up data. There is also a possibility that some patients may have been diagnosed with d-ICH in other regions where we could not access their medical records. However, since data on neurosurgical interventions and mortality were captured in nationwide registries, our analysis would not have missed any cases related to these outcomes. The only potentially missed cases would be those of d-ICH that required admission for ICU care.
The majority of patients in our study did not undergo repeat CT scan, as this is not routinely performed in line with SNC guidelines. Consequently, this study should not be used to interpret the true incidence of d-ICH in general but only of those cases causing clinical concern. The prevalence of clinically relevant d-ICH, which is the primary objective of our study, should remain unaffected by this limitation. For the same reason, the high ratio of d-ICH being clinically relevant (two out of five, 40%) should be interpreted with caution, as the denominator is likely larger.
Conclusion
In patients with head trauma, on oral anticoagulation, the incidence of clinically relevant delayed intracranial hemorrhage was found to be less than one in a thousand, with detection occurring four days or later after initial presentation. This challenges the effectiveness of the 24-hour observation period recommended by the Scandinavian Neurotrauma Committee guidelines, suggesting a need to reassess these guidelines to optimise care and resource allocation.
Data availability
Due to the nature of this retrospective cohort study, data sharing is restricted. The data contain sensitive patient information protected under national privacy regulations and patient confidentiality agreements. As such, the dataset for this study is not publicly available. Access is limited to the research team to maintain patient privacy and adhere to ethical standards. Researchers interested in the study methodology or other non-sensitive materials can contact the corresponding author.
Abbreviations
- CI :
-
Confidence Interval
- CT :
-
Computed Tomography
- d-ICH :
-
Delayed Intracranial Hemorrhage
- DOAC :
-
Direct Oral Anticoagulant
- ED :
-
Emergency Department
- ICH :
-
Intracranial Hemorrhage
- INR :
-
Prothrombin time, international normalized ratio
- QR :
-
Quartile Range
- NICE :
-
National Institute for Health and Care Excellence
- OAC :
-
Oral Anticoagulants
- SDH :
-
Subdural Hematoma
- SNC :
-
Scandinavian Neurotrauma Committee
References
Wändell P, et al. Venous thromboembolism 2011–2018 in Stockholm: a demographic study. J Thromb Thrombolysis. 2019;48:668–73.
Munir MB, et al. Contemporary clinical and economic outcomes among oral anticoagulant treated and untreated elderly patients with atrial fibrillation: insights from the United States Medicare database. PLoS ONE. 2022;17:e0263903.
Swedish National Board of Social Affairs and Health. Läkemedelsförsäljning i Sverige – analys och prognos 2022–2025 ([Swedish] Drug prescriptions - analysis and prognosis 2022–2025). Socialstyrelsen 2022-4-7858. (2022).
Ramanathan DM, McWilliams N, Schatz P, Hillary FG. Epidemiological shifts in elderly traumatic brain injury: 18-year trends in Pennsylvania. J Neurotrauma. 2012;29:1371–8.
Thompson HJ, McCormick WC, Kagan SH. Traumatic brain Injury in older adults: Epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54:1590–5.
Haydel MJ, et al. Indications for computed tomography in patients with minor Head Injury. N Engl J Med. 2000;343:100–5.
Stiell IG, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391–6.
Barbosa RR, et al. Evaluation and management of mild traumatic brain injury: an Eastern Association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012;73:S307.
Gil-Jardiné C, et al. Management of patients suffering from mild traumatic brain injury 2023. Anaesthesia Crit Care Pain Medicine. 2023;42:101260.
Wiegele M et al. Diagnostic and therapeutic approach in adult patients with traumatic brain injury receiving oral anticoagulant therapy: an Austrian interdisciplinary consensus statement. Crit Care 23, (2019).
Undén J, Ingebrigtsen T, Romner B, the Scandinavian Neurotrauma Committee (SNC). Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med. 2013;11:50.
National Institue of Health and Care Excellence. NICE guideline NG232, Head injury: assessment and early management. Available at: https://www.nice.org.uk/guidance/ng232/chapter/recommendations (2023-05-18)
Sousa P, Uva AS, Serranheira F, Uva MS, Nunes C. Patient and hospital characteristics that influence incidence of adverse events in acute public hospitals in Portugal: a retrospective cohort study. Int J Qual Health Care. 2018;30:132–7.
San Jose-Saras D, et al. Inappropriate hospital admission as a risk factor for the subsequent development of adverse events: a cross-sectional study. BMC Med. 2023;21:312.
Keeble E, Roberts HC, Williams CD, Van Oppen J, Conroy SP. Outcomes of hospital admissions among frail older people: a 2-year cohort study. Br J Gen Pract. 2019;69:e555–60.
Benedetti S, et al. In old anticoagulated patients with mild traumatic brain injury, a 24-h observation period should not be recommended without evidence of a clear benefit: a retrospective study of delayed hemorrhagic versus iatrogenic complications. Intern Emerg Med. 2023. https://doi.org/10.1007/s11739-023-03435-0
Miller J, et al. Delayed intracranial hemorrhage in the anticoagulated patient: a systematic review. J Trauma Acute Care Surg. 2015;79:310.
Sergeant ESG. Epitools Epidemiological Calculators. Ausvet. https://epitools.ausvet.com.au/ (2018).
Chenoweth JA, Gaona SD, Faul M, Holmes JF, Nishijima DK. Incidence of delayed intracranial hemorrhage in older patients after Blunt Head Trauma. JAMA Surg. 2018;153:570–5.
Li J. Admit all anticoagulated head-injured patients? A million dollars Versus your Dime. You make the call. Ann Emerg Med. 2012;59:457–9.
Puzio TJ, et al. Delayed intracranial hemorrhage following Blunt Head Trauma while on direct oral anticoagulants: a systematic review and Meta-analysis. J Am Coll Surg. 2021;232:1007–e10165.
Verschoof MA, et al. Evaluation of the yield of 24-h close observation in patients with mild traumatic brain injury on anticoagulation therapy: a retrospective multicenter study and meta-analysis. J Neurol. 2018;265:315–21.
Bergenfeldt H, Forberg JL, Lehtinen R, Anefjäll E, Vedin T. Delayed intracranial hemorrhage after head trauma seems rare and rarely needs intervention—even in antiplatelet or anticoagulation therapy. Int J Emerg Med. 2023;16:54.
Broderick M, Tripodi G, Dwyer K. Utility of repeat Head Computed Tomography in detecting delayed intracranial hemorrhage in falls on direct oral anticoagulants. Am Surgeon™. 2023;00031348231206582. https://doi.org/10.1177/00031348231206582
Soleimani T, Mosher B, Ochoa-Frongia L, Stevens P, Kepros JP. Delayed intracranial hemorrhage after Blunt Head Injury with direct oral anticoagulants. J Surg Res. 2021;257:394–8.
Mourad M, Senay A, Kharbutli B. The utility of a second head CT scan after a negative initial CT scan in head trauma patients on new direct oral anticoagulants (DOACs). Injury. 2021;52:2571–5.
Booij R, Budde RPJ, Dijkshoorn ML, van Straten M. Technological developments of X-ray computed tomography over half a century: user’s influence on protocol optimization. Eur J Radiol. 2020;131:109261.
Perry JJ, Dowlatshahi D, Eagles D. Prolonged observation or routine reimaging in older patients following a head injury is not justified. Can J Emerg Med. 2022;24:795–6.
Acknowledgements
Not applicable.
Funding
This study was also a part of the AIR Lund (Artificially Intelligent use of Registers at Lund University) research environment and received funding from the Swedish Research Council (VR; grant no. 2019-00198) as well as funding from the European Regional Development Fund InterReg ÖKS and Region Skåne.
Open access funding provided by Lund University.
Author information
Authors and Affiliations
Contributions
LA: Conceptualization, Methodology, Data Analysis, Writing – Original Draft, editing. AB: Methodology, Data Management, Writing – Review & Editing. TV: Conceptualization, Writing – Review & Editing, Supervision. JB: Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition. UE: Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition. JLF: Conceptualization, Writing – Review & Editing, Supervision, Project Administration, Funding Acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been performed in accordance with the declaration of Helsinki and was approved by Ethics Review Authority (Dnr 2019–05783, 2021 − 01836, 2021–04541). Active consent was waived by the Ethics Review Authority, but patients had the option to decline participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
André, L., Björkelund, A., Ekelund, U. et al. The prevalence of clinically relevant delayed intracranial hemorrhage in head trauma patients treated with oral anticoagulants is very low: a retrospective cohort register study. Scand J Trauma Resusc Emerg Med 32, 42 (2024). https://doi.org/10.1186/s13049-024-01214-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-024-01214-0