Abstract
Trauma is the number one cause of death among Americans between the ages of 1 and 46 years, costing more than $670 billion a year. Following death related to central nervous system injury, hemorrhage accounts for the majority of remaining traumatic fatalities. Among those with severe trauma that reach the hospital alive, many may survive if the hemorrhage and traumatic injuries are diagnosed and adequately treated in a timely fashion. This article aims to review the recent advances in pathophysiology management following a traumatic hemorrhage as well as the role of diagnostic imaging in identifying the source of hemorrhage. The principles of damage control resuscitation and damage control surgery are also discussed. The chain of survival for severe hemorrhage begins with primary prevention; however, once trauma has occurred, prehospital interventions and hospital care with early injury recognition, resuscitation, definitive hemostasis, and achieving endpoints of resuscitation become paramount. An algorithm is proposed for achieving these goals in a timely fashion as the median time from onset of hemorrhagic shock and death is 2 h.
Similar content being viewed by others
Introduction
According to the National Trauma Institute, trauma is the number one cause of death among Americans between the ages of 1 and 46 years, costing $670 billion a year in health care dollars [1]. Hemorrhage is estimated to account for more than 60,000 deaths in the United States and 1.5 million deaths worldwide each year resulting in nearly 75 million years of life lost [2]. The median time from onset of hemorrhagic shock to death is 2 h [3]. Following central nervous system injury, hemorrhage is the leading cause of death in trauma patients [4,5,6]; however, hemorrhage is amenable to interventions for reducing morbidity and mortality [7, 8]. Among those with severe multisystem trauma, early in-hospital mortality is increased by continued hemorrhage, which leads to a vicious triad of coagulopathy, hypothermia, and acidosis in the setting of incomplete or inappropriate resuscitation [9,10,11].
The military experience including research from the wars in Iraq and Afghanistan affirmed the need for improved methods of hemorrhage control [12, 13]. Department of Defense analysis of battlefield mortality demonstrated that one in four pre-hospital combat deaths and one in two in-hospital combat deaths were potentially preventable [14, 15]. This article aims to review recent advances in our understanding of the pathophysiology of traumatic hemorrhage, the role of diagnostic imaging modalities in the timely identification of hemorrhage sources, the principles of damage control resuscitation (DCR), as well as definitive hemostasis and damage control surgery (DCS). Finally, the endpoints of trauma resuscitation are summarized, and a chain of survival algorithm is proposed to achieve these endpoints in a timely manner. Although our understanding of the pathophysiology and management principles related to traumatic hemorrhage continues to improve, many questions remain unanswered in improving survival, and further study is needed.
Pathophysiology
In terms of large-volume bleeding, the following body locations or surface sources must be considered: thoracic cavity, peritoneal cavity, retroperitoneal space (e.g., pelvic fracture), muscle or subcutaneous tissue (e.g., long-bone fracture) and external hemorrhage (e.g., scalp laceration, open fracture site) (Fig. 1a) [16]. Hemorrhage and hemorrhagic shock cause inadequate oxygen delivery and activate several homeostatic mechanisms designed to preserve perfusion to vital organs. These complex events occur at the genomic, cellular, tissue, and whole-organ levels (Fig. 1b).
Pathophysiology of traumatic hemorrhagic shock. a Traumatic hemorrhage five locations. b Traumatic hemorrhagic response. (1) Genomic response. Up-regulated anti-inflammatory genes with rapid recovery. Up-regulated pro- inflammatory genes leads to complications and death. (2) Cellular response. Anerobic metabolism with damage to mitochondria, smooth endoplasmic reticulum (SER) and rough endoplasmic reticulum (RER), leading to cellular homeostasis failure. (3) Tissue response. Local hemostatic plug formation with conversion of fibrinogen to fibrin. Distant coagulopathy with hyperfibrinolysis and diffuse coagulopathy. (4) Organ response. Moderate hemorrhage with end-organ damage and Exsanguination leading to death. aPC activated protein C, CARS compensatory anti-inflammatory response syndrome, DAMPs damage-associated molecular patterns, DNA deoxyribonucleic acid, Pi inorganic phosphate, C3H6O3 lactic acid, MOF multi organ failure, O2–, OH–, oxygen radicals, RE respiratory enzymes, SIRS systemic inflammatory response syndrome, tPA tissue plasminogen activator, ↑ increased
At the genomic level, proinflammatory and anti-inflammatory innate immunity genes are up-regulated while adaptive immunity genes are simultaneously down-regulated in the early hours post-injury [17]. In addition, it has been demonstrated that the systemic inflammatory response syndrome (SIRS) and compensatory anti-inflammatory response syndrome (CARS) occur simultaneously rather than sequentially, as previously thought [17, 18]. In patients that survive, it is likely that anti-inflammatory innate immunity genomic changes translate into the phenotypic changes of innate SIRS, which is followed by relative immunosuppression, termed compensatory anti-inflammatory response syndrome (CARS), and eventual recovery without complications [17]. In patients with poor outcomes, further investigation is required regarding whether proinflammatory changes in gene expression reflect an ongoing or repeated inflammatory stimulus leading to multi-organ failure (MOF) [18]. Current understanding is most consistent with a non-resolving inflammation hypothesis among these patients which leads to SIRS, MOF, and early death [19].
At the cellular level, hemorrhage results in inadequate oxygen delivery, and as cells transition to anaerobic metabolism, there is accumulation of oxygen radicals (O2–, OH–), inorganic phosphate (Pi) and lactic acid (C3H6O3). This causes lipid peroxidation of membranes with increased permeability to Ca2+ and subsequent breakdown of mitochondria, smooth endoplasmic reticulum (SER) and rough endoplasmic reticulum (RER) [20, 21]. Cellular disruption causes release of damage-associated molecular patterns (known as DAMPs or alarmins), including mitochondrial DNA and formyl peptides, and incites a systemic inflammatory response similar to sepsis [22, 23]. Anaerobic respiration ultimately leads to mitochondrial dysfunction resulting in diminished ATP supplies, cellular homeostasis failure, and eventual cell death through necrosis from membrane rupture, apoptosis, or necroptosis [24].
At the tissue level, hemorrhage, and shock cause both adaptive and maladaptive changes within the vascular endothelium and blood. At the hemorrhagic site, the endothelium and blood act synergistically; the clotting cascade and platelets are activated leading to formation of a hemostatic plug [25, 26]. However, remote from the site of bleeding, sympathoadrenal activation and the mounting oxygen debt induces endotheliopathy with shedding of the glycocalyx barrier leading to excess autoheparinization with activation of protein C (aPC) and inactivation of factors V and VIII to prevent microvascular thrombosis [26,27,28]. Similarly, release of tissue plasminogen activator (tPA) results in increased plasmin activity leading to pathologic hyperfibrinolysis and diffuse coagulopathy [26, 28]. Hemorrhage induced decreases in platelet number and function as well as margination also contribute to the coagulopathy [29,30,31].
At the whole-organ level, moderate hemorrhagic shock causes vasoconstriction with hypoperfusion leading to end-organ damage in survivors. However, severe shock with exsanguination can cause cerebral anoxia and fatal arrhythmias leading to death [3, 32]. Iatrogenic factors like overzealous resuscitation with cold, acidic crystalloid not only dilute the concentration of clotting factors but also exacerbate the ‘lethal triad’ of heat loss, acidosis, and coagulopathy (Fig. 1) [33,34,35,36].
Diagnostic imaging in traumatic hemorrhage
Imaging plays a vital role in the identification of hemorrhagic sources as well as the response to therapeutic interventions. Diagnostic imaging techniques of critical importance in identifying specific pathology (modality no 1 to 7) and assessing cardiovascular hemodynamics (modality no 8 to 11) for patients presenting with traumatic hemorrhage are summarized in Table 1 along with pertinent findings and key values. Portable chest, pelvic radiographs (Fig. 2a, b) and the Focused Assessment with Sonography for Trauma (FAST) are the standard of care in the initial bedside evaluation of traumatic injuries [16]. As compared to X-rays and FAST, computed tomography (CT) are more sensitive in evaluating important anatomical details and altered hemodynamics [37,38,39]. In hemodynamically stable patients with multiple injuries, CT technology can comprehensively detect trauma to the chest, abdomen, and pelvis, as well as active bleeding with sensitivity and specificity approaching 100% [38, 40,41,42,43]. Multidetector CT scan is considered the gold standard in the assessment of cardiac, vascular, small bowel and mesenteric injuries [40, 42, 43]. In trauma patients, the FAST utilizes a standard order of views or windows to evaluate the pericardial, peritoneal, and pleural cavities [44]. FAST can determine the presence of pathologic hemopericardium or pericardial effusion (sensitivity 83.3% to 100% and specificity 94% to 99.7%) (Fig. 2c, Additional file 1: Video 1), hemothorax (sensitivity 83% to 92% and specificity 98% to 100%) (Fig. 2d, Additional file 2: Video 2), intraabdominal hemoperitoneum (sensitivity 63 to 100 percent) (Fig. 3a, b; Additional file 3: Video 3, Additional file 4: Video 4), and pelvic hemorrhage (Fig. 3c, d; Additional file 5: Video 5, Additional file 6: Video 6) [44,45,46,47,48,49,50,51].
a Chest X-ray AP view. Right sided hemothorax, right lateral pneumothorax and subcutaneous emphysema. b Pelvic AP view. Widening of right sacroiliac joint with right sacral fracture and vertical shift (potential vascular injury), bilateral superior inferior pubic rami fracture (risk for bladder injury), left acetabular fracture. c Subxiphoid view of the heart (2D). Large pericardial effusion causing tamponade. d Right thoracic view at the diaphragm with a right hemothorax. Thoracic spine visualized above the diaphragm (spine sign). Normally, the thoracic spine is obscured by air within the lung. D diaphragm, HT hemothorax, L liver, LV left ventricle, PE pericardial effusion, PT pneumothorax, RV right ventricle, SE subcutaneous emphysema, SI sacroiliac joint, SS spine sign, T thrombus, TS thoracic spine
a Right upper quadrant view (RUQV) of the abdomen. Anechoic hemoperitoneum in the hepatorenal space. b Left upper quadrant view (LUQV) of the abdomen. Anechoic hemoperitoneum in the splenorenal space. c Pelvic sagittal view. Anechoic hemoperitoneum cephalad and posterior to the bladder. d Pelvic transverse view. Anechoic hemoperitoneum posterior to the bladder. B bladder, HP hemoperitoneum, L liver, LK left kidney, RL right kidney, S spleen
Hypovolemia can be identified using measurements of both the inferior vena cava (IVC) diameter and IVC collapsibility index (IVCCI) with respiration (Fig. 4a, b; Additional file 7: Video 7) [52,53,54,55]. Among these hypovolemic patients, the echocardiogram may demonstrate a small and under-filled left ventricle with preserved or hyperdynamic function (Fig. 4c, d; Additional file 8: Video 8) [56]. In the peri-operative as well as postoperative period, the cause of shock and required treatment with fluid and/or inotropes therapy can be monitored using left ventricle internal diameter at end-diastole (LVIDD), LV end-diastolic volume (LVEDV), left ventricle end-systolic volume (LVESV), left ventricle ejection fraction (LVEF), and right ventricle fraction area change (RVFAC) (Table 1, modality no 8 to 11 and Additional file 9: Video 9) [54, 56,57,58].
a TTE sagittal view of IVC long axis during inspiration; b IVC during expiration. Collapses > 50% with respiration provide insight into the fluid status of an adult trauma patient. c TEE transgastric short axis view during diastole; d systole. Severe left ventricular hypovolemia and papillary muscle kissing sign during systole. HP hepatic vein, IVC inferior vena cava, L liver, LV left ventricle, RA right atrium, RV right ventricle
Chain of survival
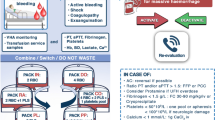
The chain of survival for patients with severe hemorrhage begins with goals of primary prevention. Following a traumatic event, focus shifts to prehospital hemorrhage control; once the patient arrives at the hospital, timely recognition of shock, resuscitation, definitive hemostasis, and achieving endpoints of resuscitation factor into the outcome. Primary prevention involves developing programs for industry and community-based violence prevention as well as increasing workplace and motor vehicle safety awareness and compliance with safety gear (seatbelt and helmet). The community-based trauma education includes B-Con (Bleeding Control) Basic or ‘Stop the Bleeding’ course, (a course aimed at educating first responders and the general public on how to stop severe bleeding and potentially save lives in an emergency) [59], Prehospital Trauma Life Support (PHTLS) Course [60], Rural Trauma Team Development Course (RTTDC) [61], and Advanced Trauma Life Support (ATLS) Student Course [16]. The prehospital and hospital chain of survival care involves applying the principles of the Damage Control Resuscitation (DCR), definite hemostasis and Damage Control Surgery (DCS), and achieving endpoints of hemostasis in a timely fashion (Fig. 5).
Damage control resuscitation (DCR)
-
a.
Pre-hospital care
Priorities for prehospital care include (1) minimizing further blood loss, (2) providing limited/delayed fluid resuscitation with permissive hypotension, (3) preventing hypothermia, and (4) rapidly transporting the patient to a facility that can provide definitive care. Tourniquet application proximal to the sites of hemorrhage in the extremities, pelvic binder placement for suspected pelvic fracture, and hemostatic dressing therapy for bleeding wounds at junctional locations (e.g., groin, axilla) can minimize blood loss and save lives [62,63,64,65,66,67]. A study by Bickell et al. examining the effects of delaying resuscitation (i.e., withholding intravenous fluid until the moment of definitive hemostasis) demonstrated improved survival, fewer complications, and reduced length of hospital stay compared with immediate resuscitation in patients with penetrating torso injuries [68]. Maintaining the principle of ‘permissive hypotension’ (systolic blood pressure 80–90 mm Hg) with low volume crystalloid boluses offers early survival advantages in blunt urban trauma patients [69].
The presence of concomitant moderate to severe traumatic brain injury (TBI) may complicate the management. Hypotension (systolic blood pressure < 90 mmHg) and hypoxia (PaO2 < 60 mmHg) were associated with a higher likelihood of a poor outcome in TBI patients [70, 71]. The Brain Trauma Foundation (BTF) and World Society of Emergency Surgery (WSES) guidelines for the management of severe TBI recommend maintaining SBP at ≥ 100 [72, 73]. However, hypotensive patients with TBI frequently have other traumatic injuries to internal organs, lungs, limbs, or the spinal cord [16], and DCR (temporarily maintaining SBP < 90 mm Hg, prevent clot disruption and re-bleeding) with immediate intervention to control severe hemorrhage may be lifesaving in these patients [68, 69].
Trauma in geriatric patients may create a special diagnostic challenge as their myocardium is less sensitive to catecholamines, and they have increased systemic vascular resistance. This results in a less profound tachycardic and hypotensive response to hemorrhage which could lead to clinical misinterpretation [74]. Among geriatric trauma patients, heart rates above 90 beats per minute and systolic blood pressure less than 110 mmHg correlate with increased mortality [74]. Older individuals are more likely to have chronic heart and lung disease. Many geriatric trauma patients may be receiving anticoagulants, antiplatelet agents, beta blockers, calcium channel blockers, and glucocorticoids for heart and lung disease. Pre-injury anticoagulants, beta blockade and glucocorticoid therapy has been shown to increase the odds of death among these trauma patients [75,76,77]. The United States Centers for Disease Control (CDC) suggests direct transport to a trauma center for any injured patient 65 or older with a systolic blood pressure < 110 mmHg [78].
Prehospital blood product resuscitation is undergoing investigation in military trauma patients with promising results [79]. In civilian trauma, the RePHILL trial was inconclusive [80] and the two large, randomized control trials (PAMPER and COMBAT) examining the benefit of prehospital plasma transfusion were contradictory. The PAMPER trial favored prehospital transfusion [81] and the COMBAT showed no benefit [82] but the post hoc analysis from 2 randomized clinical trials support a survival benefit in plasma transfusion group when transport times are longer than 20 min [83]. The CRASH-2 trial favors early administration of TXA (within 3 h of injury) to reduce the risk of death in bleeding trauma patients and is highly cost-effective [84].
The past decade has seen tremendous development of local/topical and injectable hemostatic agents for prehospital and hospital hemorrhage control [85]. Several intracavitary foams are under development to stop bleeding from a noncompressible abdominal hemorrhage [85]. ResQFoam is currently under clinical trial review to demonstrate the safety, effectiveness, and benefit-risk profile for the treatment of emergent, exsanguinating, intra-abdominal hemorrhage resulting in Class III or IV hemorrhagic shock due to trauma (https://clinicaltrials.gov/ct2/show/NCT02880163). ClotFoam, another type of intracavitary foam, is under a phase 1 clinical trial for hemostasis in liver bleeding (https://clinicaltrials.gov/ct2/show/NCT02264730). Several new hemostatic agents are under development, each with their own pros and cons based on the type of injury, severity of bleeding, wound size and configuration, location on the body, accessibility to the bleeding site, and the patient’s coagulation function [85].
Prevention of the lethal triad of hypothermia, acidosis, and coagulopathy is of paramount importance. Hypothermia should be managed using warmed blankets and warmed intravenous fluids; [86] however, these prehospital measures should not delay the transfer of a patient from the scene to the hospital [87]. In military combat, the morbidity and mortality were lower when the critically injured were transported in ≤ 60 min [87]. During prolong field care (more than 4 h), hemorrhage control (tourniquets) with blood transfusion, airway, and ventilatory support are potentially lifesaving and highly time-critical, resource-intensive interventions [88].
-
b.
Hospital care
In-hospital management starts with assembling a highly functional, multidisciplinary trauma team with individuals drawn from the specialties of anesthesia, emergency medicine, surgery, nursing, and radiology with support from the blood bank, laboratory, and patient care assistants. An experienced physician team leader receives input from the individual providers and coordinates assessment and management [89]. The Advanced Trauma Life Support (ATLS) student manual emphasizes early recognition of hemorrhagic shock, rapidly controlling the source of hemorrhage, and restoring the patient’s intravascular volume and oxygen-carrying capacity. Trauma patients can lose up to 30 percent of their blood volume before significant drops in blood pressure are manifested (Additional file 10) [16]. On arrival in the emergency department, the initial assessment and treatment are performed simultaneously in the seriously injured patient according to standard ATLS protocols. The primary survey begins with stabilization of the patient's airway, breathing, circulation, disability assessment and exposure/environmental control (ABCDEs; i.e., primary survey) [16, 90]. During the secondary survey, patients are evaluated from head-to-toe based upon their hemodynamic status and the mechanism of injury (blunt and/or penetrating) with consideration that hypotension is secondary to hemorrhage unless proven otherwise [16].
An initial aggressive resuscitation approach should be pursued with all adult trauma patients with hemorrhage, including geriatric trauma patients [91]. Vascular access is obtained as rapidly as possible with two large-bore (16-gauge or larger) intravenous (IV) lines. When difficulty arises in placing IV lines, ultrasound guided peripheral venous catheterization or central line catheterization (size 8 French), intraosseous devices, and distal saphenous vein cutdowns offer effective alternatives [92,93,94]. Initial laboratory evaluation includes blood for cross match, complete blood count (baseline hemoglobin or hematocrit and platelets), complete metabolic panel, coagulation studies [thromboelastography (TEG) and rotational thromboelastometry (ROTEM) when available], serum lactate, and arterial blood gas (serum bicarbonate for base deficit) [30, 95].
Subsequent emergency care includes activation of massive-transfusion (MT) protocols and transfusing equal amounts of packed red cells, fresh frozen plasma, and platelets in a ratio of 1:1:1 (PROPPR trial) during the early empiric phase of resuscitation. Pharmaceutical adjuncts such as calcium and tranexamic acid are important components in optimizing hemostasis [84, 96,97,98,99,100,101]. The Eastern Association for the Surgery of Trauma (EAST) guidelines compare MT with a high ratio (1:1:1) of fresh frozen plasma, platelets, and red blood cells (relatively more plasma and platelet) vs. a low ratio 1:1:2 (relatively less plasma and platelets) [101]. The EAST qualitative analysis indicated an early mortality benefit to targeting a high ratio [97, 102] due to a more frequent achievement of hemostasis [97], decreased death from truncal hemorrhage [103] or exsanguination [97]. Before the 1970s, whole blood (WB) was the resuscitation fluid of choice for bleeding trauma patients [104]. The recent data from the military and civilian trauma literature suggesting better global hemostasis using WB rather than blood components has led to a renewed interest in WB [96, 101, 105]. The EAST guidelines recommend that patients in hemorrhagic shock would benefit from high ratio DCR, if not whole blood [101] while transitioning to a laboratory-based resuscitation strategy as results become available [97, 101].
During the early hours of hospital care, the MT contents or blood products contain the anticoagulant citrate, which the liver rapidly metabolizes in healthy persons. In patients receiving MT, citrate may become toxic with life-threatening hypocalcemia and progressive coagulopathy [100, 106]. Empirical calcium dosing (e.g., 1 g of calcium chloride after administration of 4 units of RBC and/or FFP) [106] should be paired with frequent measurements of electrolyte levels to prevent hypo or hypercalcemia. [100] In the first 6 h, the isotonic crystalloid administration should be limited to 3 L to reduce the risk of respiratory failure, acute kidney injury, abdominal and extremity compartment syndromes, coagulopathy, and possibly mortality [107,108,109]. Off-label use of procoagulant hemostatic adjuncts including activated recombinant factor VII, tranexamic acid, prothrombin complex concentrate, and fibrinogen concentrate should be based on careful interpretation of the original studies and current guidelines [101]. The presence of massive transfusion protocols along with their timely activation and damage-control resuscitation (DCR) provide a decrease in mortality in trauma patients [101, 110].
Definitive hemostasis and damage control surgery (DCS)
Trauma patients with severe bleeding require timely, definitive hemostasis with surgery or angiography with embolization as prolonged time to hemostasis has been linked to increased blood-transfusion requirements and increased mortality [111, 112]. From the outset, it is important to identify the cavity with the most significant bleeding using diagnostic imaging or invasive modalities (FAST, DPL, thoracostomy) as poorly ordered surgical exploration delays definitive hemostasis and increases the risk of death [113]. Patients with extremity bleeding requiring a tourniquet and/or multicavity torso hemorrhage should not stay in the emergency department for more than 10 min [111].
Massive hemothorax (≥ 1500 ml) is identified during primary survey and initially treated with tube thoracostomy. Patient physiology should be the primary indications for surgical intervention rather than absolute numbers of initial or persistent output. Immediate bloody drainage of ≥ 20 mL/kg (approximately 1500 mL) or continuous bleeding of ≥ 3 mL/kg/hour (approximately 200 mL/hr for 2 to 4 h) may be considered an indication for surgical thoracotomy but adequate scientific evidence is lacking and need further research [16, 114, 115].
In abdominal trauma patients, surgical judgment and timing is important, and the following indications are commonly used to determine the need for laparotomy [116,117,118]: blunt abdominal trauma with hypotension and a positive FAST (Fig. 3a, b; Additional file 3: Video 3, Additional file 4: Video 4), or clinical evidence of intraperitoneal bleeding/peritonitis without another source of bleeding [16, 117,118,119]. Patients with penetrating wounds of the abdomen with associated hypotension, peritonitis, bleeding from the stomach (NG tube aspirate), rectum, genitourinary tract, or evisceration may require emergent laparotomy [120, 121]. Decisions on gunshot injuries are based on the trajectory, cavitation effect, and possible bullet fragmentation [120]. Gunshot wounds that by physical examination or routine imaging demonstrate penetration of the peritoneal cavity or viscera and vascular area of the retroperitoneum usually require laparotomy [120, 122]. Blunt or penetrating solid organ injury in hemodynamically stable patients can often be managed nonoperatively but should be admitted to the hospital for careful observation and evaluated by serial physical examinations and/or contrast-enhanced CT [122,123,124,125,126].
Patients with hypotension and pelvic fracture have high mortality rates and necessitate a team rescue effort of trauma surgeons, orthopedic surgeons, and interventional radiologists or vascular surgeons [127, 128]. The hemorrhage commonly involves disruption of the posterior osseous ligamentous complex (i.e., sacroiliac, sacrospinous, sacrotuberous, and fibromuscular pelvic floor), evidenced by a sacral fracture, a sacroiliac fracture, and/or dislocation of the sacroiliac joint [16, 127, 128]. Based on injury force, pelvic fractures are classified into four types (Table 1) and can predict those patients at high risk for massive hemorrhage [16, 127, 129]. Initial hemorrhage control is achieved through mechanical stabilization of the pelvic ring and external counter pressure by internal rotation of the lower limbs with application of a sheet or pelvic binder at the level of the greater trochanters of the femur [16]. Subsequent intervention can include pre-peritoneal packing, external fixator placement, and angiographic embolization can be used to control pelvic venous as well as arterial hemorrhage. [127, 128, 130]
The Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) therapeutic intervention for hemorrhage is in the early stages of evaluation, but it may decrease the amount of bleeding distal to the occluded site and provide a window of opportunity for resuscitation and definitive hemorrhage control [131,132,133,134]. A consensus panel recommended utilization of REBOA in patients with an initial blood pressure of < 90 mmHg that do not respond or respond only transiently to resuscitation [135]. Controlled partial REBOA is now technically possible, and the use of partial REBOA is also supported by expert panel who suggest that the most viable tool in prolonging the potential use of REBOA while attempting to avoid the dangers of distal ischemia is early partial occlusion (transitioning to partial occlusion after a short period of full occlusion) [135]. REBOA is associated with significantly reduced mortality when compared with no method of aortic occlusion, but prolonged occlusion times are associated with increased mortality [133]. Further studies are needed to determine the tolerable duration of balloon inflation, type of the balloon, ideal timing of REBOA placement, and the eligible patients who may benefit from REBOA. Preclinical studies evaluating these and other REBOA techniques are ongoing [136].
Resuscitation and management of life-threatening injuries takes precedence over the extremity or musculoskeletal injury [16, 137]. It is essential to recognize and manage musculoskeletal arterial injuries, compartment syndrome, open fractures, crush injuries, and dislocations in a timely manner [16, 137]. The mortality rate for arterial injuries is 2.2% for upper extremity and 7.7% for lower extremity injuries [138]. A staged approach to hemorrhage control is utilized by applying direct pressure, splints, tourniquets (250 mm Hg in an upper extremity and 400 mm Hg in a lower extremity), and immediate operative surgical repair when musculoskeletal injury is the source of hemodynamic instability [137]. Hemorrhage from scalp wounds may be extensive and can be controlled by applying direct pressure, cauterization, and ligation of large vessels through appropriate usage of sutures, clips, or staples [139].
In the 1980s, the concept of ‘damage control surgery’ (DCS) was initiated in severely injured patients with multisystem trauma. This concept can be broken down into distinct phases [140, 141]. Phase 0 includes rapid transport and triage for treatment (eg, operating room, interventional suite). Phase 1 encompasses surgery to arrest the hemorrhage, limit contamination, and maintain optimal blood flow to vital organs and the extremities. Operative time is limited to minimize further exacerbation of the ‘lethal triad’ of coagulopathy, hypothermia, and acidosis. Phase 2 is resuscitation in the ICU, and Phase 3 is a staged approach to definite repair of all injuries based upon the patient's physiologic status (Additional file 10). Finally, Phase 4 involves closure of the abdomen or other soft tissue wounds and delayed complex reconstructive surgery, should the primary fascia closure not be achieved during the initial hospitalization [141]. DCR and DCS are associated with a survival advantage and shorter ICU length of stay in patients with severe hemorrhage [142]. The phases and principles of Damage Control Resuscitation (DCR) and Damage Control Surgery (DCS) as well as an algorithm to achieve these goals are summarized in Fig. 5.
Endpoints of resuscitation
The traditional ATLS course standard of care markers of successful resuscitation includes restoration of normal blood pressure (BP), heart rate (HR), and urine output [16]. However, approximately 85% of severely injured trauma victims still have evidence of inadequate tissue oxygenation after normalization of BP, HR, and urine output based upon ongoing metabolic acidosis or gastric mucosal ischemia [143]. The Eastern Association for the Surgery of Trauma (EAST) guidelines for endpoints of resuscitation fall into 2 categories: global and regional [144]. The patients who achieve supranormal global oxygen delivery goals (cardiac index (CI) > 4.5 l/min/m2, oxygen delivery (DO2) > 600 ml/min/m2, and oxygen consumption (VO2) > 170 ml/min/m2) may have a better chance of survival than those who do not achieve these goals but there is no convincing evidence that attempting to attain these goals directly improves survival [145, 146]. Trying to achieve supranormal oxygen delivery goals can led to over-resuscitation, open abdomens, longer ventilation time and increased mortality [107, 108]. Other considerations for global endpoints of resuscitation include normalization of base deficit (normal range ± 2 mEq/L), lactic acid (normal range 0.5–1 mmol/L), and end-tidal carbon dioxide (normal range 35–45 mmHg) [11, 147], yet these values have failed to demonstrate conclusive survival advantage [144].
Regional endpoints include monitoring gastric ischemia using gastric tonometry or intramucosal pH (pHi, normal range ≥ 7.35) and sublingual pCO2 (normal range 45.2 ± 0.7 mm Hg). The normalization of pHi or pCO2 gap can predict better outcome [148,149,150]. Skeletal muscle and subcutaneous tissue pO2 (normal range 80–100 mmHg), pCO2 (normal range 35–45 mmHg), and pH (normal range 7.03 ± 0.02) can be monitored to demonstrate decreased regional blood flow by using near infrared spectroscopy or tissue electrodes [151, 152]. Preliminary data suggest that they may have potential for predicting risk of MOF and death after trauma [144]. Numerous resuscitation endpoint parameters have been studied but they failed to show clear mortality benefits and more work is needed [144].
Discussion
Traumatic hemorrhagic represents a serious therapeutic problem that results in high patient mortality when not managed properly. The time from injury to hospital admission, diagnosis, resuscitation, and definite hemostasis should be as abbreviated as possible. The pathophysiology of traumatic hemorrhage is complex, and imaging modalities are important to identify the source of bleeding. The application of damage control resuscitation (DCR), definitive hemostasis, and damage control surgery (DCS) have shown promising results in trauma patients. The guidelines for endpoints of resuscitation are developed but too limited in their scope to show clear outcome benefit at this point. Significant work remains in reducing the morbidity and mortality associated with traumatic hemorrhagic in areas of primary prevention, early recognition and accurate diagnosis, resuscitation therapy with hemostasis, and determination of resuscitation endpoints.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
National Trauma Institute, available at https://www.nattrauma.org/trauma-statistics-facts/. Accessed on November, 2022.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. https://doi.org/10.1016/S0140-6736(12)61728-0.
Tisherman SA, Schmicker RH, Brasel KJ, Bulger EM, Kerby JD, Minei JP, Powell JL, Reiff DA, Rizoli SB, Schreiber MA. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586–90. https://doi.org/10.1097/SLA.0000000000000837.
National Trauma Institute SC. http://www.nationaltraumainstitute.org/home/trauma_statistics.html,.Published 2015, Accessed January Dec 14, 2020. .
Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93. https://doi.org/10.1097/00005373-199502000-00006.
Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg. 2010;34(1):158–63. https://doi.org/10.1007/s00268-009-0266-1.
Stewart RM, Myers JG, Dent DL, Ermis P, Gray GA, Villarreal R, Blow O, Woods B, McFarland M, Garavaglia J, Root HD, Pruitt BA Jr. Seven hundred fifty-three consecutive deaths in a level I trauma center: the argument for injury prevention. J Trauma. 2003;54(1):66–70. https://doi.org/10.1097/00005373-200301000-00009. (discussion -1).
Schoeneberg C, Schilling M, Hussmann B, Schmitz D, Lendemans S, Ruchholtz S. Preventable and potentially preventable deaths in severely injured patients: a retrospective analysis including patterns of errors. Eur J Trauma Emerg Surg. 2017;43(4):481–9. https://doi.org/10.1007/s00068-016-0670-9.
Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, Neugebauer E, Bouillon B, Society AGPotGT. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298–304. https://doi.org/10.1016/j.injury.2006.10.003.
Balvers K, Van der Horst M, Graumans M, Boer C, Binnekade JM, Goslings JC, Juffermans NP. Hypothermia as a predictor for mortality in trauma patients at admittance to the Intensive Care Unit. J Emerg Trauma Shock. 2016;9(3):97–102. https://doi.org/10.4103/0974-2700.185276.
Gale SC, Kocik JF, Creath R, Crystal JS, Dombrovskiy VY. A comparison of initial lactate and initial base deficit as predictors of mortality after severe blunt trauma. J Surg Res. 2016;205(2):446–55. https://doi.org/10.1016/j.jss.2016.06.103.
Morrison JJ, Stannard A, Rasmussen TE, Jansen JO, Tai NR, Midwinter MJ. Injury pattern and mortality of noncompressible torso hemorrhage in UK combat casualties. J Trauma Acute Care Surg. 2013;75(2 Suppl 2):S263–8. https://doi.org/10.1097/TA.0b013e318299da0a.
Stannard A, Morrison JJ, Scott DJ, Ivatury RA, Ross JD, Rasmussen TE. The epidemiology of noncompressible torso hemorrhage in the wars in Iraq and Afghanistan. J Trauma Acute Care Surg. 2013;74(3):830–4. https://doi.org/10.1097/TA.0b013e31827a3704.
Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S431–7. https://doi.org/10.1097/TA.0b013e3182755dcc.
Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, Wade CE, Simmons J, Mace J, Mabry R, Bolenbaucher R, Blackbourne LH. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1 Suppl):S4-8. https://doi.org/10.1097/TA.0b013e318221147b.
American College of Surgeons, Committe on Trauma, Advanced Trauma Life Support (ATLS) student course manual. 9th ed. American College of Surgeons. Chicago: 2012:69.
Tompkins RG. Genomics of injury: The Glue Grant experience. J Trauma Acute Care Surg. 2015;78(4):671–86. https://doi.org/10.1097/TA.0000000000000568.
Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG, Inflammation, Host Response to Injury Large-Scale Collaborative Research P. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. https://doi.org/10.1084/jem.20111354.
Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–82. https://doi.org/10.1016/j.cell.2010.02.029.
Barbee RW, Reynolds PS, Ward KR. Assessing shock resuscitation strategies by oxygen debt repayment. Shock. 2010;33(2):113–22. https://doi.org/10.1097/SHK.0b013e3181b8569d.
Chaudry IH. Cellular mechanisms in shock and ischemia and their correction. Am J Physiol. 1983;245(2):R117–34. https://doi.org/10.1152/ajpregu.1983.245.2.R117.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. https://doi.org/10.1038/nature08780.
Wenceslau CF, McCarthy CG, Goulopoulou S, Szasz T, NeSmith EG, Webb RC. Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med Hypotheses. 2013;81(4):532–5. https://doi.org/10.1016/j.mehy.2013.06.026.
Iwata S, Tanaka A, Ozawa K. Alterations in the proton ATPase activity of rat liver mitochondria after hemorrhagic shock. J Lab Clin Med. 1992;120(3):420–7.
White NJ, Ward KR, Pati S, Strandenes G, Cap AP. Hemorrhagic blood failure: oxygen debt, coagulopathy, and endothelial damage. J Trauma Acute Care Surg. 2017;82(61):S41–9. https://doi.org/10.1097/TA.0000000000001436.
Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128(8):1043–9. https://doi.org/10.1182/blood-2016-01-636423.
Britten MW, Lumers L, Tominaga K, Peters J, Dirkmann D. Glycocalyx components affect platelet function, whole blood coagulation, and fibrinolysis: an in vitro study suggesting a link to trauma-induced coagulopathy. BMC Anesthesiol. 2021;21(1):83. https://doi.org/10.1186/s12871-021-01300-1.
Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: the past, present, and future. J Thromb Haemost. 2019;17(6):852–62. https://doi.org/10.1111/jth.14450.
Spann AP, Campbell JE, Fitzgibbon SR, Rodriguez A, Cap AP, Blackbourne LH, Shaqfeh ESG. The effect of hematocrit on platelet adhesion: experiments and simulations. Biophys J. 2016;111(3):577–88. https://doi.org/10.1016/j.bpj.2016.06.024.
Brown LM, Call MS, Margaret Knudson M, Cohen MJ, Trauma Outcomes G, Holcomb JB, Wade CE, Brasel KJ, Vercruysse G, MacLeod J, Dutton RP, Hess JR, Duchesne JC, McSwain NE, Muskat P, Johannigamn J, Cryer HM, Tillou A, Pittet JF, De Moya MA, Schreiber MA, Tieu B, Brundage S, Napolitano LM, Brunsvold M, Brunsvold M, Beilman G, Peitzman AB, Zenait MS, Sperry J, Alarcon L, Croce MA, Minei JP, Kozar R, Gonzalez EA, Stewart RM, Cohn SM, Mickalek JE, Bulger EM, Cotton BA, Nunez TC, Ivatury R, Meredith JW, Miller P, Pomper GJ, Marin B. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71(2 Suppl 3):S337–42. https://doi.org/10.1097/TA.0b013e318227f67c.
Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, Nelson MF, Cohen MJ. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–9. https://doi.org/10.1097/TA.0b013e318256deab.
Tisherman SA, Alam HB, Rhee PM, Scalea TM, Drabek T, Forsythe RM, Kochanek PM. Development of the emergency preservation and resuscitation for cardiac arrest from trauma clinical trial. J Trauma Acute Care Surg. 2017;83(5):803–9. https://doi.org/10.1097/TA.0000000000001585.
Roche AM, James MF, Bennett-Guerrero E, Mythen MG. A head-to-head comparison of the in vitro coagulation effects of saline-based and balanced electrolyte crystalloid and colloid intravenous fluids. Anesth Analg. 2006;102(4):1274–9. https://doi.org/10.1213/01.ane.0000197694.48429.94.
Maegele M, Schochl H, Cohen MJ. An update on the coagulopathy of trauma. Shock. 2014;41(Suppl 1):21–5. https://doi.org/10.1097/SHK.0000000000000088.
Reynolds BR, Forsythe RM, Harbrecht BG, Cuschieri J, Minei JP, Maier RV, Moore EE, Billiar EE, Peitzman AB, Sperry JL, Inflammation, Host Response to Injury I. Hypothermia in massive transfusion: have we been paying enough attention to it? J Trauma Acute Care Surg. 2012;73(2):486–91.
Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J Trauma. 1997;42(5):857–61. https://doi.org/10.1097/00005373-199705000-00016. (discussion 61–2).
Aukema TS, Beenen LF, Hietbrink F, Leenen LP. Initial assessment of chest X-ray in thoracic trauma patients: awareness of specific injuries. World J Radiol. 2012;4(2):48–52. https://doi.org/10.4329/wjr.v4.i2.48.
Langdorf MI, Medak AJ, Hendey GW, Nishijima DK, Mower WR, Raja AS, Baumann BM, Anglin DR, Anderson CL, Lotfipour S, Reed KE, Zuabi N, Khan NA, Bithell CA, Rowther AA, Villar J, Rodriguez RM. Prevalence and Clinical import of thoracic injury identified by chest computed tomography but not chest radiography in blunt trauma: multicenter prospective cohort study. Ann Emerg Med. 2015;66(6):589–600. https://doi.org/10.1016/j.annemergmed.2015.06.003.
Guillamondegui OD, Pryor JP, Gracias VH, Gupta R, Reilly PM, Schwab CW. Pelvic radiography in blunt trauma resuscitation: a diminishing role. J Trauma. 2002;53(6):1043–7. https://doi.org/10.1097/00005373-200212000-00002.
Fang JF, Wong YC, Lin BC, Hsu YP, Chen MF. Usefulness of multidetector computed tomography for the initial assessment of blunt abdominal trauma patients. World J Surg. 2006;30(2):176–82. https://doi.org/10.1007/s00268-005-0194-7.
Cinquantini F, Tugnoli G, Piccinini A, Coniglio C, Mannone S, Biscardi A, Gordini G, Di Saverio S. Educational review of predictive value and findings of computed tomography scan in diagnosing bowel and mesenteric injuries after blunt trauma: correlation with trauma surgery findings in 163 patients. Can Assoc Radiol J. 2017;68(3):276–85. https://doi.org/10.1016/j.carj.2016.07.003.
Baxi AJ, Restrepo C, Mumbower A, McCarthy M, Rashmi K. Cardiac Injuries: a review of multidetector computed tomography findings. Trauma Mon. 2015;20(4):e19086. https://doi.org/10.5812/traumamon.19086.
Raniga SB, Mittal AK, Bernstein M, Skalski MR, Al-Hadidi AM. Multidetector CT in vascular injuries resulting from pelvic fractures: a primer for diagnostic radiologists. Radiographics. 2019;39(7):2111–29. https://doi.org/10.1148/rg.2019190062.
American Institute of Ultrasound in M, American College of Emergency P. AIUM practice guideline for the performance of the focused assessment with sonography for trauma (FAST) examination. J Ultrasound Med. 2014;33(11):2047–56. https://doi.org/10.7863/ultra.33.11.2047.
American College of Emergency P. Emergency ultrasound imaging criteria compendium. American College of Emergency Physicians. Ann Emerg Med. 2006;48(4):487–510. https://doi.org/10.1016/j.annemergmed.2006.07.946.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, Hung J, Garcia MJ, Kronzon I, Oh JK, Rodriguez ER, Schaff HV, Schoenhagen P, Tan CD, White RD. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26(9):965-1012 e15. https://doi.org/10.1016/j.echo.2013.06.023.
Dolich MO, McKenney MG, Varela JE, Compton RP, McKenney KL, Cohn SM. 2,576 ultrasounds for blunt abdominal trauma. J Trauma. 2001;50(1):108–12. https://doi.org/10.1097/00005373-200101000-00019.
Zhou J, Huang J, Wu H, Jiang H, Zhang H, Prasoon P, Xu Y, Bai Y, Qiu J, Zeng Y. Screening ultrasonography of 2,204 patients with blunt abdominal trauma in the Wenchuan earthquake. J Trauma Acute Care Surg. 2012;73(4):890–4. https://doi.org/10.1097/TA.0b013e318256dfe1.
Chan KK, Joo DA, McRae AD, Takwoingi Y, Premji ZA, Lang E, Wakai A. Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. Cochrane Database Syst Rev. 2020;7:013031. https://doi.org/10.1002/14651858.CD013031.pub2.
Brooks A, Davies B, Smethhurst M, Connolly J. Emergency ultrasound in the acute assessment of haemothorax. Emerg Med J. 2004;21(1):44–6. https://doi.org/10.1136/emj.2003.005438.
Holmes JF, Harris D, Battistella FD. Performance of abdominal ultrasonography in blunt trauma patients with out-of-hospital or emergency department hypotension. Ann Emerg Med. 2004;43(3):354–61. https://doi.org/10.1016/j.annemergmed.2003.09.011.
Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14(3):181–5. https://doi.org/10.1007/s10140-007-0602-4.
Lyon M, Blaivas M, Brannam L. Sonographic measurement of the inferior vena cava as a marker of blood loss. Am J Emerg Med. 2005;23(1):45–50. https://doi.org/10.1016/j.ajem.2004.01.004.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010.
Brennan JM, Ronan A, Goonewardena S, Blair JE, Hammes M, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1(4):749–53. https://doi.org/10.2215/CJN.00310106.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G, American Society of Echocardiography's G, Standards C, European Association of E. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. https://doi.org/10.1016/j.echo.2005.10.005.
Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, Troughton RW. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28(1):40–56. https://doi.org/10.1016/j.echo.2014.09.009.
Ristow B, Na B, Ali S, Whooley MA, Schiller NB. Left ventricular outflow tract and pulmonary artery stroke distances independently predict heart failure hospitalization and mortality: the Heart and Soul Study. J Am Soc Echocardiogr. 2011;24(5):565–72. https://doi.org/10.1016/j.echo.2010.12.024.
Chernock B, Anjaria D, Traba C, Chen S, Nasser W, Fox A, Holland B, Lamba S. Integrating the Bleeding Control Basic course into medical school curriculum. Am J Surg. 2020;219(4):660–4. https://doi.org/10.1016/j.amjsurg.2019.04.023.
Johansson J, Blomberg H, Svennblad B, Wernroth L, Melhus H, Byberg L, Michaelsson K, Karlsten R, Gedeborg R. Prehospital Trauma Life Support (PHTLS) training of ambulance caregivers and impact on survival of trauma victims. Resuscitation. 2012;83(10):1259–64. https://doi.org/10.1016/j.resuscitation.2012.02.018.
Dennis BM, Vella MA, Gunter OL, Smith MD, Wilson CS, Patel MB, Nunez TC, Guillamondegui OD. Rural Trauma Team Development Course decreases time to transfer for trauma patients. J Trauma Acute Care Surg. 2016;81(4):632–7. https://doi.org/10.1097/TA.0000000000001188.
Eilertsen KA, Winberg M, Jeppesen E, Hval G, Wisborg T. Prehospital tourniquets in civilians: a systematic review. Prehosp Disaster Med. 2021;36(1):86–94. https://doi.org/10.1017/S1049023X20001284.
Schroll R, Smith A, McSwain NE Jr, Myers J, Rocchi K, Inaba K, Siboni S, Vercruysse GA, Ibrahim-Zada I, Sperry JL, Martin-Gill C, Cannon JW, Holland SR, Schreiber MA, Lape D, Eastman AL, Stebbins CS, Ferrada P, Han J, Meade P, Duchesne JC. A multi-institutional analysis of prehospital tourniquet use. J Trauma Acute Care Surg. 2015;79(1):10–4. https://doi.org/10.1097/TA.0000000000000689. (discussion 4).
Schweigkofler U, Wohlrath B, Trentzsch H, Horas K, Hoffmann R, Wincheringer D. Is there any benefit in the pre-hospital application of pelvic binders in patients with suspected pelvic injuries? Eur J Trauma Emerg Surg. 2021;47(2):493–8. https://doi.org/10.1007/s00068-019-01239-6.
Pierrie SN, Seymour RB, Wally MK, Studnek J, Infinger A, Hsu JR, Evidence-based Musculoskeletal I, Trauma C. Pilot randomized trial of pre-hospital advanced therapies for the control of hemorrhage (PATCH) using pelvic binders. Am J Emerg Med. 2021;42:43–8. https://doi.org/10.1016/j.ajem.2020.12.082.
Bulger EM, Snyder D, Schoelles K, Gotschall C, Dawson D, Lang E, Sanddal ND, Butler FK, Fallat M, Taillac P, White L, Salomone JP, Seifarth W, Betzner MJ, Johannigman J, McSwain N Jr. An evidence-based prehospital guideline for external hemorrhage control: American College of Surgeons Committee on Trauma. Prehosp Emerg Care. 2014;18(2):163–73. https://doi.org/10.3109/10903127.2014.896962.
Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251(2):217–28. https://doi.org/10.1097/SLA.0b013e3181c3bcca.
Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9. https://doi.org/10.1056/NEJM199410273311701.
Schreiber MA, Meier EN, Tisherman SA, Kerby JD, Newgard CD, Brasel K, Egan D, Witham W, Williams C, Daya M, Beeson J, McCully BH, Wheeler S, Kannas D, May S, McKnight B, Hoyt DB, Investigators ROC. A controlled resuscitation strategy is feasible and safe in hypotensive trauma patients: results of a prospective randomized pilot trial. J Trauma Acute Care Surg. 2015;78(4):687–95. https://doi.org/10.1097/TA.0000000000000600. (discussion 95–7).
Butcher I, Maas AI, Lu J, Marmarou A, Murray GD, Mushkudiani NA, McHugh GS, Steyerberg EW. Prognostic value of admission blood pressure in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):294–302. https://doi.org/10.1089/neu.2006.0032.
Fuller G, Hasler RM, Mealing N, Lawrence T, Woodford M, Juni P, Lecky F. The association between admission systolic blood pressure and mortality in significant traumatic brain injury: a multi-centre cohort study. Injury. 2014;45(3):612–7. https://doi.org/10.1016/j.injury.2013.09.008.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the management of severe traumatic brain injury. Fourth Edition Neurosurg. 2017;80(1):6–15. https://doi.org/10.1227/NEU.0000000000001432.
Picetti E, Rossi S, Abu-Zidan FM, Ansaloni L, Armonda R, Baiocchi GL, Bala M, Balogh ZJ, Berardino M, Biffl WL, Bouzat P, Buki A, Ceresoli M, Chesnut RM, Chiara O, Citerio G, Coccolini F, Coimbra R, Di Saverio S, Fraga GP, Gupta D, Helbok R, Hutchinson PJ, Kirkpatrick AW, Kinoshita T, Kluger Y, Leppaniemi A, Maas AIR, Maier RV, Minardi F, Moore EE, Myburgh JA, Okonkwo DO, Otomo Y, Rizoli S, Rubiano AM, Sahuquillo J, Sartelli M, Scalea TM, Servadei F, Stahel PF, Stocchetti N, Taccone FS, Tonetti T, Velmahos G, Weber D, Catena F. WSES consensus conference guidelines: monitoring and management of severe adult traumatic brain injury patients with polytrauma in the first 24 hours. World J Emerg Surg. 2019;14:53. https://doi.org/10.1186/s13017-019-0270-1.
Heffernan DS, Thakkar RK, Monaghan SF, Ravindran R, Adams CA Jr, Kozloff MS, Gregg SC, Connolly MD, Machan JT, Cioffi WG. Normal presenting vital signs are unreliable in geriatric blunt trauma victims. J Trauma. 2010;69(4):813–20. https://doi.org/10.1097/TA.0b013e3181f41af8.
Neideen T, Lam M, Brasel KJ. Preinjury beta blockers are associated with increased mortality in geriatric trauma patients. J Trauma. 2008;65(5):1016–20. https://doi.org/10.1097/TA.0b013e3181897eac.
Ahmed N, Kuo YH. Early risk stratification of mortality in the geriatric patients who are at high risk for bleeding and fall from a ground level: an analysis of the national data. J Inj Violence Res. 2022;14(3):173–81. https://doi.org/10.5249/jivr.v14i3.1628.
Grossman MD, Miller D, Scaff DW, Arcona S. When is an elder old? Effect of preexisting conditions on mortality in geriatric trauma. J Trauma. 2002;52(2):242–6. https://doi.org/10.1097/00005373-200202000-00007.
Sasser SM, Hunt RC, Faul M, Sugerman D, Pearson WS, Dulski T, Wald MM, Jurkovich GJ, Newgard CD, Lerner EB, Centers for Disease C, Prevention. Guidelines for field triage of injured patients: recommendations of the National Expert Panel on Field Triage, 2011. MMWR Recomm Rep. 2012;61(RR-1):1–20.
Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, Gurney J, Butler FK Jr, Gross K, Stockinger ZT. Association of prehospital blood product transfusion during medical evacuation of combat casualties in Afghanistan with acute and 30-day survival. JAMA. 2017;318(16):1581–91. https://doi.org/10.1001/jama.2017.15097.
Crombie N, Doughty HA, Bishop JRB, Desai A, Dixon EF, Hancox JM, Herbert MJ, Leech C, Lewis SJ, Nash MR, Naumann DN, Slinn G, Smith H, Smith IM, Wale RK, Wilson A, Ives N, Perkins GD, Re Pcg. Resuscitation with blood products in patients with trauma-related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Haematol. 2022;9(4):e250–e61. https://doi.org/10.1016/S2352-3026(22)00040-0.
Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Witham WR, Putnam AT, Duane TM, Alarcon LH, Callaway CW, Zuckerbraun BS, Neal MD, Rosengart MR, Forsythe RM, Billiar TR, Yealy DM, Peitzman AB, Zenati MS, Group PAS. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315-26. https://doi.org/10.1056/NEJMoa1802345
Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, Chin T, Burlew CC, Pieracci F, West FB, Fleming CD, Ghasabyan A, Chandler J, Silliman CC, Banerjee A, Sauaia A. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392(10144):283–91. https://doi.org/10.1016/S0140-6736(18)31553-8.
Pusateri AE, Moore EE, Moore HB, Le TD, Guyette FX, Chapman MP, Sauaia A, Ghasabyan A, Chandler J, McVaney K, Brown JB, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA, Witham WR, Putnam AT, Sperry JL. Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: a post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg. 2020;155(2):e195085. https://doi.org/10.1001/jamasurg.2019.5085.
Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, Ramos M, Cairns J, Guerriero C. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1–79. https://doi.org/10.3310/hta17100.
Peng HT. Hemostatic agents for prehospital hemorrhage control: a narrative review. Mil Med Res. 2020;7(1):13. https://doi.org/10.1186/s40779-020-00241-z.
Haverkamp FJC, Giesbrecht GG, Tan E. The prehospital management of hypothermia—an up-to-date overview. Injury. 2018;49(2):149–64. https://doi.org/10.1016/j.injury.2017.11.001.
Kotwal RS, Howard JT, Orman JA, Tarpey BW, Bailey JA, Champion HR, Mabry RL, Holcomb JB, Gross KR. The effect of a golden hour policy on the morbidity and mortality of combat casualties. JAMA Surg. 2016;151(1):15–24. https://doi.org/10.1001/jamasurg.2015.3104.
Shackelford SA, Del Junco DJ, Riesberg JC, Powell D, Mazuchowski EL, Kotwal RS, Loos PE, Montgomery HR, Remley MA, Gurney JM, Keenan S. Case-control analysis of prehospital death and prolonged field care survival during recent US military combat operations. J Trauma Acute Care Surg. 2021;91(2S Suppl 2):S186–93. https://doi.org/10.1097/TA.0000000000003252.
Georgiou A, Lockey DJ. The performance and assessment of hospital trauma teams. Scand J Trauma Resusc Emerg Med. 2010;18:66. https://doi.org/10.1186/1757-7241-18-66.
Ferrada P, Vanguri P, Anand RJ, Whelan J, Duane T, Aboutanos M, Malhotra A, Ivatury R. A, B, C, D, echo: limited transthoracic echocardiogram is a useful tool to guide therapy for hypotension in the trauma bay–a pilot study. J Trauma Acute Care Surg. 2013;74(1):220–3. https://doi.org/10.1097/TA.0b013e318278918a.
Jacobs DG, Plaisier BR, Barie PS, Hammond JS, Holevar MR, Sinclair KE, Scalea TM, Wahl W, Group EPMGW. Practice management guidelines for geriatric trauma: the EAST Practice Management Guidelines Work Group. J Trauma. 2003;54(2):391–416. https://doi.org/10.1097/01.TA.0000042015.54022.BE.
Dunning J, Williamson J. Ultrasonic guidance and the complications of central line placement in the emergency department. Emerg Med J. 2003;20(6):551–2. https://doi.org/10.1136/emj.20.6.551.
Lee MM, Anand S, Loyd JW. Saphenous Vein Cutdown. StatPearls. Treasure Island (FL) 2021.
Tyler JA, Perkins Z, De’Ath HD. Intraosseous access in the resuscitation of trauma patients: a literature review. Eur J Trauma Emerg Surg. 2021;47(1):47–55. https://doi.org/10.1007/s00068-020-01327-y.
Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, Barnett CC, Bensard DD, Biffl WL, Burlew CC, Johnson JL, Pieracci FM, Jurkovich GJ, Banerjee A, Silliman CC, Sauaia A. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–9. https://doi.org/10.1097/SLA.0000000000001608.
Kornblith LZ, Howard BM, Cheung CK, Dayter Y, Pandey S, Busch MP, Pati S, Callcut RA, Vilardi RF, Redick BJ, Nelson MF, Cohen MJ. The whole is greater than the sum of its parts: hemostatic profiles of whole blood variants. J Trauma Acute Care Surg. 2014;77(6):818–27. https://doi.org/10.1097/TA.0000000000000354.
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, Cohen MJ, Cotton BA, Fabian TC, Inaba K, Kerby JD, Muskat P, O'Keeffe T, Rizoli S, Robinson BR, Scalea TM, Schreiber MA, Stein DM, Weinberg JA, Callum JL, Hess JR, Matijevic N, Miller CN, Pittet JF, Hoyt DB, Pearson GD, Leroux B, van Belle G, Group PS. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-82. https://doi.org/10.1001/jama.2015.12
Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. https://doi.org/10.1097/TA.0b013e3181271ba3.
Taeuber I, Weibel S, Herrmann E, Neef V, Schlesinger T, Kranke P, Messroghli L, Zacharowski K, Choorapoikayil S, Meybohm P. Association of intravenous tranexamic acid with thromboembolic events and mortality: a systematic review, meta-analysis, and meta-regression. JAMA Surg. 2021. https://doi.org/10.1001/jamasurg.2021.0884.
MacKay EJ, Stubna MD, Holena DN, Reilly PM, Seamon MJ, Smith BP, Kaplan LJ, Cannon JW. Abnormal calcium levels during trauma resuscitation are associated with increased mortality, increased blood product use, and greater hospital resource consumption: a pilot investigation. Anesth Analg. 2017;125(3):895–901. https://doi.org/10.1213/ANE.0000000000002312.
Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, Dubose JJ, Fox EE, Inaba K, Rodriguez CJ, Holcomb JB, Duchesne JC. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–17. https://doi.org/10.1097/TA.0000000000001333.
Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, Cotton BA, Matijevic N, Muskat P, Myers JG, Phelan HA, White CE, Zhang J, Rahbar MH, Group PS. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127-36. https://doi.org/10.1001/2013.jamasurg.387
Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58. https://doi.org/10.1097/SLA.0b013e318185a9ad.
Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. J Trauma. 1971;11(4):275–85. https://doi.org/10.1097/00005373-197104000-00001.
Shea SM, Staudt AM, Thomas KA, Schuerer D, Mielke JE, Folkerts D, Lowder E, Martin C, Bochicchio GV, Spinella PC. The use of low-titer group O whole blood is independently associated with improved survival compared to component therapy in adults with severe traumatic hemorrhage. Transfusion. 2020;60(Suppl 3):S2–9. https://doi.org/10.1111/trf.15696.
Byerly S, Inaba K, Biswas S, Wang E, Wong MD, Shulman I, Benjamin E, Lam L, Demetriades D. Transfusion-related hypocalcemia after trauma. World J Surg. 2020;44(11):3743–50. https://doi.org/10.1007/s00268-020-05712-x.
Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Valdivia A, Sailors RM, Moore FA. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138(6):637–42. https://doi.org/10.1001/archsurg.138.6.637. (discussion 42–3).
Jones DG, Nantais J, Rezende-Neto JB, Yazdani S, Vegas P, Rizoli S. Crystalloid resuscitation in trauma patients: deleterious effect of 5L or more in the first 24h. BMC Surg. 2018;18(1):93. https://doi.org/10.1186/s12893-018-0427-y.
Zitek T, Ataya R, Farino L, Mohammed S, Miller G. Is the use of greater than 1 L of intravenous crystalloids associated with worse outcomes in trauma patients? Am J Emerg Med. 2021;40:32–6. https://doi.org/10.1016/j.ajem.2020.12.013.
Johnson JW, Gracias VH, Schwab CW, Reilly PM, Kauder DR, Shapiro MB, Dabrowski GP, Rotondo MF. Evolution in damage control for exsanguinating penetrating abdominal injury. J Trauma. 2001;51(2):261–9. https://doi.org/10.1097/00005373-200108000-00007. (discussion 9-71).
Meizoso JP, Ray JJ, Karcutskie CAT, Allen CJ, Zakrison TL, Pust GD, Koru-Sengul T, Ginzburg E, Pizano LR, Schulman CI, Livingstone AS, Proctor KG, Namias N. Effect of time to operation on mortality for hypotensive patients with gunshot wounds to the torso: the golden 10 minutes. J Trauma Acute Care Surg. 2016;81(4):685–91. https://doi.org/10.1097/TA.0000000000001198.
Tesoriero RB, Bruns BR, Narayan M, Dubose J, Guliani SS, Brenner ML, Boswell S, Stein DM, Scalea TM. Angiographic embolization for hemorrhage following pelvic fracture: is it “time” for a paradigm shift? J Trauma Acute Care Surg. 2017;82(1):18–26. https://doi.org/10.1097/TA.0000000000001259.
Clarke DL, Gall TM, Thomson SR. Double jeopardy revisited: clinical decision making in unstable patients with, thoraco-abdominal stab wounds and potential injuries in multiple body cavities. Injury. 2011;42(5):478–81. https://doi.org/10.1016/j.injury.2010.06.027.
Karmy-Jones R, Jurkovich GJ, Nathens AB, Shatz DV, Brundage S, Wall MJ Jr, Engelhardt S, Hoyt DB, Holcroft J, Knudson MM. Timing of urgent thoracotomy for hemorrhage after trauma: a multicenter study. Arch Surg. 2001;136(5):513–8. https://doi.org/10.1001/archsurg.136.5.513.
Mowery NT, Gunter OL, Collier BR, Diaz JJ Jr, Haut E, Hildreth A, Holevar M, Mayberry J, Streib E. Practice management guidelines for management of hemothorax and occult pneumothorax. J Trauma. 2011;70(2):510–8. https://doi.org/10.1097/TA.0b013e31820b5c31.
Clarke JR, Trooskin SZ, Doshi PJ, Greenwald L, Mode CJ. Time to laparotomy for intra-abdominal bleeding from trauma does affect survival for delays up to 90 minutes. J Trauma. 2002;52(3):420–5. https://doi.org/10.1097/00005373-200203000-00002.
Harvin JA, Maxim T, Inaba K, Martinez-Aguilar MA, King DR, Choudhry AJ, Zielinski MD, Akinyeye S, Todd SR, Griffin RL, Kerby JD, Bailey JA, Livingston DH, Cunningham K, Stein DM, Cattin L, Bulger EM, Wilson A, Undurraga Perl VJ, Schreiber MA, Cherry-Bukowiec JR, Alam HB, Holcomb JB. Mortality after emergent trauma laparotomy: a multicenter, retrospective study. J Trauma Acute Care Surg. 2017;83(3):464–8. https://doi.org/10.1097/TA.0000000000001619.
Nishijima DK, Simel DL, Wisner DH, Holmes JF. Does this adult patient have a blunt intra-abdominal injury? JAMA. 2012;307(14):1517–27. https://doi.org/10.1001/jama.2012.422.
Dharap SB, Noronha J, Kumar V. Laparotomy for blunt abdominal trauma-some uncommon indications. J Emerg Trauma Shock. 2016;9(1):32–6. https://doi.org/10.4103/0974-2700.173866.
Biffl WL, Moore EE. Management guidelines for penetrating abdominal trauma. Curr Opin Crit Care. 2010;16(6):609–17. https://doi.org/10.1097/MCC.0b013e32833f52d2.
Biffl WL, Kaups KL, Cothren CC, Brasel KJ, Dicker RA, Bullard MK, Haan JM, Jurkovich GJ, Harrison P, Moore FO, Schreiber M, Knudson MM, Moore EE. Management of patients with anterior abdominal stab wounds: a Western Trauma Association multicenter trial. J Trauma. 2009;66(5):1294–301. https://doi.org/10.1097/TA.0b013e31819dc688.
Navsaria PH, Nicol AJ, Edu S, Gandhi R, Ball CG. Selective nonoperative management in 1106 patients with abdominal gunshot wounds: conclusions on safety, efficacy, and the role of selective CT imaging in a prospective single-center study. Ann Surg. 2015;261(4):760–4. https://doi.org/10.1097/SLA.0000000000000879.
Stassen NA, Bhullar I, Cheng JD, Crandall ML, Friese RS, Guillamondegui OD, Jawa RS, Maung AA, Rohs TJ, Jr., Sangosanya A, Schuster KM, Seamon MJ, Tchorz KM, Zarzuar BL, Kerwin AJ, Eastern Association for the Surgery of T. Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S294–300. https://doi.org/10.1097/TA.0b013e3182702afc.
Stassen NA, Bhullar I, Cheng JD, Crandall M, Friese R, Guillamondegui O, Jawa R, Maung A, Rohs TJ, Jr., Sangosanya A, Schuster K, Seamon M, Tchorz KM, Zarzuar BL, Kerwin A, Eastern Association for the Surgery of T. Nonoperative management of blunt hepatic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S288–93. https://doi.org/10.1097/TA.0b013e318270160d.
Como JJ, Bokhari F, Chiu WC, Duane TM, Holevar MR, Tandoh MA, Ivatury RR, Scalea TM. Practice management guidelines for selective nonoperative management of penetrating abdominal trauma. J Trauma. 2010;68(3):721–33. https://doi.org/10.1097/TA.0b013e3181cf7d07.
Inaba K, Branco BC, Moe D, Barmparas G, Okoye O, Lam L, Talving P, Demetriades D. Prospective evaluation of selective nonoperative management of torso gunshot wounds: when is it safe to discharge? J Trauma Acute Care Surg. 2012;72(4):884–91. https://doi.org/10.1097/TA.0b013e31824d1068.
Cullinane DC, Schiller HJ, Zielinski MD, Bilaniuk JW, Collier BR, Como J, Holevar M, Sabater EA, Sems SA, Vassy WM, Wynne JL. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture–update and systematic review. J Trauma. 2011;71(6):1850–68. https://doi.org/10.1097/TA.0b013e31823dca9a.
Costantini TW, Coimbra R, Holcomb JB, Podbielski JM, Catalano R, Blackburn A, Scalea TM, Stein DM, Williams L, Conflitti J, Keeney S, Suleiman G, Zhou T, Sperry J, Skiada D, Inaba K, Williams BH, Minei JP, Privette A, Mackersie RC, Robinson BR, Moore FO, Group APFS. Current management of hemorrhage from severe pelvic fractures: results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg. 2016;80(5):717–23; https://doi.org/10.1097/TA.0000000000001034. (discussion 23–5(.
Cryer HM, Miller FB, Evers BM, Rouben LR, Seligson DL. Pelvic fracture classification: correlation with hemorrhage. J Trauma. 1988;28(7):973–80.
Cothren CC, Osborn PM, Moore EE, Morgan SJ, Johnson JL, Smith WR. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: a paradigm shift. J Trauma. 2007;62(4):834–9. https://doi.org/10.1097/TA.0b013e31803c7632. (discussion 9–42).
Morrison JJ, Ross JD, Houston RT, Watson JD, Sokol KK, Rasmussen TE. Use of resuscitative endovascular balloon occlusion of the aorta in a highly lethal model of noncompressible torso hemorrhage. Shock. 2014;41(2):130–7. https://doi.org/10.1097/SHK.0000000000000085.
Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, Rasmussen TE. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013;153(6):848–56. https://doi.org/10.1016/j.surg.2012.12.001.
Brenner M, Inaba K, Aiolfi A, DuBose J, Fabian T, Bee T, Holcomb JB, Moore L, Skarupa D, Scalea TM, Group AAS. Resuscitative endovascular balloon occlusion of the aorta and resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the American association for the surgery of trauma's aortic occlusion in resuscitation for trauma and acute care surgery registry. J Am Coll Surg. 2018;226(5):730-40. https://doi.org/10.1016/j.jamcollsurg.2018.01.044
Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Ishida T, Okada Y, Kon Y, Oka K, Ishida K, Toyoda Y, Funabiki T, Investigators D-I. Early arterial access for resuscitative endovascular balloon occlusion of the aorta is related to survival outcome in trauma. J Trauma Acute Care Surg. 2018;85(3):507–11. https://doi.org/10.1097/TA.0000000000002004.
Borger van der Burg BLS, Kessel B, DuBose JJ, Horer TM, Hoencamp R. Consensus on resuscitative endovascular balloon occlusion of the Aorta: a first consensus paper using a Delphi method. Injury. 2019;50(6):1186–91. https://doi.org/10.1016/j.injury.2019.04.024.
Daniel L, Christopher M, Dominic F, Kaitlin M, Jason B, Matthew M, Matthew E, Jason P. Partial resuscitative endovascular balloon occlusion of the aorta via the tri-lobe balloon catheter. J Surg Res. 2021;260:20–7. https://doi.org/10.1016/j.jss.2020.11.056.
Langer V. Management of major limb injuries. ScientificWorldJournal. 2014;2014:640430. https://doi.org/10.1155/2014/640430.
Tan TW, Joglar FL, Hamburg NM, Eberhardt RT, Shaw PM, Rybin D, Doros G, Farber A. Limb outcome and mortality in lower and upper extremity arterial injury: a comparison using the National Trauma Data Bank. Vasc Endovasc Surg. 2011;45(7):592–7. https://doi.org/10.1177/1538574411415125.
Arne BC. Management of scalp hemorrhage and lacerations. J Spec Oper Med. 2012;12(1):11–6.
Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197(5):532–5. https://doi.org/10.1097/00000658-198305000-00005.
Rotondo MF, Schwab CW, McGonigal MD, Phillips GR 3rd, Fruchterman TM, Kauder DR, Latenser BA, Angood PA. “Damage control”: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375–82 (discussion 82–3).
Duchesne JC, Kimonis K, Marr AB, Rennie KV, Wahl G, Wells JE, Islam TM, Meade P, Stuke L, Barbeau JM, Hunt JP, Baker CC, McSwain NE Jr. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma. 2010;69(1):46–52. https://doi.org/10.1097/TA.0b013e3181df91fa.
Abou-Khalil B, Scalea TM, Trooskin SZ, Henry SM, Hitchcock R. Hemodynamic responses to shock in young trauma patients: need for invasive monitoring. Crit Care Med. 1994;22(4):633–9. https://doi.org/10.1097/00003246-199404000-00020.
Tisherman SA, Barie P, Bokhari F, Bonadies J, Daley B, Diebel L, Eachempati SR, Kurek S, Luchette F, Carlos Puyana J, Schreiber M, Simon R. Clinical practice guideline: endpoints of resuscitation. J Trauma. 2004;57(4):898–912. https://doi.org/10.1097/01.ta.0000133577.25793.e5.
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176–86. https://doi.org/10.1378/chest.94.6.1176.
Velmahos GC, Demetriades D, Shoemaker WC, Chan LS, Tatevossian R, Wo CC, Vassiliu P, Cornwell EE 3rd, Murray JA, Roth B, Belzberg H, Asensio JA, Berne TV. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Ann Surg. 2000;232(3):409–18. https://doi.org/10.1097/00000658-200009000-00013.
Tyburski JG, Collinge JD, Wilson RF, Carlin AM, Albaran RG, Steffes CP. End-tidal CO2-derived values during emergency trauma surgery correlated with outcome: a prospective study. J Trauma. 2002;53(4):738–43. https://doi.org/10.1097/00005373-200210000-00020.
Ivatury RR, Simon RJ, Islam S, Fueg A, Rohman M, Stahl WM. A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg. 1996;183(2):145–54.
Weil MH, Nakagawa Y, Tang W, Sato Y, Ercoli F, Finegan R, Grayman G, Bisera J. Sublingual capnometry: a new noninvasive measurement for diagnosis and quantitation of severity of circulatory shock. Crit Care Med. 1999;27(7):1225–9. https://doi.org/10.1097/00003246-199907000-00001.
Povoas HP, Weil MH, Tang W, Sun S, Kamohara T, Bisera J. Decreases in mesenteric blood flow associated with increases in sublingual PCO2 during hemorrhagic shock. Shock. 2001;15(5):398–402. https://doi.org/10.1097/00024382-200115050-00011.
Tatevossian RG, Wo CC, Velmahos GC, Demetriades D, Shoemaker WC. Transcutaneous oxygen and CO2 as early warning of tissue hypoxia and hemodynamic shock in critically ill emergency patients. Crit Care Med. 2000;28(7):2248–53. https://doi.org/10.1097/00003246-200007000-00011.
Torella F, Cowley RD, Thorniley MS, McCollum CN. Regional tissue oxygenation during hemorrhage: can near infrared spectroscopy be used to monitor blood loss? Shock. 2002;18(5):440–4. https://doi.org/10.1097/00024382-200211000-00009.
Acknowledgements
Not applicable.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
RKL conceived, design and wrote the manuscript, SPC participated in the design and helped to write the article, JAB helped design the manuscript and provided the figures and videos, RL participated in the design, draft and coordinated the work, MZH carried out the work of drawing the figures and tables, JH helped design the manuscript and provided the figures and videos, IF carried out the work of drawing the figures and tables, JRB participated in the design, draft and coordinated the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Video 1
. Subxiphoid view of the heart. Large pericardial effusion causing tamponade. L, liver; LV; left ventricle; PE, pericardial effusion; RV, right ventricle; T, thrombus.
Additional file 2. Video 2
. Right thoracic view at the diaphragm with a right hemothorax. Thoracic spine visualized above the diaphragm. Normally, the thoracic spine is obscured by air within the lung. HT, hemothorax; L, liver; D, diaphragm; TS, thoracic spine; SS, spine sign.
Additional file 3. Video 3
. Right upper quadrant viewof the abdomen. Anechoic hemoperitoneum in the hepatorenal space. HP, hemoperitoneum; L, liver; RL, right kidney.
Additional file 4. Video 4
. Left upper quadrant viewof the abdomen. Anechoic hemoperitoneum in the splenorenal space. HP, hemoperitoneum; LK, left kidney; S, spleen.
Additional file 5. Video 5.
Pelvic sagittal view. Anechoic hemoperitoneum cephalad and posterior to the bladder. HP, hemoperitoneum; B, bladder.
Additional file 6. Video 6.
Pelvic transverse view. Anechoic hemoperitoneum posterior to the bladder. HP, hemoperitoneum; B, bladder.
Additional file 7. Video 7
. TTE sagittal view of IVC long axis. IVC collapses > 50% with respiration provide insight into the fluid status of an adult trauma patient. IVC, inferior vena cava, HP, hepatic vein, RA, right atrium, L, liver.
Additional file 8. Video 8
. TEE transgastric short axis view.diastole;systole. Severe left ventricular hypovolemia and papillary muscle kissing sign during systole. LV, left ventricle; RV, right ventricle.
Additional file 9. Video 9
. Apical 4-chamber view. Intravascular volume status and function: Reduced fractional area change indicating both RV and LV dysfunction. LV, left ventricle; MV, mitral valve; RV, right ventricle.
Additional file 10: Table S2
. Classification of Hemorrhagic Shock.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Latif, R.K., Clifford, S.P., Baker, J.A. et al. Traumatic hemorrhage and chain of survival. Scand J Trauma Resusc Emerg Med 31, 25 (2023). https://doi.org/10.1186/s13049-023-01088-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-023-01088-8