Abstract
Background
There is little published data investigating non-invasive cardiac output monitoring in the emergency department (ED). We assess here the accuracy of five non-invasive methods in detecting fluid responsiveness in the ED: (1) common carotid artery blood flow, (2) suprasternal aortic Doppler, (3) bioreactance, (4) plethysmography with digital vascular unloading method, and (5) inferior vena cava collapsibility index. Left ventricular outflow tract echocardiography derived velocity time integral is the reference standard. This follows an assessment of feasibility and repeatability of these methods in the same cohort of ED patients.
Methods
This is a prospective observational study of non-invasive methods for assessing fluid responsiveness in the ED. Participants were non-ventilated ED adult patients requiring intravenous fluid resuscitation. Sensitivity and specificity of each method in determining the fluid responsiveness status of participants is determined in comparison to the reference standard.
Results
Thirty-three patient data sets were included for analysis. The specificity and sensitivity to detect fluid responders was 46.2 and 45% for common carotid artery blood flow (CCABF), 61.5 and 63.2% for suprasternal artery Doppler (SSAD), 46.2 and 50% for bioreactance, 50 and 41.2% for plethysmography vascular unloading technique (PVUT), and 63.6 and 47.4% for inferior vena cava collapsibility index (IVCCI), respectively. Analysis of agreement with Cohen’s Kappa − 0.08 for CCABF, 0.24 for SSAD, − 0.04 for bioreactance, − 0.08 for PVUT, and 0.1 for IVCCI.
Conclusion
In this study, non-invasive methods were not found to reliably identify fluid responders. Non-invasive methods of identifying fluid responders are likely to play a key role in improving patient outcome in the ED in fluid depleted states such as sepsis. These results have implications for future studies assessing the accuracy of such methods.
Similar content being viewed by others
Background
The aim of intravenous fluid therapy is to increase cardiac output and therefore to increase oxygen delivery to hypo-perfused organs. Resuscitation with inadequate intravenous fluid may risk inadequate organ oxygen delivery. Over resuscitation may precipitate iatrogenic tissue oedema leading to compartment syndromes and decreased oxygen delivery, with consequent increases in mortality [1,2,3]. Early-stage fluid resuscitation is commonly guided by physiological parameters such as capillary refill time, pulse rate, and blood pressure. Biochemical parameters such as lactate and acid-base assessment may also be used to guide fluid doses [4]. Neither approach offers a sensitive or specific proxy for organ perfusion, so in-precisely guiding fluid dosing [5,6,7,8,9].
Fluid responsiveness is commonly defined as a stroke volume increase of at least 10% following a fluid bolus of 200-500mls delivered over 10–15 min [10]. Around one to two thirds of emergency department (ED) patients thought to require fluid resuscitation are not fluid responsive and risk harm from aggressive fluid administration [11]. Identifying which patients respond to fluids (and continue to do so) has the potential to individualise fluid delivery and improve outcomes [12]. In intensive care units, invasive monitoring of cardiac output is frequently used to guide fluid administration, with methods including pulmonary artery catheterisation (PAC), arterial pulse pressure analysis or oesophageal Doppler [13]. However, these methods are invasive and unsuitable for routine monitoring in the ED [14, 15].
Non-invasive cardiac output monitoring methods are emerging in the ED and in the pre-hospital environment [16, 17]. These methods include common carotid artery blood flow monitoring (CCABF) [18], suprasternal aortic Doppler (SSAD) [19, 20], plethysmography using the vascular unloading technique (PVUT) [21, 22], and thoracic bioreactance [23,24,25]. Inferior vena cava collapsibility index (IVCCI), while not measuring cardiac output, has been studied as an indicator of fluid responsiveness [25,26,27]. Stroke volume measured by left ventricular outflow tract velocity time integral (LVOT VTI) [28, 29] is the most widely studied of these techniques, including validation against the PAC, which is widely referred to as the gold standard for cardiac output monitoring. LVOT VTI is widely accepted as a non-invasive method to assess stroke volume and cardiac output, and guide fluid therapy [30,31,32,33,34,35]. We thus arbitrarily assigned the LVOT VTI as reference standard in the current study whist being aware of its limitations in terms of operator experience/skill.
The clinical value of a diagnostic test is assessed by a multi-phase process which includes assessing its feasibility, repeatability, accuracy, impact on patient outcomes and cost. We have previously reported on the feasibility and repeatability of the above methods in these same cohort of ED patients [36]. We report here the diagnostic accuracy of these techniques determined by the sensitivity and specificity of each in identifying fluid responders as identified by LVOT VTI.
Methods
Study protocol
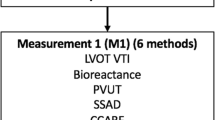
The study was a prospective observational diagnostic accuracy study. The Standards for Reporting of Diagnostic Accuracy Studies (STARD) were followed [37]. Accuracy was determined by sensitivity and specificity of each method in identifying fluid responders as determined by the assigned reference standard LVOT VTI. Participants were placed in a semi-recumbent position at 30 degrees on a trolley. The stroke volume was simultaneously measured by LVOT VTI, CCABF, bioreactance, and PVUT (measurement round 1 - M1) (Fig. 1). The IVCCI was also measured. A fluid bolus of 250–500 mls of crystalloid was then delivered over 15 min or less. A measurement margin of error of up to 10% in the estimated volume delivered, and up to 1 min in delivering the fluid bolus, was deemed acceptable. A post-fluid measurement round with all six methods was then conducted (M2). Fluid responsiveness was defined as a stroke volume increase of 10% or more from M1 to M2 by LVOT VTI using Dinh et al’s method [38]. Stolz et al’s method was used to measure CCABF [39]; an CCABF increase of 10% identified fluid responsiveness in this index test. The Fremantle criteria were used to measure stroke volume by SSAD [40]. IVCCI was measured in B mode with the minimal and maximal IVC diameters during respiration measured 1 cm distal to the hepatic-caval junction or 2-3 cm distal to the atrial-caval junction as guided by previous studies [26, 27, 41, 42]. IVCCI was calculated with the following formula:
Study participant pathway. SV = stroke volume; CO = cardiac output; PVUT = plethysmography vascular unloading technique; CCABF = common carotid artery blood flow; IVCCI = inferior vena cava collapsibility index; LVOT VTI = left ventricular outflow tract velocity time integral; SSAD = suprasternal aortic Doppler. Immediate intervention required signifies here a systolic blood pressure < 80 mmHg including traumatic or cardiogenic shock, and ventricular or supraventricular tachycardia)
Stroke volume by PVUT and bioreactance was measured following the manufacturer’s instructions.
Participants
All participants recruited for the previously reported feasibility and repeatability analysis were also considered for inclusion in the present method accuracy analysis [36]. Briefly, potential participants were screened for inclusion and exclusion criteria by the clinical team on arrival to the ED to minimise selection bias. Patients requiring immediate intervention were excluded (systolic blood pressure < 80 mmHg including traumatic or cardiogenic shock, and ventricular or supraventricular tachycardia). All eligible patients were then referred to the research team for consent. All patients attending the ED during the study period during the hours of 09:00 to 20:00 Monday to Friday between August to October were eligible for recruitment. Patients having received fluids in a pre-hospital setting were not excluded from the study.
Equipment
LVOT VTI was measured with a uSmart 3300 ultrasound system (Terason, Burlington, MA, USA). Carotid Doppler traces were assessed by a Sonosite EDGE (Sonosite, Bothwell, WA, USA). Suprasternal aortic Doppler traces were obtained using the USCOM-1A (Pty Ltd., Coffs Harbour, NSW, Australia). PVUT was assessed with a LiDCO continuous non-invasive arterial pressure device (LiDCO plus and CNAP, LiDCO Ltd., London, UK). Bioreactance was assessed with a Cheetah Medical device (Cheetah Medical, Portland, OR, USA).
Operator training
Three operators (DM, SG, SS) with no prior ultrasound experience were trained to operate all six non-invasive monitoring methods during the same pre-study training programme as previously described [36]. Briefly, this consisted of the standard UK Level 1 ultrasound course followed by 50 measurements for all four ultrasound methods on volunteers. Competency for each method was confirmed through triggered assessment by the regional Royal College of Emergency Medicine lead for ultrasound training. Bioreactance and PVUT training was provided by the respective manufacturers for 2 h each. This training programme ensured that operators’ experience on all methods was identical and achievable by junior residents and ED nurses. Interobserver reliability for LVOT VTI amongst operators was satisfactory and reported in a separate study [43].
Statistical analysis
Measurement data were collected onto a REDCap database (Vanderbilt University, Nashville, TN, USA) and analysed with SPSS v24 (IBM, New York City, NY, USA) and STATA 15.1 (StataCorp, College Station, TX, US). P < 0.05 (two-tailed) was considered statistically significant. To compare proportions of fluid responders with an expected sensitivity of at least 0.80 for all index tests and a minimum acceptable lower confidence limit of 0.50, an a priori minimum sample size of 28 was required [44]. Mann-Whitney, Chi square and Fisher exact tests were used where appropriate. Agreement between the reference standard and the index tests was also analysed with Cohen’s kappa (k) as described in previous studies of cardiac output/stroke volume assessment methods [45,46,47]. A kappa value of 1 shows perfect agreement between methods (0.8–1: strong agreement, 0.7–0.8: good agreement, 0.5–0.7: moderate agreement, < 0.5: poor agreement). Specificity and sensitivity values for each method were also calculated.
Ethics, consent and permissions
This study was approved by the East of England Research Ethics Committee (REC reference 15/EE/0227; IRAS project ID 172012). Informed consent by each subject was required for participation in this study.
Results
Of 76 participants recruited in the primary study [36], 33 received 250-500mls of crystalloid over 15 min or less and were included for assessment of accuracy (Fig. 2). Reasons for exclusion included a prescribed change in fluid delivery rate by the clinical care team, interruption of fluid monitoring for patient transfer, and rate-limited fluid infusion equipment. 60.6% (20/33) of participants were identified as fluid responders (Table 1).
The measure of agreement between the reference standard (LVOT VTI) and test method to identify fluid responders was expressed as a kappa value (Table 2). A four-quadrant plot illustrates the concordance of directional change for each patient as measured by each test method as compared to LVOT VTI. An exclusion zone for small changes in stroke volume is applied to exclude changes of stroke volume of less than 10% (Fig. 3). To assess the accuracy of IVCCI in identifying fluid responders, the optimal IVCCI cut-off was 40%. A receiver operating characteristic (ROC) curve was plotted (Fig. 3f).
Accuracy of each method was assessed by its agreement with LVOT VTI (left ventricular outflow tract velocity time integral) in identifying fluid responders. Changes in stroke volume (ΔSV) after fluid challenge as determined by LVOT VTI are plotted against CCABF (common carotid artery blood flow) (a), SSAD (suprasternal aortic Doppler) (b), bioreactance (c), and PVUT (plethysmography vascular unloading technique) (d). A 10% exclusion zone is marked by a square in the concordance plots. Dots in the right upper quadrant (red area) indicate agreement between LVOT VTI and the test method in identifying fluid responders. IVCCI was assessed with a dot plot (e) which shows the IVCCI values of fluid responders (FR) on the left and fluid non-responders (NR) on the right. The receiver operating characteristic curve (f) had an area under the receiver operating curve (AUROC) of 0.464 (p = 0.747) [95% CI 0.264–0.675]
Discussion
This study finds that non-invasive cardiac output methods have poor agreement in identifying fluid responders in spontaneously breathing ED patients.
We identified no studies defining the accuracy of bioreactance in assessing fluid responsiveness in spontaneously breathing ED patients. To date studies performed heterogenous results. Squara et al. reported good agreement between cardiac output as measured by bioreactance and PAC in 110 patients [24]. However, accuracy to identify fluid responders was poor when studied on a subset of 23 patients with an assortment of interventions (7 rapid infusion challenges, 6 dobutamine challenges, 6 high PEEP stops, and 4 adrenaline infusions). Marque et al. also compared bioreactance to PAC in a group 19 mechanically ventilated patients who underwent rapid fluid infusion or passive leg raise (PLR) [48]. Bioreactance was found to have a sensitivity of 91% and a specificity of 95% in identifying fluid responders (no confidence intervals reported). Similarly, Galarza et al. reported reasonable accuracy in identifying fluid responders amongst 32 ICU patients with a sensitivity of 92% (95% CI: 62–91%) and a specificity of 80% (95% CI: 56–94%) when using an upgraded bioreactance device against PAC [49]. However, Kupersztych-Hagege et al. reported that the AUROC for bioreactance when compared to PAC to identify fluid responders amongst 48 critically ill patients was not significantly different to 0.5 (p = 0.77; no confidence intervals reported) [50]. The study was criticised for its methodology [51, 52]. Similarly, in a cohort of 22 patients under anaesthesia prior to elective intra-abdominal surgery, Conway et al. reported a sensitivity of 75% and a specificity of 69% in identifying fluid responders against oesophageal Doppler monitoring (no confidence intervals reported) [53]. Lastly, De Pascale et al. reported a sensitivity of 80% (95% CI: 56.3–94.3) and specificity of 82.6% (95% CI: 68.6–92.2) in 21 patients under anaesthesia prior to elective pelvic surgery against oesophageal Doppler monitoring [54].
In the current study, PVUT showed no agreement with LVOT VTI in identifying fluid responders. Movement of the digit on which the cuff device is placed was found to delay calibration and interfere with PVUT measurements. Other studies have investigated PVUT in its accuracy in predicting fluid responsiveness through pulse pressure variation (PPV), rather than measuring stroke volume change. Biais et al. reported that in 35 patients under general anaesthesia, a baseline 15% PPV threshold measured through CNAP identified fluid responders with a sensitivity of 76% (95% CI: 53–92%) and a specificity of 93% (95% CI: 66–99%) [55]. Similarly, Monnet et al. reported a sensitvity of 82% (95% CI: 57–96%) and a specificity of 91% (95% CI: 71–99%) for CNAP-derived PPV against pulse pressure analysis in 39 critically ill mechanically ventilated patients [56].
Previous studies on CCABF were conducted on healthy volunteers [57, 58], ICU patients [18, 59,60,61], and peri-operative patients [62, 63]. To our knowledge no studies have investigated the accuracy of SSAD in identifying fluid responders in spontaneously breathing patients in any clinical setting. Marik et al’s retrospective data review of 34 ICU patients found that CCABF identified fluid responders, using PLR as a surrogate for fluid loading, with a sensitivity of 94% and a specificity of 86% (no confidence levels or AUROC reported) [18]. 19 (56%) participants were mechanically ventilated, reducing variations in intra-thoracic pressure compared to spontaneous breathing potentially improving CCABF accuracy. However, Roehrig et al’s study of 33 post-operative mechanically ventilated patients reported an AUROC of 0.54 (95% CI: 0.32–0.76) using PAC as a reference method. The authors suggest that cerebral blood flow autoregulation possibly explains why CCABF did not reflect cardiac output changes after PLR [63]. PLR is a low-cost, easily accessible, and well validated alternative to an intravenous fluid bolus to assess fluid responsiveness. However, the latter is still first line practice in many UK EDs, and was therefore selected as the method of choice in this study.
Although SSAD is reported to reliably detect a 7.5% blood volume loss in healthy volunteers few studies have investigated its accuracy to detect fluid responsiveness [64, 65]. A meta-analysis on SSAD accuracy in mechanically ventilated patients by Chong and Peyton found an overall cardiac output percentage error of 42.7% (95% CI: 38.5–46.9%) in comparison to PAC [66]. The authors hypothesized that inaccuracy arises from operators obtaining a suboptimal angle of incidence on the direction of aortic blood flow.
For IVCCI accuracy, a recent systematic review by Seccombe at al identified six studies conducted in spontaneously breathing patients with sepsis. The authors reported that the high level of heterogeneity amongst study designs prevented pooling of results [67]. The lack of studies conducted in non-ICU settings, where most patients with sepsis are managed, also contributed to poor generalisability of results. The heterogeneity of techniques in measuring IVCCI has been previously discussed [36]. Four studies used LVOT VTI as the reference method, one used bioreactance, and one used systolic blood pressure. The study of Lanspa et al. was the only one based in the ED. The authors reported an IVCCI sensitivity of 100%, a specificity of 67% (no CI reported), and an AUROC of 0.83 (95% CI: 0.58–1.00) to detect fluid responders amongst 14 patients using LVOT VTI as the reference standard [26].
Judicious intravenous fluid delivery plays an important role in increasing oxygen delivery to hypoperfused organs and avoiding fluid overload. As discussed above, few studies have investigated non-invasive methods to identify fluid responsive states in spontaneously breathing patients. This group represents the majority of patients with sepsis who receive intravenous fluid therapy and consequently requires further study. Overall, reviewed studies report low to moderate accuracy across all methods.
Study limitations
This study has several limitations. A window of 15 min was specified in the protocol to allow all 6 measures to be taken in each round as patients generally do not tolerate more than three measures to be taken at once. Therefore, not all measures occurred simultaneously. This window of time could be sufficient for a physiological change of stroke volume to occur, especially in patients in which fluid is quickly re-distributed to extra-vascular compartments. This would increase the chances of a significant agreement between methods being missed when it actually exists (type II error). All measurements were taken as efficiently as possible to mitigate this effect. A second limitation was that the order with which methods were used during the measurement windows did not vary. The least restrictive methods were deployed first to minimise distress in patients. Anecdotally, bioreactance and PVUT were best tolerated, followed by IVCCI, CCABF, LVOT VTI and SSAD. There is little evidence available on duration of the effect of a fluid bolus in fluid depleted patients. It is reasonable to assume that this duration varies with the cause of the fluid depletion and its severity. Monnet and Teboul found that a PLR manoeuvre, during which the patient’s leg are raised by 45 degrees to increase venous return to the heart, induced its maximal preload effect within 90 s in critically ill patients with a subsequent return to baseline [68]. It is uncertain how long an increased preload, either by PLR or fluid bolus, is maintained for. Lastly, LVOT VTI was our chosen reference standard for this study. The method suffers from poor performance in the hands of inexperienced operators. The study’s operators were trained using a supervised and standardised training programme based on current best practice to mitigate this effect.
Conclusion
This study showed that there was poor agreement between the non-invasive methods used to measure SV/CO (bioreactance, PVUT CCABF, LVOT VTI and SSAD) and LVOT VTI in identifying fluid responders. Multiple studies have shown suboptimal agreement between methods used to assess stroke volume/cardiac output. Our data should not be interpreted as seeing one device as offering more accurate measurements than another but that there is limited agreement between the devices used in this study. Further studies are required to assess the accuracy of these non-invasive haemodynamic methods in spontaneously breathing patients in the ED prior to conducting large outcome trials based on their use.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CCA:
-
Common carotid artery
- CCABF:
-
Common carotid arterial blood flow
- CNAP:
-
Continuous non-invasive arterial pressure
- ED:
-
Emergency department
- ICU:
-
Intensive care units
- IVC:
-
Inferior vena cava
- IVCCI:
-
Inferior vena cava collapsibility index
- LVOT VTI:
-
Left ventricular outflow tract velocity time integral
- PVUT:
-
Plethysmography with vascular unloading technique
- SBP:
-
Systolic blood pressure
- SSAD:
-
Suprasternal aortic Doppler
- USCOM:
-
Ultrasound cardiac output monitor
References
Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest. 2000;117(6):1749–54.
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–65.
Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–9.
Javed A, Guirgis FW, Sterling SA, Puskarich MA, Bowman J, Robinson T, et al. Clinical predictors of early death from sepsis. J Crit Care. 2017;42:30–4.
McGee S. Abernethy Wr, Simel D. the rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281:1022–9.
Fortes MB, Owen JA, Raymond-Barker P, Bishop C, Elghenzai S, Oliver SJ, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. J Am Med Dir Assoc. 2015;16(3):221–8.
Nowak RM, Sen A, Garcia AJ, Wilkie H, Yang JJ, Nowak MR, et al. The inability of emergency physicians to adequately clinically estimate the underlying hemodynamic profiles of acutely ill patients. Am J Emerg Med. 2012;30(6):954–60.
Maurer C, Wagner JY, Schmid RM, Saugel B. Assessment of volume status and fluid responsiveness in the emergency department: a systematic approach. Med Klin Intensivmed Notfmed. 2017;112(4):326–33.
Wo CC, Shoemaker WC, Appel PL, Bishop MH, Kram HB, Hardin E. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit Care Med. 1993;21(2):218–23.
Marik PE. Fluid responsiveness and the six guiding principles of fluid resuscitation. Crit Care Med. 2016;44(10):1920–2.
Harris T, Coats TJ, Elwan MH. Fluid therapy in the emergency department: an expert practice review. Emerg Med J. 2018;35(8):511–5.
Elwan MH, Roshdy A, Elsharkawy EM, Eltahan SM, Coats TJ. The haemodynamic dilemma in emergency care: is fluid responsiveness the answer? A systematic review. Scand J Trauma Resusc Emerg Med. 2017;25(1):25.
Pugsley J, Lerner AB. Cardiac output monitoring: is there a gold standard and how do the newer technologies compare? Semin Cardiothorac Vasc Anesth. 2010;14(4):274–82.
Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366(9484):472–7.
Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. Jama. 2005;294(13):1625–33.
Laher AE, Watermeyer MJ, Buchanan SK, Dippenaar N, Simo NC, Motara F, et al. A review of hemodynamic monitoring techniques, methods and devices for the emergency physician. Am J Emerg Med. 2017;35:1335.
Hou PC, Filbin MR, Napoli A, Feldman J, Pang PS, Sankoff J, et al. Cardiac output monitoring managing intravenous therapy (COMMIT) to treat emergency department patients with Sepsis. Shock. 2016;46(2):132–8.
Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364–70.
Horster S, Stemmler HJ, Strecker N, Brettner F, Hausmann A, Cnossen J, et al. Cardiac output measurements in septic patients: comparing the accuracy of USCOM to PiCCO. Crit Care Res Pract. 2012;2012:270631.
van Lelyveld-Haas LE, van Zanten AR, Borm GF, Tjan DH. Clinical validation of the non-invasive cardiac output monitor USCOM-1A in critically ill patients. Eur J Anaesthesiol. 2008;25(11):917–24.
Hahn R, Rinosl H, Neuner M, Kettner SC. Clinical validation of a continuous non-invasive haemodynamic monitor (CNAP™ 500) during general anaesthesia. Br J Anaesth. 2012;108(4):581–5.
Ilies C, Bauer M, Berg P, Rosenberg J, Hedderich J, Bein B, et al. Investigation of the agreement of a continuous non-invasive arterial pressure device in comparison with invasive radial artery measurement. Br J Anaesth. 2012;108(2):202–10.
Raval NY, Squara P, Cleman M, Yalamanchili K, Winklmaier M, Burkhoff D. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput. 2008;22(2):113–9.
Squara P, Rotcajg D, Denjean D, Estagnasie P, Brusset A. Comparison of monitoring performance of bioreactance vs. pulse contour during lung recruitment maneuvers. Crit Care. 2009;13(4):R125.
Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40:845.
Lanspa MJ, Grissom CK, Hirshberg EL, Jones JP, Brown SM. Applying dynamic parameters to predict hemodynamic response to volume expansion in spontaneously breathing patients with septic shock. Shock. 2013;39(2):155–60.
Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16(5):R188.
Dittmann H, Voelker W, Karsch KR, Seipel L. Influence of sampling site and flow area on cardiac output measurements by Doppler echocardiography. J Am Coll Cardiol. 1987;10(4):818–23.
Seo H, Yamagishi M, Haque SA, Mohibullah AK, Majumder A-S, Nakatani S, et al. An enhanced method for measuring cardiac output using Doppler color flow echocardiography. Jpn Circ J. 1997;61(11):905–11.
Preau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38(3):819–25.
Biais M, Vidil L, Sarrabay P, Cottenceau V, Revel P, Sztark F. Changes in stroke volume induced by passive leg raising in spontaneously breathing patients: comparison between echocardiography and Vigileo/FloTrac device. Crit Care. 2009;13(6):R195.
Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125–32.
Temporelli PL, Scapellato F, Eleuteri E, Imparato A, Giannuzzi P. Doppler echocardiography in advanced systolic heart failure: a noninvasive alternative to Swan-Ganz catheter. Circ Heart Fail. 2010;3(3):387–94.
Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Crit Care. 2016;20:274.
Mandeville JC, Colebourn CL. Can transthoracic echocardiography be used to predict fluid responsiveness in the critically ill patient? A systematic review. Crit Care Res Pract. 2012;2012:513480.
McGregor D, Sharma S, Gupta S, Ahmad S, Godec T, Harris T. Emergency department non-invasive cardiac output study (EDNICO): a feasibility and repeatability study. Scand J Trauma Resusc Emerg Med. 2019;27(1):30.
Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799.
Dinh VA, Ko HS, Rao R, Bansal RC, Smith DD, Kim TE, et al. Measuring cardiac index with a focused cardiac ultrasound examination in the ED. Am J Emerg Med. 2012;30(9):1845–51.
Stolz LA, Mosier JM, Gross AM, Douglas MJ, Blaivas M, Adhikari S. Can emergency physicians perform common carotid Doppler flow measurements to assess volume responsiveness? West J Emerg Med. 2015;16(2):255–9.
Dey I, Sprivulis P. Emergency physicians can reliably assess emergency department patient cardiac output using the USCOM continuous wave Doppler cardiac output monitor. Emerg Med Australas. 2005;17(3):193–9.
Akkaya A, Yesilaras M, Aksay E, Sever M, Atilla OD. The interrater reliability of ultrasound imaging of the inferior vena cava performed by emergency residents. Am J Emerg Med. 2013;31(10):1509–11.
De Lorenzo RA, Holbrook-Emmons VL. Ultrasound measurement of inferior vena cava diameters by emergency department nurses. Adv Emerg Nurs J. 2014;36(3):271–8.
Bussmann BM, Sharma S, McGregor D, Hulme W, Harris T. Observational study in healthy volunteers to define interobserver reliability of ultrasound haemodynamic monitoring techniques performed by trainee doctors. Eur J Emerg Med. 2019;26(3):217–223.
Flahault A, Cadilhac M, Thomas G. Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol. 2005;58(8):859–62.
Eiferman DS, Davido HT, Howard JM, Gerckens J, Evans DC, Cook CH, et al. Two methods of hemodynamic and volume status assessment in critically ill patients: a study of disagreement. J Intensive Care Med. 2016;31(2):113–7.
Duus N, Shogilev DJ, Skibsted S, Zijlstra HW, Fish E, Oren-Grinberg A, et al. The reliability and validity of passive leg raise and fluid bolus to assess fluid responsiveness in spontaneously breathing emergency department patients. J Crit Care. 2015;30(1):217.e1–5.
Phan TD, Kluger R, Wan C, Wong D, Padayachee A. A comparison of three minimally invasive cardiac output devices with thermodilution in elective cardiac surgery. Anaesth Intensive Care. 2011;39(6):1014–21.
Marque S, Cariou A, Chiche JD, Squara P. Comparison between Flotrac-Vigileo and bioreactance, a totally noninvasive method for cardiac output monitoring. Crit Care. 2009;13(3):R73.
Galarza L, Mercado P, Teboul JL, Girotto V, Beurton A, Richard C, et al. Estimating the rapid haemodynamic effects of passive leg raising in critically ill patients using bioreactance. Br J Anaesth. 2018;121(3):567–73.
Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961–6.
Denman WT, Hutchison C, Levy B. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2014;112:943–4.
Squara P. Bioreactance for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2014;112:942.
Conway DH, Hussain OA, Gall I. A comparison of noninvasive bioreactance with oesophageal Doppler estimation of stroke volume during open abdominal surgery: an observational study. Eur J Anaesthesiol. 2013;30(8):501–8.
De Pascale G, Singer M, Brealey D. Comparison of stroke volume measurement between non-invasive bioreactance and esophageal Doppler in patients undergoing major abdominal-pelvic surgery. J Anesth. 2017;31(4):545–51.
Biais M, Stecken L, Martin A, Roullet S, Quinart A, Sztark F. Automated, continuous and non-invasive assessment of pulse pressure variations using CNAP((R)) system. J Clin Monit Comput. 2017;31(4):685–92.
Monnet X, Dres M, Ferre A, Le Teuff G, Jozwiak M, Bleibtreu A, et al. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth. 2012;109(3):330–8.
Sidor M, Premachandra L, Hanna B, Nair N, Misra A. Carotid flow as a surrogate for cardiac output measurement in hemodynamically stable participants. J Intensive Care Med. 2018. https://doi.org/10.1177/0885066618775694.
Peachey T, Tang A, Baker EC, Pott J, Freund Y, Harris T. The assessment of circulating volume using inferior vena cava collapse index and carotid Doppler velocity time integral in healthy volunteers: a pilot study. Scand J Trauma Resusc Emerg Med. 2016;24(1):108.
Barjaktarevic I, Toppen WE, Hu S, Aquije Montoya E, Ong S, Buhr R, et al. Ultrasound assessment of the change in carotid corrected flow time in fluid responsiveness in undifferentiated shock. Crit Care Med. 2018;46(11):e1040–e6.
Girotto V, Teboul JL, Beurton A, Galarza L, Guedj T, Richard C, et al. Carotid and femoral Doppler do not allow the assessment of passive leg raising effects. Ann Intensive Care. 2018;8(1):67.
Jalil B, Thompson P, Cavallazzi R, Marik P, Mann J, El-Kersh K, et al. Comparing changes in carotid flow time and stroke volume induced by passive leg raising. Am J Med Sci. 2018;355(2):168–73.
Kim DH, Shin S, Kim N, Choi T, Choi SH, Choi YS. Carotid ultrasound measurements for assessing fluid responsiveness in spontaneously breathing patients: corrected flow time and respirophasic variation in blood flow peak velocity. Br J Anaesth. 2018;121(3):541–9.
Roehrig C, Govier M, Robinson J, Aneman A. Carotid Doppler flowmetry correlates poorly with thermodilution cardiac output following cardiac surgery. Acta Anaesthesiol Scand. 2017;61(1):31–8.
O'Loughlin E, Ward M, Crossley A, Hughes R, Bremner AP, Corcoran T. Evaluation of the utility of the Vigileo FloTrac() , LiDCO() , USCOM and CardioQ() to detect hypovolaemia in conscious volunteers: a proof of concept study. Anaesthesia. 2015;70(2):142–9.
Bussmann BM, Hulme W, Tang A, Harris T. Investigating the ability of non-invasive measures of cardiac output to detect a reduction in blood volume resulting from venesection in spontaneously breathing subjects. Scand J Trauma Resusc Emerg Med. 2018;26(1):104.
Chong SW, Peyton PJ. A meta-analysis of the accuracy and precision of the ultrasonic cardiac output monitor (USCOM). Anaesthesia. 2012;67(11):1266–71.
Seccombe A, McCluskey L, Moorey H, Lasserson D, Sapey E. Assessing fluid resuscitation in adults with sepsis who are not mechanically ventilated: a systematic review of diagnostic test accuracy studies. J Gen Intern Med. 2019;34:1874.
Monnet X, Teboul JL. Passive leg raising. Intensive Care Med. 2008;34(4):659–63.
Acknowledgements
We would like to thank all the participants and their families. Thanks to nursing and medical staff at the Royal London Hospital Emergency Department. Special thanks to Jason Pott, Fiona Bennin, and Prof Stephen Thomas for their support.
Funding
The study was funded by the Royal College of Emergency Medicine.
For the duration of the study, the research team received equipment from:
Terason (Burlington, MA, USA): one uSmart 3300 ultrasound system.
Pty Ltd. (Coffs Harbour, NSW, Australia): one USCOM-1A system.
LiDCO Ltd. (London, UK): one LiDCO+ and CNAP system.
Cheetah Medical (Portland, OR, USA): one Cheetah Medical NICOM system.
Author information
Authors and Affiliations
Contributions
DM was principal investigator and compiled the final manuscript. SS, SG, and SA collected, entered and analysed clinical data. TG provided statistical analysis of the data. TH was chief investigator, designed the study protocol and provided quality assurance. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the NRES Committee East of England - Essex.
Study reference 15/EE/0227.
Consent for publication
All participants consented for study data to be published.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
McGregor, D., Sharma, S., Gupta, S. et al. Emergency department non-invasive cardiac output study (EDNICO): an accuracy study. Scand J Trauma Resusc Emerg Med 28, 8 (2020). https://doi.org/10.1186/s13049-020-0704-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13049-020-0704-5