Abstract
Background
This study aimed to develop and evaluate radiomics models to predict CD27 expression and clinical prognosis before surgery in patients with serous ovarian cancer (SOC).
Methods
We used transcriptome sequencing data and contrast-enhanced computed tomography images of patients with SOC from The Cancer Genome Atlas (n = 339) and The Cancer Imaging Archive (n = 57) and evaluated the clinical significance and prognostic value of CD27 expression. Radiomics features were selected to create a recursive feature elimination-logistic regression (RFE-LR) model and a least absolute shrinkage and selection operator logistic regression (LASSO-LR) model for CD27 expression prediction.
Results
CD27 expression was upregulated in tumor samples, and a high expression level was determined to be an independent protective factor for survival. A set of three and six radiomics features were extracted to develop RFE-LR and LASSO-LR radiomics models, respectively. Both models demonstrated good calibration and clinical benefits, as determined by the receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis. The LASSO-LR model performed better than the RFE-LR model, owing to the area under the curve (AUC) values of the ROC curves (0.829 vs. 0.736). Furthermore, the AUC value of the radiomics score that predicted the overall survival of patients with SOC diagnosed after 60 months was 0.788 using the LASSO-LR model.
Conclusion
The radiomics models we developed are promising noninvasive tools for predicting CD27 expression status and SOC prognosis. The LASSO-LR model is highly recommended for evaluating the preoperative risk stratification for SOCs in clinical applications.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Ovarian cancer (OC) is the second leading cause and deadliest type of gynecological malignancy worldwide, estimated to account for 19,710 new cases and 13,270 deaths in the US in 2023 [1]. This debilitating disease is highly heterogeneous and comprises multiple distinct histologic subtypes with different risk factors, origins, etiologies, molecular biology, and prognoses. Serous ovarian cancer (SOC) is the most common histological subtype with the least favorable prognosis [2]. Despite intense efforts and progress in therapeutic options over the past few decades, the overall survival (OS) for patients with OC is far from satisfactory [3]. Classic prognostic indicators such as clinicopathological characteristics, serum carbohydrate antigen 125 (CA125), ultrasound, and computed tomography (CT) can no longer meet the clinical needs of precision medicine. Therefore, urgent progress is required to explore novel prognostic markers that are beneficial for stratifying patients to provide new indicators for precision medicine.
The cluster of differentiation 27 gene (CD27, also known as TNFRSF7) is a member of the tumor necrosis factor receptor superfamily (TNFRSF) and frequently induces both costimulatory and apoptosis-inducing molecules to facilitate anti-tumor and anti-infection immunity [4,5,6]. CD27 plays a crucial role in regulating B-cell activation and immunoglobulin synthesis [10, 11] by interacting with its only natural ligand CD70 (CD27L), which is transiently expressed on antigen-activated immune cells [4, 7, 8]. This interaction transduces signals leading to the activation of pathways, including nuclear factor-kappa B and mitogen-activated protein kinase 8/c-jun N-terminal kinase [5, 9]. Moreover, CD27 is expressed on tumor-infiltrating lymphocytes (TILs) and can transmit signals to T and NK cells across a variety of tumors [5]. Agonism of the costimulatory CD27-CD70 pathway has been studied as a promising target for therapeutic intervention in various tumor types [10]. For instance, varilumab, a CD27 agonizing monoclonal antibody, has been extensively studied as a monotherapy and checkpoint inhibitor therapy in several hematologic and solid tumor types, including Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, and OC [10].
Images, such as CT images, may contain mineable information, reflecting the underlying pathophysiology of a tumoral tissue [11]. As an emerging translation field, radiomics investigates the association between quantitative high-dimensional data extracted from imaging examinations and clinical data to construct a prediction model, which eventually improves evidence-based personalized medical decision-making [12, 13]. In recent years, radiomics has shown great promise in predicting tumor diagnosis, molecular subtype, therapeutic effect, and survival in patients with various tumor types, including breast cancer [14, 15], lung cancer [16, 17], and OC [18,19,20]. A systematic review demonstrated that radiomics models have shown promising results as predictors of OS and progression-free survival (PFS) in patients with OC, but larger studies are needed to demonstrate clinical applicability [20]. However, no study has used radiomics features to predict the expression levels of CD27 and evaluate its prognostic value in SOC.
In this study, we innovatively constructed radiomics models based on preoperative CT and clinical information for the noninvasive prediction of CD27 expression and survival prognosis in patients with SOC. Using bioinformatics analysis, the underlying molecular mechanism of CD27 and its possible association with the immune microenvironment were investigated.
Methods
Study cohort and data acquisition
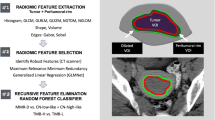
To investigate the prognostic value of CD27 expression and construct radiomics prediction models, this retrospective study used imaging (including clinical and follow-up data) and gene expression data (including clinical and follow-up data) from The Cancer Imaging Archive (TCIA, https://www.cancerimagingarchive.net/) and The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.Gov/), respectively. Three hundred thirty-nine patients with pathologically confirmed primary SOC from the TCGA dataset were enrolled in the study. Among these, 57 qualified cases had preoperative CT images stored in the TCIA. TCGA data were used to evaluate the prognostic value of CD27 expression, and the images were used for feature extraction and radiomics model construction. The exclusion criteria included non-primary SOC cases, samples with incomplete clinical or genomic data, samples with poor-quality images, and images with no corresponding clinical or genomic data. A flowchart of this study is shown in Fig. 1.
Identification of CD27 as a DEG in SOC
Based on the expression of CD27 in the TCGA dataset, all subjects included in the study were dichotomized into CD27high and CD27low groups by the cutoff values calculated using the R package “survminer.” UCSC Xena (https://xenabrowser.net/datapages/) RNA-Seq data in the “transcripts per kilobase million” (TPM) format were processed uniformly using Toil (portable open-source workflow software) [21]. RNA-seq data from SOC in TCGA and normal samples from the GTEx databank were extracted. To compare the CD27 levels between groups, the RNA-seq data in the TPM format was log2 transformed, and the R package “ggplot2” was used for visualization. The correlation between CD27 expression and the clinical characteristics was analyzed using Spearman’s rank correlation coefficients.
Kaplan–Meier survival curve analysis illustrated changes in the survival rates among groups. The log-rank test was used to compare the statistical difference of survival rates in different groups. The R package “survival” was used for each variable, and the R package “survminer” was used for conclusion and visualization.
Univariate and multivariate Cox regression analyses were used to investigate the relationships between one or more study factors and survival outcomes. The R packages “survival” and “forestplot” were used in the aforementioned analyses.
A subgroup analysis was performed using univariate COX regression analysis to determine the role of CD27 expression (CD27high and CD27low groups) in the prognosis of patients in different covariate subgroups. The R packages “cmprsk”, “survival” and “forestplot” were used in the subgroup analysis.
Correlation analysis between CD27 expression and immune-cell infiltration and immune genes
The gene expression files of the SOC samples were uploaded to the CIBERSORTx database (https://cibersortx.stanford.edu/), and immune cell infiltration was measured for each sample. The correlation between CD27 expression and immune cell infiltration or immune gene expression was calculated using Spearman’s rank correlation coefficient.
GO and KEGG enrichment analysis
To further identify the differentially enriched pathways between the CD27high and CD27low groups, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analyses were employed. In this study, we used GO and KEGG analyses to visualize the top 10 and 20 significantly enriched pathways, respectively. The R package “clusterProfiler” was used for enrichment analyses and “org.Hs.eg.db” for ID conversion.
Tumor segmentation and feature extraction
Fifty-seven qualified SOC cases with preoperative CT images from TCIA and bioinformatics data from TCGA were divided and labeled into high- and low- expression groups using the cutoff value (1.0959) of CD27 expression calculated by the R package “survminer.” Volumes of interest were delineated along each tumor contour by one radiologist and repeated by another radiologist in 10 randomly selected patients. A set of 107 radiomics features was extracted using pyRadiomics and standardized. The intraclass correlation coefficient (ICC) was used to assess the inter-reader reproducibility for both image segmentation and radiomics feature extraction. Radiomics features with an ICC ≥ 0.75 were selected for further exploration.
Construction and evaluation of RFE-LR model and LASSO-LR model
Before modeling, feature selection was conducted using recursive feature elimination (RFE) implemented by the R package “caret” and the repeat (1,000 times) least absolute shrinkage and selection operator (LASSO) method implemented by the R package “glmnet”, respectively, to find the optimal set of features for an accurate model. Using the glm function from R package “stats,” the radiomics features screened out by the RFE algorithm and LASSO algorithm were fitted using the logistic regression (LR) algorithm to establish a dichotomous model for predicting CD27 expression.
The performance of the models was evaluated, and an internal 5-fold cross-validation was performed. Receiver operating characteristic (ROC) and precision recall (PR) curves were plotted to assess diagnostic performance. The calibration degree of the model was demonstrated using calibration curves and the Hosmer–Lemeshow goodness-of-fit testing method. The clinical usefulness of the model was evaluated using a decision curve analysis (DCA). This radiomics model can generate the prediction probability of gene expression levels (radiomics score, Rad_score). The R packages “pROC,” “measures,” “ResourceSelection” and “modEvA” were used in these analyses. Wilcoxon test was used to test the difference of Rad_score between CD27high and CD27low groups, implemented by the R package “ggplot2.”
Comparison of RFE-LR model and LASSO-LR model
To determine which model was better, the Delong test was used to compare the area under the curve (AUC) values before and after validation. The PR curve is also an important reference. The LASSO-LR model performed better and was used for further analyses.
We incorporated the Rad_score in the LASSO-LR model into clinical data to obtain 57 SOC cases from the TCGA database with the corresponding Rad_score. A time-dependent ROC curve was constructed to demonstrate predictive capability at different time points. A time-based AUC for predicting patient survival at different time points after SOC diagnosis was also established.
Results
Patient characteristics
In total, 339 patients with SOC from the TCGA database were included in our study (Fig. 1). The cut-off expression level was 1.096, dichotomizing patients into CD27high (n = 141) and CD27low (n = 198) groups. The study cohort statistics of the groups are presented in Table 1. A significant difference in the distribution of residual tumor disease was noted between the CD27high and CD27low groups (P = 0.033). However, no significant differences were observed in variables such as age, FIGO stage, lymphatic invasion, venous invasion, histologic grade, or chemotherapy condition between the two groups (all P > 0.05).
Clinical significance of CD27 in SOC
As shown in Fig. 2A, CD27 expression levels were significantly higher in SOC samples from the TCGA project than in normal ovary samples from the GTEx databank (P < 0.001). The median survival times in the CD27low and CD27high groups were 42.6 (95% confidence interval [CI], 38.6–46.3) and 52.1 (95% CI, 41.6–64.4) months, respectively. Kaplan–Meier survival analysis showed that a high expression level of CD27 was associated with a better prognosis in terms of OS than a low expression level (P = 0.013, Fig. 2B). The results of the univariate Cox regression analysis indicated that variables, including high expression levels of CD27 (hazard ratio [HR] = 0.699, 95% CI:0.527–0.929, P = 0.013) and chemotherapy (HR = 0.47, 95% CI:0.288–0.766, P = 0.002), were protective factors for SOC. However, Tumor_residual_disease: 1–10 mm (HR = 1.909, 95% CI:1.186–3.074, P = 0.008) or Tumor_residual_disease:10 mm (HR = 2.264, 95% CI:1.363–3.762, P = 0.002) correlated with poorer survival than No Macroscopic disease (Fig. 2C). After adjustment for other covariates included in the multivariate COX regression analysis, a high expression level of CD27 (HR = 0.566, 95% CI:0.418–0.766, P < 0.001) and chemotherapy (HR = 0.341, 95% CI:0.203–0.571, P < 0.001) were independent protective factors for survival, and Tumor_residual_disease: 1–10 mm (HR = 1.743, 95% CI:1.067–2.848, P = 0.026) or Tumor_residual_disease: 10 mm~ (HR = 2.296, 95% CI:1.359–3.879, P = 0.002) were independent risk factors (Fig. 2D). In the univariate subgroup analysis, a high CD27 expression level was protective in patients with SOC with the characteristics: aged ≤ 50 years (HR = 0.631, 95% CI:0.412–0.966, P = 0.034), FIGO_stage: III/IV (HR = 0.704, 95% CI:0.528–0.94, P = 0.017), Histologic_grade: G3/G4/GX (HR = 0.653, 95% CI:0.483–0.883, P = 0.006), Tumor_residual_disease: 1–10 mm (HR = 0.506, 95% CI:0.334–0.765, P = 0.001) and Chemotherapy: YES (HR = 0.704, 95% CI:0.522–0.949, P = 0.021) (Fig. 2E). Moreover, CD27 expression levels were significantly associated with Tumor_residual_disease (P = 0.036; Fig. 2F).
CD27 expression in SOC and its association with clinical characteristics. (A) CD27 expression was upregulated in SOC tissues than in normal tissues (∗∗∗P < 0.001). (B) Kaplan–Meier analysis showed that low CD27 expression was associated with a poor OS of patients with SOC (P = 0.013). (C) Univariate Cox regression analyses. (D) Multivariate Cox regression analyses. (E) Subgroup analyses. (F) Correlation analysis between CD27 expression level and clinical characteristics of SOC. SOC, serous ovarian carcinoma; OS, overall survival
CD27 expression correlates with immune-cell infiltration and immune genes in SOC
Correlation heat maps revealed that CD27 positively correlated with macrophages (M0, M1, and M2) and T cells, including T cells CD8, T cells follicular helper, regulatory T cells (Tregs), CD4 memory testing, and T cells CD4 memory activated, while negatively correlated with eosinophils and dendritic cell activation (Fig. 3A, all P < 0.01). Furthermore, CD27 expression had a significant positive correlation with the expression levels of some immune-related genes, including CTLA4, PDCD1, CD70, CD86, CD48, CD28, and CD80 (Fig. 3B, all P < 0.01).
Correlation of the immune-cell infiltration, DEGs, and CD27 expression in SOC. (A) Correlation heat map between CD27 expression and immune-cell infiltration in SOC in CIBERSORTx database. (B) Correlation between CD27 expression and immune-related genes. (C) GO enrichment analysis of the DEGs related to CD27. (D) KEGG enrichment analysis of the DEGs related to CD27. SOC, serous ovarian carcinoma; GO, gene ontology; DEGs, differentially expressed genes; KEGG, Kyoto Encyclopedia of Genes and Genomes
GO and KEGG enrichment analysis of DEGs associated with CD27 in SOC
GO enrichment analysis indicated that differentially expressed genes (DEGs) in the CD27high and CD27low groups were significantly enriched in pathways, including complement activation, T cell receptor complex, and immunoglobulin receptor binding (Fig. 3C). KEGG enrichment analysis revealed that the DEGs were significantly enriched in cytokine-cytokine receptor interactions (Fig. 3D).
Construction and evaluation of RFE-LR model
In total, 57 patients with SOC from the TCIA database were included in this study. The median ICC value of the image radiomics features was 0.975, and 104 out of 107 features (97.2% of the total features) with ICC ≥ 0.75 were enrolled for further analysis. After feature reduction using the RFE algorithm (Fig. 4A), three features remained for model construction. Their importance is shown in Table 2; Fig. 4B. The RFE-LR model showed favorable predictive ability, as shown by the ROC curve. This model produced an AUC of 0.736 (Fig. 4C) and 0.725 after internal 5-fold cross-validation (Fig. 4D); the PR-AUC of this model was 0.642 (Supplementary Fig. 1). Calibration curves and Hosmer–Lemeshow goodness-of-fit testing indicated that our prediction fits well with the actual CD27 expression levels (P = 0.957, Fig. 4E). DCA analysis showed the high clinical practicality of the model (Fig. 4F). The AUC values before and after cross-validation showed no statistically significant differences (P = 0.867). Moreover, the Rad_score was significantly higher in the CD27high group than in the CD27low group (P < 0.01; Fig. 4G).
Construction and evaluation of the RFE-LR model. (A) Radiomics features with statistical differences using the RFE algorithm. (B) Importance of the selected features in the RFE-LR model. (C) ROC curve analysis of the RFE-LR model. (D) Cross-validation ROC curve analysis of the RFE-LR model. (E) Calibration-curve analysis of the RFE-LR model. (F) Hosmer–Lemeshow goodness-of-fit testing. (G) Prediction of CD27 expression level using the RFE-LR model. RFE, recursive feature elimination; LR, logistic regression; ROC, receiver operating characteristic curve
Construction and evaluation of LASSO-LR model
Six radiomics features were selected for inclusion in the LASSO-LR model (Fig. 5A). Figure 5B; Table 3 show the importance of the selected radiomics features. The LASSO-LR model showed a favorable predictive capacity, with an AUC of 0.829 (Fig. 5C) and an AUC after internal 5-fold cross-validation of 0.783 (Fig. 5D). The PR-AUC of this model was 0.780 (Supplementary Fig. 2). According to the calibration curves and Hosmer-Lemeshow goodness-of-fit testing, the LASSO-LR model revealed high conformity in predicting CD27 expression levels compared with the actual value (P = 0.833, Fig. 5E). DCA analysis displayed preferable clinical practicality for the model (Fig. 5F). No statistical difference was observed in the AUC values before and after cross-validation (P = 0.566). The Rad_score distribution was remarkably different in the CD27high and CD27low groups, with a higher Rad_score in the CD27high group (P < 0.001; Fig. 5G).
Construction and evaluation of LASSO-LR model. (A) Radiomics features with statistical differences using repeat LASSO algorithm. (B) Importance of the selected features in the LASSO-LR model. (C) ROC curve analysis of the LASSO-LR model. (D) Cross-validation ROC curve analysis of the LASSO model. (E) Calibration-curve analysis of the LASSO-LR model. (F) Hosmer–Lemeshow goodness-of-fit testing. (G) Prediction of CD27 expression level using the LASSO-LR model. LASSO, least absolute shrinkage and selection operator; LR, logistic regression; ROC, receiver operating characteristic curve
Model selection and survival analysis
We compared the AUC values before and after validation in the RFE-LR and LASSO-LR models, respectively, using the Delong test, and no statistical difference was observed (P > 0.05). Based on the ROC (AUC:0.736 vs. 0.829; cross-validation AUC:0.725 vs. 0.783) and PR curves (AUC:0.642 vs. 0.780) of the two models, we found that the LASSO-LR model was superior to the RFE-LR model. Therefore, the LASSO-LR model was used for subsequent prognostic analyses. The results of time-dependent ROC curve analysis indicated that the AUC values of the Rad_score predicting OS at 36 months and 60 months were 0.625 and 0.788, respectively (Fig. 6A). The AUC increased over time (Fig. 6B).
Prediction of OS in SOC using LASSO-LR model. (A) Time-dependent ROC curve constructed using the AUC value of the main variable at each time point (36 and 60 months). (B) The AUC value at different time points. OS, overall survival; SOC, serous ovarian carcinoma; LASSO, least absolute shrinkage and selection operator; LR, logistic regression; ROC, receiver operating characteristic curve; AUC, area under the curve
Discussion
The gold standard treatment for patients with epithelial ovarian cancer (EOC) comprises complete cytoreductive surgery, followed by platinum-based adjuvant chemotherapy with or without maintenance therapy [22]. A residual tumor of less than 1 cm is considered optimal after debulking surgery, and the amount of residual tumor tissue remains the strongest prognostic factor for survival [23]. Preoperative assessment of the scope and timing of primary surgery is a key issue. However, conventional CT techniques are insufficient for accurate and individualized imaging, and imaging analysis combined with biomarkers is currently a new hotspot. Our study developed CT-based radiomics models to preoperatively and noninvasively predict CD27 expression levels and prognosis in patients with SOC, which is beneficial for personalized clinical decision-making.
However, few studies have investigated the role of CD27 in OC. Swiderska et al. found that CD27 could be a potential biomarker for the diagnosis of OC and is an unfavorable prognostic factor for OC [24]. CD27 + TILs were associated with improved prognosis in high-grade SOC [25]. Guo et al. observed that higher CD27 expression indicated greater sensitivity to cisplatin treatment [26]. As the ligand for CD27, CD70 expression can be induced by the platinum treatment of OC cells [27]. These results suggest that antibody-drug or antibody-drug conjugates in the CD27-CD70 pathway may be promising immunotherapeutic regimens for patients with cisplatin-resistant OC [26, 27]. CD27 is expressed on TILs [5], and a less differentiated TIL phenotype (CD27+TIL) is associated with favorable survival after incomplete cytoreductive surgery [25]. Varlilumab may have potential therapeutic implications in the treatment of OC [10, 28]. In a Phase I/II dose-escalation and cohort expansion study (NCT02335918), the combination of anti-PD1/PD-L1 antibody (nivolumab) and varlilumab resulted in 5 of 49 (10%) patients with OC achieving partial remission (PR), and 19 of 49 (39%) achieving stable disease (SD) [28]. Additionally improved clinical outcomes in a subset of patients, particularly patients with OC, have been associated with increased tumor expression of PD-L1 and CD8 + TILs [28]. Herein, CD27 was identified as a differentially expressed prognosis-related gene in SOC, and its expression was associated with immune cell infiltration and immune genes, such as CTLA4, PDCD1, and CD70. Consequently, CD27 signaling pathway targeting strategies may provide new insights into SOC.
The success of radiomics has been reported in its application in evaluating of ovarian masses [29], categorizing cancer subtypes [19, 30,31,32], predicting metastasis [33,34,35], recurrence [36], and survival [20, 29] in OC. Notably, radiomics models could predict the expression of targets and clinical outcomes in OC [18, 37,38,39]. Combining clinical and radiomics models may improve model performance when predicting BRCA mutations and PFS in OC [18]. Habitat radiomics using positron emission tomography/CT imaging can accurately predict Ki-67 status and stratify the prognosis of patients with OC [37]. Gao et al. showed that radiomics signatures from CT images can differentiate between the PD-1 expression status and OS in patients with OC [38]. A recent study revealed that a CT-based radiomics model using LASSO regression analysis could predict C-C motif chemokine receptor type 5 (CCR5) expression and survival in OC [39].
Targeting CD27 in OC treatment has a potential application. However, the detection method of CD27 is limited. The study constructed radiomics models to predict CD27 expression in OC, aiming to provide a non-invasive method for detecting CD27 and a tool for dynamic monitoring of molecular expression, thereby providing a useful strategy for precision medicine. Herein, the RFE-LR and LASSO-LR models based on CD27 expression, radiomics, and clinical features were developed, and the performance of the two models were compared. Both models showed the capability and applicability of noninvasively predicting the expression of CD27 in SOC. Furthermore, the LASSO-LR model performed better than the RFE-LR model in predicting the prognosis. In line with the literature [18, 37,38,39], our study demonstrates that combining biomarker-based features with standard radiomics offers a convenient and feasible strategy for improving prognosis prediction.
This study has some limitations. A limitation is its retrospective nature, so the findings require further validation. Another limitation is that all images were downloaded from a public dataset, and the sample size was relatively small. Hence, a multicenter study with good reproducibility should be conducted using independent cohorts.
Conclusion
The expression levels of CD27 significantly influenced the clinical prognosis of patients with SOC. Radiomics models based on CT signatures and clinical data can preoperatively discriminate CD27 expression levels and predict the prognosis of patients with SOC, providing a practical and noninvasive tool for predicting survival prognosis in patients with SOC.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Kaku T, Ogawa S, Kawano Y, Ohishi Y, Kobayashi H, Hirakawa T, et al. Histological classification of ovarian cancer. Med Electron Microsc. 2003;36:9–17.
Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773.
Starzer AM, Berghoff AS. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open. 2020;4:e000629.
Bullock TN. Stimulating CD27 to quantitatively and qualitatively shape adaptive immunity to cancer. Curr Opin Immunol. 2017;45:82–8.
Liu W, Maben Z, Wang C, Lindquist KC, Li M, Rayannavar V, et al. Structural delineation and phase-dependent activation of the costimulatory CD27:CD70 complex. J Biol Chem. 2021;297:101102.
Lens SM, Tesselaar K, van Oers MH, van Lier RA. Control of lymphocyte function through CD27-CD70 interactions. Semin Immunol. 1998;10:491–9.
Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, et al. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40.
Oshikawa Y, Makino T, Nakayama M, Sawamura S, Makino K, Kajihara I, et al. Increased CD27 expression in the skins and sera of patients with systemic sclerosis. Intractable Rare Dis Res. 2020;9:99–103.
Lutfi F, Wu L, Sunshine S, Cao X. Targeting the CD27-CD70 pathway to improve outcomes in both checkpoint immunotherapy and allogeneic hematopoietic cell transplantation. Front Immunol. 2021;12:715909.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–77.
Lambin P, Zindler J, Vanneste BG, De Voorde LV, Eekers D, Compter I, et al. Decision support systems for personalized and participative radiation oncology. Adv Drug Deliv Rev. 2017;109:131–53.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62.
Li H, Robinson K, Lan L, Baughan N, Chan CW, Embury M et al. Temporal Machine Learning Analysis of prior mammograms for breast Cancer risk prediction. Cancers (Basel). 2023;15.
Zhang S, Shao H, Li W, Zhang H, Lin F, Zhang Q et al. Intra- and peritumoral radiomics for predicting malignant BiRADS category 4 breast lesions on contrast-enhanced spectral mammography: a multicenter study. Eur Radiol. 2023.
Yoo J, Lee J, Cheon M, Kim H, Choi YS, Pyo H et al. Radiomics Analysis of (18)F-FDG PET/CT for prognosis prediction in patients with Stage III Non-small Cell Lung Cancer undergoing Neoadjuvant Chemoradiation Therapy followed by surgery. Cancers (Basel). 2023;15.
Zhou C, Hou L, Tang X, Liu C, Meng Y, Jia H, et al. CT-based radiomics nomogram may predict who can benefit from adaptive radiotherapy in patients with local advanced-NSCLC patients. Radiother Oncol. 2023;183:109637.
Avesani G, Tran HE, Cammarata G, Botta F, Raimondi S, Russo L et al. CT-Based Radiomics and Deep learning for BRCA mutation and progression-free survival prediction in Ovarian Cancer using a Multicentric dataset. Cancers (Basel). 2022;14.
Li C, Wang H, Chen Y, Zhu C, Gao Y, Wang X, et al. Nomograms of combining MRI multisequences Radiomics and clinical factors for differentiating high-Grade from Low-Grade Serous Ovarian Carcinoma. Front Oncol. 2022;12:816982.
Rizzo S, Manganaro L, Dolciami M, Gasparri ML, Papadia A, Del Grande F. Computed Tomography based Radiomics as a predictor of Survival in Ovarian Cancer patients: a systematic review. Cancers (Basel). 2021;13.
Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol. 2017;35:314–6.
Querleu D, Planchamp F, Chiva L, Fotopoulou C, Barton D, Cibula D, et al. European Society of Gynaecological Oncology (ESGO) guidelines for ovarian Cancer surgery. Int J Gynecol Cancer. 2017;27:1534–42.
Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;2011:CD007565.
Swiderska J, Kozlowski M, Gaur M, Pius-Sadowska E, Kwiatkowski S, Machalinski B et al. Clinical significance of BTLA, CD27, CD70, CD28 and CD80 as diagnostic and prognostic markers in Ovarian Cancer. Diagnostics (Basel). 2022;12.
Wouters MC, Komdeur FL, Workel HH, Klip HG, Plat A, Kooi NM, et al. Treatment regimen, Surgical Outcome, and T-cell differentiation influence Prognostic Benefit of Tumor-infiltrating lymphocytes in high-Grade Serous Ovarian Cancer. Clin Cancer Res. 2016;22:714–24.
Guo Y, Yang N, Li G, Yin X, Dong L. Identification of an Immune Gene-based cisplatin response model and CD27 as a therapeutic target against Cisplatin Resistance for Ovarian Cancer. J Immunol Res. 2022;2022:4379216.
Shiomi M, Matsuzaki S, Serada S, Matsuo K, Mizuta-Odani C, Jitsumori M, et al. CD70 antibody-drug conjugate: a potential novel therapeutic agent for ovarian cancer. Cancer Sci. 2021;112:3655–68.
Sanborn RE, Pishvaian MJ, Callahan MK, Weise A, Sikic BI, Rahma O et al. Safety, tolerability and efficacy of agonist anti-CD27 antibody (varlilumab) administered in combination with anti-PD-1 (nivolumab) in advanced solid tumors. J Immunother Cancer. 2022;10.
Zhang H, Mao Y, Chen X, Wu G, Liu X, Zhang P, et al. Magnetic resonance imaging radiomics in categorizing ovarian masses and predicting clinical outcome: a preliminary study. Eur Radiol. 2019;29:3358–71.
Qian L, Ren J, Liu A, Gao Y, Hao F, Zhao L, et al. MR imaging of epithelial ovarian cancer: a combined model to predict histologic subtypes. Eur Radiol. 2020;30:5815–25.
Jian J, Li Y, Pickhardt PJ, Xia W, He Z, Zhang R, et al. MR image-based radiomics to differentiate type iota and type IotaIota epithelial ovarian cancers. Eur Radiol. 2021;31:403–10.
Yao F, Ding J, Lin F, Xu X, Jiang Q, Zhang L, et al. Nomogram based on ultrasound radiomics score and clinical variables for predicting histologic subtypes of epithelial ovarian cancer. Br J Radiol. 2022;95:20211332.
Song XL, Ren JL, Yao TY, Zhao D, Niu J. Radiomics based on multisequence magnetic resonance imaging for the preoperative prediction of peritoneal metastasis in ovarian cancer. Eur Radiol. 2021;31:8438–46.
Yu XY, Ren J, Jia Y, Wu H, Niu G, Liu A, et al. Multiparameter MRI Radiomics Model predicts preoperative peritoneal carcinomatosis in Ovarian Cancer. Front Oncol. 2021;11:765652.
Chen HZ, Wang XR, Zhao FM, Chen XJ, Li XS, Ning G, et al. The Development and Validation of a CT-Based Radiomics Nomogram to Preoperatively Predict Lymph Node Metastasis in High-Grade Serous Ovarian Cancer. Front Oncol. 2021;11:711648.
Li HM, Gong J, Li RM, Xiao ZB, Qiang JW, Peng WJ, et al. Development of MRI-Based Radiomics Model to predict the risk of recurrence in patients with Advanced High-Grade Serous Ovarian Carcinoma. AJR Am J Roentgenol. 2021;217:664–75.
Wang X, Xu C, Grzegorzek M, Sun H. Habitat radiomics analysis of pet/ct imaging in high-grade serous ovarian cancer: application to Ki-67 status and progression-free survival. Front Physiol. 2022;13:948767.
Gao L, Jiang W, Yue Q, Ye R, Li Y, Hong J, et al. Radiomic model to predict the expression of PD-1 and overall survival of patients with ovarian cancer. Int Immunopharmacol. 2022;113:109335.
Wan S, Zhou T, Che R, Li Y, Peng J, Wu Y, et al. CT-based machine learning radiomics predicts CCR5 expression level and survival in ovarian cancer. J Ovarian Res. 2023;16:1.
Acknowledgements
Not applicable.
Funding
This work was supported by the Beijing Natural Science Foundation (No. 7222202) and National Key Research and Development Program of China (No. 2022YFC2704204).
Author information
Authors and Affiliations
Contributions
C.Z. conceived the study, performed data analysis and wrote the manuscript. H.C., Y.L., and X.H.C. supervised the experiments and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Considering that all data and images from TCIA and TCGA were anonymized and publicly available, the requirements for ethical review and informed consent were waived.
Consent for publication
Not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., Cui, H., Li, Y. et al. Predicting CD27 expression and clinical prognosis in serous ovarian cancer using CT-based radiomics. J Ovarian Res 17, 131 (2024). https://doi.org/10.1186/s13048-024-01456-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-024-01456-7