Abstract
Background
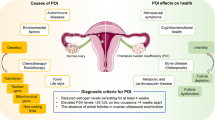
The etiology of premature ovarian insufficiency, that is, the loss of ovarian activity before 40 years of age, is complex. Studies suggest that genetic factors are involved in 20–25% of cases. The aim of this study was to explore the oligogenic basis of premature ovarian insufficiency.
Results
Whole-exome sequencing of 93 patients with POI and whole-genome sequencing of 465 controls were performed. In the gene-burden analysis, multiple genetic variants, including those associated with DNA damage repair and meiosis, were more common in participants with premature ovarian insufficiency than in controls. The ORVAL-platform analysis confirmed the pathogenicity of the RAD52 and MSH6 combination.
Conclusions
The results of this study indicate that oligogenic inheritance is an important cause of premature ovarian insufficiency and provide insights into the biological mechanisms underlying premature ovarian insufficiency.
Similar content being viewed by others
Background
Premature ovarian insufficiency (POI), characterized by the decline of ovarian function before 40 years of age, affects 3.7% of women globally [1]. It is highly heterogeneous, ranging from ovarian dysgenesis and primary amenorrhea to post-pubertal secondary amenorrhea, with elevated serum gonadotropin levels and hypoestrogenism [2]. Long-term complications include osteoporosis, cardiovascular/neurological disease, and cancer. The development of POI in patients with secondary amenorrhea can be a gradual process that encompasses occult, biochemical and overt stages [3]. Because of the irreversible nature of the decline in ovarian function, there is no effective method of restoring and improving ovarian function. Therefore, researchers should focus on early recognition, early diagnosis, and early intervention in disease development in patients with POI, which will subsequently be of great significance in improving the quality of life of patients in the near future and preventing complications in the long term. Its etiology is complex, involving genetic, immune, infectious, and iatrogenic factors [4]. Genetic factors have been identified in 20–25% of cases [5].
Whole-exome sequencing has been used to identify novel candidate genes for POI associated with various biological functions [6]. However, no genes have been implicated in more than 5% of cases, except for BMP15, FMR1, and NOBOX [7]. Genome-wide association studies (GWASs) have identified several single-nucleotide polymorphisms [8,9,10,11,12,13,14,15]. However, the cohorts were small, the results varied among populations, and the suggested candidate genes tend to lack biological evidence of a direct association with the ovary. Although whole-exome sequencing and GWASs have revealed a part of the genetic basis of POI, > 50% of cases are idiopathic [5]. The delayed intervention is a consequence of the failure to diagnose at an earlier stage [3]. Genetic testing can provide families with important information about the risks and etiology of POI [16]. None of the mutations in one or a few genes or a particular genetic mechanism explains most of the pathophysiological mechanisms of POI, and some of the challenges that have arisen in genetic studies have not yet been plausibly explained (e.g., despite familial onset of the disease, majority of patients present with sporadic cases, and some candidate genes are incomplete in families with autosomal dominant inheritance), suggesting the need to look at the genetic background of POI development from a new genetic perspective, which suggests that new causative mechanisms remain to be explored. The exploration of therapeutic targets, such as in vitro activation, holds significant importance in the context of research and providing guidance for genetic counseling and pregnancy planning [16].
The inheritance of a trait (or disease) by a few genes is defined as oligogenic inheritance. It is an intermediate state between monogenic and polygenic inheritance [17]. Since the first report of retinitis pigmentosa as a digenic disease in 1994 [18], 207 digenic or oligogenic diseases have been reported [19]. Several studies [20,21,22] have reported the possibility of oligogenic inheritance in POI. The oligogenic inheritance pattern may be a more plausible explanation for the differences in clinical symptoms, time of onset, and severity of clinical manifestations among patients with POI. Variants at different loci in the same gene, or multiple genes with multiple variants, may contribute to the different clinical phenotypes of patients with POI. However, these studies mainly included patients with sporadic disease in European countries and did not include healthy control groups. Moreover, the authors did not validate their findings or investigate the potential mechanisms. Combinations of variants may cause POI by similar or different mechanisms and pathways. Therefore, we recruited Chinese individuals with POI and normal women (as controls) and aimed to investigate the oligogenic basis of POI, which may aid early diagnosis and treatment. The overall study design is shown in Fig. 1.
Results
POI cohort gene-burden analysis
Sequencing analysis of 93 patients with POI and 465 controls was performed. Gene-burden analysis was performed after whole-exome sequencing, quality control, and variant annotation. Genes were the basic study unit, and different groups were analyzed separately from controls. There were 7,549 variants, including 4,631 loss-of-function (included frame-shift, splice-site or stop variants) and 4,471 missense variants. In total, 2,924 variants, including 1,792 loss-of-function and 1,704 missense variants were significant in the comparison between patients and controls (P < 0.05; Additional File 1). The P-values and quantiles (calculated by ranking the genes according to P-values) are summarized in Additional File 2.
Participants heterozygous for multiple variants
Regarding the 191 POI-related genes, 35.5% (33/93) of patients with POI and 8.2% (38/465) of controls were heterozygous for more than one variant (odds ratio, 6.20 [95% confidence interval: 3.60–10.60]; P = 1.50 × 10− 10; Table 1). Overall, multiple variants were more common in patients with POI than in controls.
Additional File 3 provides an overview of the 33 patients with POI who were heterozygous for more than one variant. The proportions of patients who were heterozygous for two, three, four, and five variants were 16.1% (15/93), 10.8% (10/93), 7.5% (7/93), and 1.1% (1/93), respectively. The highest proportion of patients with POI was heterozygous for two variants.
Analysis and validation of variant combinations
Gene-burden analysis
Among 191 POI-related genes, the top 15 genes (P < 0.05) ranked using gene-burden analysis are listed in Table 2. RAD52 (P = 5.28 × 10− 4) and MSH6 (P = 5.98 × 10− 4) were enriched in patients with POI, ranking first and second, respectively. We identified RAD52 variants in 9/93 (9.7%) patients with POI; seven of these patients (77.8%) were heterozygous for an additional variant in a POI-related gene (MSH6, TEP1, POLG, MLH1, or NUP107; Additional File 4).
Validation of the RAD52 and MSH6 combination
Two patients were heterozygous for both variants; the RAD52 and MSH6 combination was not detected in the control group (P = 0.027; Additional file 5). Oligomeric network analysis using the ORVAL platform showed that RAD52 and MSH6 existed in combination with candidate disease-causing variants (Additional File 6). In CADD raw score generation, gene haploinsufficiency prediction, and biological process similarity, VarCoPP predicted that the RAD52 and MSH6 combination was pathogenic (scores of 1.0; Table 3). Using the Digenic Effect predictor, loci were classified as “true digenic” or “monogenic + modifier” (Table 3 and Additional File 6).
Protein–protein interaction (PPI) networks
PPI network analysis revealed associations of RAD52 and MSH6 with DNA damage-repair processes (such as DNA recombination, DNA repair complex, nucleotide-excision repair, double-strand break repair, and the homologous recombination pathway), suggesting their significant roles in DNA damage-repair processes (Fig. 2).
Biological functions of POI-related genes
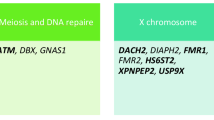
POI-related genes are involved in various biological functions, such as meiosis and DNA damage repair, gonadal formation, and ovarian development, and the encoded proteins serve as signaling molecules and transcription factors. Gene-burden analyses were performed on four gene sets with different biological functions. A significant difference was observed in the number of genes associated with meiotic and DNA repair pathways between patients with POI and controls (P = 4.04 × 10–9; Table 4).
Discussion
Whole-exome and -genome sequencing are widely used for the diagnosis and molecular analysis of POI, thereby gradually improving our understanding of its molecular basis. In the gene-burden analysis, multiple genetic variants and genetic variants associated with DNA damage repair and meiosis were more commonly found in patients with POI than in controls. The ORVAL-platform analysis supported the pathogenicity of the RAD52 and MSH6 combination. Our results indicate that oligogenic inheritance is an important cause of POI.
Regarding POI-related genes, the incidence of POI in patients with multiple variants was significantly higher than that in controls. However, some individuals in the control group also had multiple POI-related genetic variants. It is possible that variants on different alleles have different degrees of pathogenicity as some alleles may not be sufficient to cause a phenotypic trait or disease. Alternatively, one or more variants can cause POI, whereas simultaneous variants can mitigate or counteract the pathogenicity of the variant via interaction effects [23]. In this study, the RAD52 and MSH6 combination was classified as “true digenic” in one patient and as “monogenic + modifier” in another. RAD52 variants were at the same locus in both patients, whereas MSH6 variants were at different loci. This illustrates that the same gene combination with different alleles can lead to a different classification, indicating the complexity of predicting and classifying oligogenic pathogenic combinations [24]. In both patients, another variant was detected in addition to the RAD52 and MSH6 combination. However, it was difficult to predict the pathogenicity of combinations involving three or more variants. Additional data are needed to support the classification criterion of the RAD52 and MSH6 combination.
Using age of onset and FSH values as indicators of the severity of the POI phenotype, we analyzed the relationship between the age of onset (age at which oligomenorrhea or amenorrhea occurs) and FSH values of patients respectively, and the number of variants carried by patients with POI. Our results showed that a higher number of variants in patients with POI was associated with a lower age of onset (Fig. 3a), however, there were no statistical differences between groups (Fig. 3b); In addition, our results indicated a positive correlation between the value of FSH and the number of variants carried by patients with POI (Fig. 3c), and differences between groups were statistically significant (P < 0.01) (Fig. 3d). However, this analysis did not include factors such as the pathogenicity of variants and the contribution of different genes. More cases and data analyses are required to construct disease prediction models for POI, including age of onset, age at menopause, phenotype severity, master genes, and the specific relative contribution of each locus.
Relationship of age of onset, FSH and number of variants carried in patients with POI. (a) Relation between age of POI onset and the number of variants carried, (b) Comparison of age of POI onset in patients with one or no variants compared with those with two or more variants, (c) Relation between FSH and the number of variants carried in patients with POI, (d) Comparison of FSH in patients with one or no variants compared with those with two or more variants. FSH: follicle-stimulating hormone; ns: no significance; **P < 0.01
POI-related genes are involved in various biological functions, such as meiosis and DNA damage repair, gonadal formation, ovarian development, and the encoded proteins serve as signaling molecules and transcription factors [4]. RAD52 is involved in DNA double-strand break repair and mitotic/meiotic recombination [7]. RAD51, which is the known causative gene for POI [25], is in the same gene family as RAD52. MSH6 variants have been reported in patients with POI [26]. Although RAD52 variants were more commonly found in patients with POI than in controls, RAD52 has not yet been identified as a pathogenic gene for POI, and a clear pathogenic mechanism by which RAD52 causes POI has not been determined. We found TEP1 and FANCG as novel potential candidate disease-causing genes for POI. TEP1 is a part of the telomerase ribonucleoprotein complex that catalyzes the addition of new telomeres to chromosome ends [27]. A FANCG mutation has been identified in patients with Fanconi anemia of complementation group G [28]. The N-terminal nuclear localization signal of FANCA is necessary for FANCG binding, FANCC binding, sensitivity of complementary FAA lymphocytes to mitomycin C, and nuclear localization [28]. More evidence on whether TEP1 and FANCG are causative genes for POI is required. Our results confirmed that the frequencies of genetic variants associated with the meiotic and DNA repair pathways differed between patients with POI and controls. Several variants are involved in the meiotic and DNA repair pathways. Therefore, these pathways represent promising therapeutic targets.
Whole-exome sequencing is a molecular diagnostic approach that can be used to explore multiple genetic variants. In an whole-exome sequencing analysis of 2,076 participants, 101 (4.9%) were found to be heterozygous for multiple genetic variants [29]. Oligogenic inheritance models have been proposed for 207 diseases in the OLIDA database. Tools for oligogenic analysis include DiGePred, OligoPVP, and VarCoPP/ORVAL. DiGePred is a random forest classifier developed to specifically identify pathogenic variant combinations based on biological networks, along with genomic, evolutionary, and functional data. DiGePred facilitates identification of genetic factors for rare non-monogenic diseases by scoring the potential of variant combinations to cause a biallelic disease [30]. OligoPVP combines a random forest classifier and a deep neural network to predict the pathogenicity of combinations of genetic variants, using feature sets from different tools (e.g., CADD and DANN) to classify the variant combinations as pathogenic or non-pathogenic [31]. In addition, VarCoPP is a machine-learning tool that identifies pathogenic variant combinations in gene pairs (digenic/bilocus variant combinations), generating classification scores for combinations of genetic variants using 11 different biological traits [32]. ORVAL is another tool that extends VarCoPP predictions to include more features (e.g., network-based exploration) [33] and integrates different resources to predict combinations of oligogenic variants that cause disease. Several studies have used VarCoPP/ORVAL to predict variant combinations [34,35,36,37,38], as in this study. The advantages of ORVAL over other tools include the number of predicted combinations of pathogenic variants, ability to classify combinations of variants, availability of detailed information on genes at the level of functional pathways, and visual presentation of the results. In this study, we investigated the oligogenic inheritance of POI using gene-burden analysis, quantified the contributions of variants to the disease by P-value ranking, and demonstrated that the frequency of multiple POI-related genetic variants differed significantly between patients with POI and controls. However, pathogenicity prediction was not possible using gene-burden analysis. Accordingly, this approach was combined with VarCoPP/ORVAL to demonstrate the roles of multiple POI-related genetic variants in the disease and to score and classify combinations of variants in patients for pathogenicity prediction. These findings support an oligogenic inheritance model of POI and will inform oligogenic studies in more disease cohorts.
In this study, 93 participants with POI and 465 controls were recruited. The sample sizes are being expanded and data from parents and other family members are being collected to verify whether the variants are consistent with familial co-segregation.
Conclusions
Overall, we demonstrated that POI is consistent with an oligogenic inheritance model by going beyond the traditional methods of screening and validation of pathogenic genes for POI. Our findings will inform cohort-based oligogenic studies and provide insights into the biological mechanisms underlying POI. Our findings provide support for further research on the etiology of and potential therapeutic targets for POI.
Methods
POI group
In total, 93 patients with POI (Two and 91 displayed primary or secondary amenorrhea, respectively) were recruited by stage from Xiangya Second Hospital of Central South University, Hunan, China; Changsha Reproductive Medicine Hospital, Changsha, China; and Changsha Jiangwan Maternity Hospital, Changsha, China. All patients with POI were women aged < 40 years with oligomenorrhea or amenorrhea for ≥ 4 months and elevated follicle-stimulating hormone levels > 25 IU/L on two occasions > 4 weeks apart. Patients with chromosomal abnormalities, FMR1 variants, pelvic surgery, endometriosis, ovarian infection, radiotherapy or chemotherapy, and endocrine autoimmune diseases were excluded. Blood samples (2–3 mL) were collected in EDTA anticoagulation tubes. DNA was extracted and stored at − 80 °C.
Control group
In total, 465 women aged 45–65 years in the menopause stage (no amenorrhea and regular menstrual cycles before age 40) were recruited from The Third Affiliated Hospital of Southern Medical University in 2017. Whole-genome sequencing data were collected previously [39].
Whole-exome sequencing and variant calling
In total, 191 POI-related genes were obtained from the literature [6, 7, 40] and IDDB database (Additional File 7). To clarify the role of genes with different biological functions in POI etiology, the biological functions are mainly categorized into meiosis and DNA damage repair, gonadal formation, ovarian development, and signaling molecules and transcription factors according to previous literature [6, 41]. We have organized the different gene sets based on four different biological functions (Additional File 8).
Whole-exome sequencing was performed in 93 patients with POI. Genomic DNA was extracted from peripheral blood. Whole-exome sequences were captured using the SureSelect Target Enrichment System for Illumina Paired-End Sequencing Library (Agilent Technologies, Santa Clara, CA, USA). DNA sequencing was performed on the Illumina HiSeq Platform (Illumina, San Diego, CA, USA). Reads were mapped to the GRCh37 genome. Variants were annotated using GATK, ANNOVAR, and custom pipelines.
Gene-burden analysis
Gene-burden analysis of 191 POI-related genes was performed. The following filtering thresholds were applied: read depth, > 20; minor allele frequency, < 5% in GnomAD/ExAC/1000 Genomes Phase 3; and in silico prediction tools (REVEL, > 0.5; Splice Site, > 0.6; and scSNV, > 0.6). Genes were weighted using SKAT-O, and associations between genetic variants and POI were evaluated.
Prediction of oligogenic pathogenic variant combinations
ORVAL is a web-based bioinformatics platform for predicting pathogenic variant combinations. VarCoPP, the variant combination pathogenicity predictor, was used to obtain the probability of whether a particular combination of pathogenic loci was a true positive result. The Digenic Effect predictor was used to classify pathogenic variant combinations as “true digenic,” “monogenic + modifier,” or “dual molecular diagnosis [32].”
PPI networks
The GeneMANIA database (http://genemania.org/), a powerful tool for analyzing gene lists, predicting gene function, and prioritizing genes for functional analysis, was used to construct PPI networks for the genes. The database identifies genes with similar functions and assigns a value to each functional genome dataset based on the predicted value of the query by integrating multiple genomic and proteomic data. This integrated approach enhances the accuracy of gene function prediction and facilitates a deeper understanding of the complex interactions between genes within biological pathways.
Statistical analysis
Gene-burden analysis was performed using “SKAT” (R version 2.2.4; R Foundation for Statistical Computing, Vienna, Austria). Fisher’s exact test was performed using “SciPy” (Python version 1.7.0) [42]. GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. P < 0.05 was considered statistically significant.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ESHRE:
-
European Society for Human Reproduction and Embryology
- GWAS:
-
Genome-wide association study
- POI:
-
Premature ovarian insufficiency
References
Golezar S, Ramezani TF, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric. 2019;22:403–11. https://doi.org/10.1080/13697137.2019.1574738.
Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–37. https://doi.org/10.1093/humrep/dew027.
Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499–509. https://doi.org/10.1111/j.1365-2265.2007.03073.x.
De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–21. https://doi.org/10.1016/S0140-6736(10)60355-8.
Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21:787–808. https://doi.org/10.1093/humupd/dmv036.
Yang Q, Mumusoglu S, Qin Y, Sun Y, Hsueh AJ. A kaleidoscopic view of ovarian genes associated with premature ovarian insufficiency and senescence. Faseb J. 2021;35:e21753. https://doi.org/10.1096/fj.202100756R.
Jiao X, Ke H, Qin Y, Chen ZJ. Molecular Genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29:795–807. https://doi.org/10.1016/j.tem.2018.07.002.
Kang H, Lee SK, Kim MH, Song J, Bae SJ, Kim NK, et al. Parathyroid hormone-responsive B1 gene is associated with premature ovarian failure. Hum Reprod. 2008;23:1457–65. https://doi.org/10.1093/humrep/den086.
Knauff EA, Franke L, van Es MA, van den Berg LH, van der Schouw YT, Laven JS, et al. Genome-wide association study in premature ovarian failure patients suggests ADAMTS19 as a possible candidate gene. Hum Reprod. 2009;24:2372–8. https://doi.org/10.1093/humrep/dep197.
Pyun JA, Cha DH, Kwack K. LAMC1 gene is associated with premature ovarian failure. Maturitas. 2012;71:402–6. https://doi.org/10.1016/j.maturitas.2012.01.011.
Qin Y, Zhao H, Xu J, Shi Y, Li Z, Qiao J, et al. Association of 8q22.3 locus in Chinese Han with idiopathic premature ovarian failure (POF). Hum Mol Genet. 2012;21:430–6. https://doi.org/10.1093/hmg/ddr462.
Park J, Park Y, Koh I, Kim NK, Baek KH, Yun BS, et al. Association of an APBA3 missense variant with risk of premature ovarian failure in the Korean Female Population. J Pers Med. 2020;10. https://doi.org/10.3390/jpm10040193.
Oldenburg RA, van Dooren MF, de Graaf B, Simons E, Govaerts L, Swagemakers S, et al. A genome-wide linkage scan in a Dutch family identifies a premature ovarian failure susceptibility locus. Hum Reprod. 2008;23:2835–41. https://doi.org/10.1093/humrep/den278.
Qin Y, Vujovic S, Li G, Li J, Dalgleish R, Simpson JL, et al. Ethnic specificity of variants of the ESR1, HK3, BRSK1 genes and the 8q22.3 locus: no association with premature ovarian failure (POF) in Serbian women. Maturitas. 2014;77:64–7. https://doi.org/10.1016/j.maturitas.2013.09.006.
Caburet S, Zavadakova P, Ben-Neriah Z, Bouhali K, Dipietromaria A, Charon C, et al. Genome-wide linkage in a highly consanguineous pedigree reveals two novel loci on chromosome 7 for non-syndromic familial premature ovarian failure. PLoS One. 2012;7:e33412. https://doi.org/10.1371/journal.pone.0033412.
Ke H, Tang S, Guo T, Hou D, Jiao X, Li S, et al. Landscape of pathogenic mutations in premature ovarian insufficiency. Nat Med. 2023;29:483–92. https://doi.org/10.1038/s41591-022-02194-3.
Goutman SA, Hardiman O, Al-Chalabi A, Chio A, Savelieff MG, Kiernan MC, et al. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022;21:480–93. https://doi.org/10.1016/S1474-4422(21)00465-8.
Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264:1604–8. https://doi.org/10.1126/science.8202715.
Nachtegael C, Gravel B, Dillen A, Smits G, Nowe A, Papadimitriou S et al. Scaling up oligogenic diseases research with OLIDA: the Oligogenic Diseases Database. Database (Oxford). 2022;2022: https://doi.org/10.1093/database/baac023.
Bouilly J, Beau I, Barraud S, Bernard V, Azibi K, Fagart J, et al. Identification of multiple gene mutations accounts for a new Genetic Architecture of primary ovarian insufficiency. J Clin Endocrinol Metab. 2016;101:4541–50. https://doi.org/10.1210/jc.2016-2152.
Patino LC, Beau I, Carlosama C, Buitrago JC, Gonzalez R, Suarez CF, et al. New mutations in non-syndromic primary ovarian insufficiency patients identified via whole-exome sequencing. Hum Reprod. 2017;32:1512–20. https://doi.org/10.1093/humrep/dex089.
Rossetti R, Moleri S, Guizzardi F, Gentilini D, Libera L, Marozzi A, et al. Targeted next-generation sequencing indicates a frequent oligogenic involvement in primary ovarian insufficiency onset. Front Endocrinol (Lausanne). 2021;12:664645. https://doi.org/10.3389/fendo.2021.664645.
Gropman AL, Adams DR. Atypical patterns of inheritance. Semin Pediatr Neurol. 2007;14:34–45. https://doi.org/10.1016/j.spen.2006.11.007.
Deltas C. Digenic inheritance and genetic modifiers. Clin Genet. 2018;93:429–38. https://doi.org/10.1111/cge.13150.
Li D, Xu W, Wang X, Dang Y, Xu L, Lu G, et al. lncRNA DDGC participates in premature ovarian insufficiency through regulating RAD51 and WT1. Mol Ther Nucleic Acids. 2021;26:1092–106. https://doi.org/10.1016/j.omtn.2021.10.015.
Luo W, Guo T, Li G, Liu R, Zhao S, Song M, et al. Variants in homologous recombination genes EXO1 and RAD51 related with premature ovarian insufficiency. J Clin Endocrinol Metab. 2020;105. https://doi.org/10.1210/clinem/dgaa505.
Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, et al. A mammalian telomerase-associated protein. Science. 1997;275:973–7. https://doi.org/10.1126/science.275.5302.973.
de Winter JP, Waisfisz Q, Rooimans MA, van Berkel CG, Bosnoyan-Collins L, Alon N, et al. The fanconi anaemia group G gene FANCG is identical with XRCC9. Nat Genet. 1998;20:281–3. https://doi.org/10.1038/3093.
Posey JE, Harel T, Liu P, Rosenfeld JA, James RA, Coban AZ, et al. Resolution of Disease Phenotypes resulting from Multilocus genomic variation. N Engl J Med. 2017;376:21–31. https://doi.org/10.1056/NEJMoa1516767.
Mukherjee S, Cogan JD, Newman JH, Phillips JR, Hamid R, Meiler J, et al. Identifying digenic disease genes via machine learning in the undiagnosed diseases Network. Am J Hum Genet. 2021;108:1946–63. https://doi.org/10.1016/j.ajhg.2021.08.010.
Boudellioua I, Kulmanov M, Schofield PN, Gkoutos GV, Hoehndorf R, OligoPVP. Phenotype-driven analysis of individual genomic information to prioritize oligogenic disease variants. Sci Rep. 2018;8:14681. https://doi.org/10.1038/s41598-018-32876-3.
Papadimitriou S, Gazzo A, Versbraegen N, Nachtegael C, Aerts J, Moreau Y, et al. Predicting disease-causing variant combinations. Proc Natl Acad Sci U S A. 2019;116:11878–87. https://doi.org/10.1073/pnas.1815601116.
Renaux A, Papadimitriou S, Versbraegen N, Nachtegael C, Boutry S, Nowe A, et al. ORVAL: a novel platform for the prediction and exploration of disease-causing oligogenic variant combinations. Nucleic Acids Res. 2019;47:W93–8. https://doi.org/10.1093/nar/gkz437.
Zhao T, Ma Y, Zhang Z, Xian J, Geng X, Wang F, et al. Young and early-onset dilated cardiomyopathy with malignant ventricular arrhythmia and sudden cardiac death induced by the heterozygous LDB3, MYH6, and SYNE1 missense mutations. Ann Noninvasive Electrocardiol. 2021;26:e12840. https://doi.org/10.1111/anec.12840.
Mkaouar R, Abdallah L, Naouali C, Lahbib S, Turki Z, Elouej S, et al. Oligogenic inheritance underlying incomplete penetrance of PROKR2 mutations in Hypogonadotropic Hypogonadism. Front Genet. 2021;12:665174. https://doi.org/10.3389/fgene.2021.665174.
Dallali H, Kheriji N, Kammoun W, Mrad M, Soltani M, Trabelsi H, et al. Multiallelic Rare variants in BBS genes support an oligogenic ciliopathy in a non-obese Juvenile-Onset Syndromic Diabetic patient: a Case Report. Front Genet. 2021;12:664963. https://doi.org/10.3389/fgene.2021.664963.
Chen J, Ma Y, Li H, Lin Z, Yang Z, Zhang Q, et al. Rare and potential pathogenic mutations of LMNA and LAMA4 associated with familial arrhythmogenic right ventricular cardiomyopathy/dysplasia with right ventricular heart failure, cerebral thromboembolism and hereditary electrocardiogram abnormality. Orphanet J Rare Dis. 2022;17:183. https://doi.org/10.1186/s13023-022-02348-z.
Perea-Romero I, Solarat C, Blanco-Kelly F, Sanchez-Navarro I, Bea-Mascato B, Martin-Salazar E, et al. Allelic overload and its clinical modifier effect in Bardet-Biedl syndrome. NPJ Genom Med. 2022;7:41. https://doi.org/10.1038/s41525-022-00311-2.
Lv WQ, Lin X, Shen H, Liu HM, Qiu X, Li BY, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J Cachexia Sarcopenia Muscle. 2021;12:1860–70. https://doi.org/10.1002/jcsm.12788.
Wu J, Li D, Liu X, Li Q, He X, Wei J, et al. IDDB: a comprehensive resource featuring genes, variants and characteristics associated with infertility. Nucleic Acids Res. 2021;49:D1218–24. https://doi.org/10.1093/nar/gkaa753.
Franca MM, Funari M, Lerario AM, Santos MG, Nishi MY, Domenice S, et al. Screening of targeted panel genes in Brazilian patients with primary ovarian insufficiency. PLoS One. 2020;15:e240795. https://doi.org/10.1371/journal.pone.0240795.
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17:261–72. https://doi.org/10.1038/s41592-019-0686-2.
Acknowledgements
We acknowledge all individuals who participated in the study and are grateful to the Jiangwan Group for their support in the Construction Project of the Center of Reproductive Health. We thank the research team at the ORVAL platform for predicting pathogenic variants and Editage (www.editage.com) for English language editing.
Funding
This study was supported by the National Key Research and Development Program of China [grant number: 2017YFC1001100] and A Project Supported by Scientific Research Fund Of Hunan Provincial Education Department [grant number: 23A0731]. The funder had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
P.L., L.W., H.D., and H.X. conceptualized the study. P.L. wrote the main manuscript text. P.L., L.W., H.T., R.Q., Z.H., M.Z., Z.D., and HH curated the data. P.L., L.W., and H.T. conducted the formal analysis. P.L., L.W., J.G., H.D., and H.X. contributed to the investigation, methodology, and data validation. P.L. and R.Q. contributed to the data visualization. P.L. drafted the original manuscript. P.L., L.W., H.T., R.Q., Z.H., M.Z., Z.D., H.H., J.G., H.D., and H.X. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study design was approved by the Research Ethics Committee of the School of Basic Medicine, Central South University (approval number: 2022-KT110). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13048_2024_1351_MOESM6_ESM.tif
Additional File 6: Figure S2. Oligogenic combination networks and radar plots. (a, b) Oligogenic combination networks for patients 64 and 66. Gene pairs are connected if they contain at least one pathogenic variant combination. Edge color: highest pathogenicity score (highest VarCoPP score) for a variant in the pair, shown from low (yellow) to high (dark red) pathogenicity scores. (c, d) Radar plots of the prediction results of the Digenic Effect for patients 64 and 66.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Long, P., Wang, L., Tan, H. et al. Oligogenic basis of premature ovarian insufficiency: an observational study. J Ovarian Res 17, 32 (2024). https://doi.org/10.1186/s13048-024-01351-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-024-01351-1