Abstract
Polycystic ovarian syndrome (PCOS) is a genetically complex disorder that involves the interplay of multiple genes and environmental factors. It is characterized by anovulation and irregular menses and is associated with type 2 diabetes. Neuroendocrine pathways and ovarian and adrenal dysfunctions are possibly implicated in the disorder pathogenesis. The melatonin system plays a role in PCOS. Melatonin receptors are expressed on the surface of ovarian granulosa cells, and variations in the melatonin receptor genes have been associated with increased risk of PCOS in both familial and sporadic cases. We have recently reported the association of variants in MTNR1A and MTNR1B genes with familial type 2 diabetes. In this study, we aimed to investigate whether MTNR1A and MTNR1B contribute to PCOS risk in peninsular families. In 212 Italian families phenotyped for PCOS, we amplified by microarray 14 variants in the MTNR1A gene and 6 variants in the MTNR1B gene and tested them for linkage and linkage disequilibrium with PCOS. We detected 4 variants in the MTNR1A gene and 2 variants in the MTNR1B gene significantly linked and/or in linkage disequilibrium with the risk of PCOS (P < 0.05). All variants are novel and have not been reported before with PCOS or any of its related phenotypes, except for 3 variants previously reported by us to confer risk for type 2 diabetes and 1 variant for type 2 diabetes-depression comorbidity. These findings implicate novel melatonin receptor genes’ variants in the risk of PCOS with potential functional roles.
Similar content being viewed by others
Background
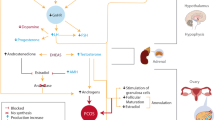
Melatonin is a pineal hormone known for its role in the regulation of circadian [1] and seasonal rhythms [2], in addition to glucose and lipid metabolism [3, 4] with a role in obesity [5], anti-inflammation and antioxidation [6,7,8].
Melatonin exerts its roles through two melatonin G protein-coupled receptors: melatonin receptor 1 A (encoded by MTNR1A gene) and melatonin receptor 1B (encoded by MTNR1B gene) [9, 10]. The two receptors are expressed on the nervous system, pancreas, liver, skeletal muscle, adipose tissue, and ovaries [6, 11]. The two melatonin receptors have been implicated in the risk of mental and metabolic disorders such as type 2 diabetes (T2D) [12,13,14] and depression (MDD) [14, 15].
Of interest, polycystic ovarian syndrome (PCOS), a complex and common hormonal disorder affecting women of reproductive age and characterized by anovulation, hyperandrogenism, and polycystic ovaries, is commonly associated with type 2 diabetes (T2D) [16] and mental traits, including anxiety [17, 18] and depression [19, 20]. PCOS, which affects approximately 6-18% of women worldwide [21] and can lead to long-term health consequences, including infertility, metabolic syndrome, cardiovascular disease, is a genetically complex disorder that involves the interplay of multiple genes and environmental factors [22]. PCOS is linked to a variety of possible pathogenetic impairments, distinct or overlapping, including the neuroendocrine pathways, and ovarian and adrenal hormonal secretions [23]. The circadian rhythm and melatonin system have been implicated in PCOS [24]. Candidate gene studies have associated PCOS with several genes, including the insulin receptor gene (INSR) [25], insulin-like growth factor (IGF) system genes, luteinizing hormone (LH) /chorionic gonadotropin receptor gene (LHCGR) [26], genes involved in androgen biosynthesis and steroid hormone metabolism (CYP11) [25], and the melatonin receptor genes (MTNR1A and MTNR1B) [27]. Melatonin receptors are expressed on the surface of ovarian granulosa cells [28] and variations in the melatonin receptor genes have been associated with increased risk of PCOS in both familial and sporadic cases [27]. We have recently reported the linkage and linkage plus association of variants in MTNR1A with familial type 2 diabetes [13] and in MTNR1B [14] with familial type 2 diabetes and type 2 diabetes-depression comorbidity. In this study, we aimed to investigate whether the MTNR1A and MTNR1B genes are in linkage to and/or linkage disequilibrium (LD, i.e., association joint to linkage) with PCOS in Italian families.
Materials and methods
We originally recruited for a type 2 diabetes (T2D) study 212 Italian families, which were later phenotyped for PCOS according to the phenotypes for PCOS necessary to meet the Rotterdam diagnostic criteria (presence of at least two of these three characteristics: chronic anovulation or oligomenorrhea, clinical or biochemical hyperandrogenism, and/or polycystic ovaries) [29]. We amplified by microarray 14 variants in the MTNR1A gene and 6 variants in the MTNR1B gene and tested them for linkage and LD with PCOS, using Pseudomarker [30] with dominant and recessive models with complete or incomplete penetrance, after excluding genotyping and Mendelian errors via PLINK [31]. The first tool (Pseudomarker) offers a robust method to simultaneously examine linkage and LD in a combination of family and singleton samples, utilizing the true pedigree relationships without relying on artificial assumptions to rectify linkage effects in statistics [30]. The second tool (PLINK) is a well-known toolset for whole genome association analysis that is both free and open-source, engineered to efficiently conduct various fundamental, large-scale analyses. We used the correlation coefficient of variants with data from the 1000 Genomes Project (https://www.internationalgenome.org/data-portal/population/TSI) to estimate the presence of LD blocks.
In-Silico Analysis. We used different bioinformatics tools to predict the risk variants’ roles in transcription factor (TF) binding (SNP Function Prediction) [32], miRNA binding (mirSNP) [33], splicing (SpliceAI) [34], and regulatory potential (RegulomeDB) [35].
Results and discussion
We detected 4 variants in the MTNR1A gene and 2 variants in the MTNR1B gene significantly linked and/or associated (LD) with the risk of PCOS (P < 0.05) (Table 1). The variants were significant across different inheritance models (Fig. 1). Two variants (rs2119883 and rs13147179) were within an LD block (Set01). All variants are novel and have not been reported before with PCOS or any of its related phenotypes (i.e., T2D, obesity, insulin resistance, metabolic syndrome, hyperglycemia, oligomenorrhea, anovulation, irregular menses, hyperandrogenism, MDD, male-pattern baldness, acne, hirsutism, infertility), excluding T2D for 3 risk variants and T2D-MDD for 1 risk variant. Within peninsular families, the same risk alleles of the two variants (MTNR1B-rs61747139 and MTNR1A-rs2119883) were previously linked to and associated with the risk of T2D [13, 14], confirming the interrelatedness of these complex phenotypes. On the other hand, the non-risk alleles of the two variants (MTNR1A-rs13147179 and MTNR1B-rs4601728) were linked and associated with T2D and T2D-MDD comorbidity respectively [13, 14], indicating multiple association at the allelic level and possibly the presence of LD with other contributing yet undetected variants. Via our bioinformatic analyses, we found that the risk allele (A) of the variant MTNR1A-rs13147179 disrupts the binding of Kruppel-like factor 5 (KLF5) which is hypomethylated in the ovarian tissue in PCOS [36], potentially extending the role of this variant to epigenetic mechanisms. We also found that the risk allele (G) of the variant MTNR1B-rs61747139 affects the binding of transcription factor AP2A (TFAP2A) which is expressed in the brain, liver, pancreas, and ovaries [37] and forms a part of the signaling network of PCOS at least in vitro [38]. PCOS patients in our study could therefore be at higher risk due to altered expression of genes in PCOS pathways. Our study therefore implicates novel melatonin receptor genes’ variants in the risk of PCOS with potential functional roles. It also offers the possibility of inhibitors of melatonin metabolism (e.g., coumarins [39]) as novel therapeutic modalities in the treatment of PCOS. However, more studies are needed to validate these results.
Data availability
The study data are available on reasonable request, and due to lacking specific patients’ consent and privacy restrictions, they are not publicly available.
References
Lewy AJ, et al. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9(5):380–92.
Bartness TJ, et al. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15(4):161–90.
Genario R, et al. Melatonin supplementation in the management of obesity and obesity-associated disorders: a review of physiological mechanisms and clinical applications. Pharmacol Res. 2021;163:105254.
Garaulet M, et al. Melatonin effects on glucose metabolism: Time to unlock the controversy. Trends Endocrinol Metab. 2020;31(3):192–204.
Guan Q et al. Mechanisms of melatonin in obesity: a review. Int J Mol Sci, 2021. 23(1).
Pandi-Perumal SR, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–53.
Ferlazzo N et al. Is melatonin the cornucopia of the 21st Century?. Antioxid (Basel), 2020. 9(11).
Chitimus DM et al. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules, 2020. 10(9).
Li DY, et al. Melatonin receptor genes in vertebrates. Int J Mol Sci. 2013;14(6):11208–23.
Emet M, et al. A review of Melatonin, its receptors and drugs. Eurasian J Med. 2016;48(2):135–41.
Slominski RM, et al. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351(2):152–66.
Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019;15(2):105–25.
Amin M, Gragnoli C. Melatonin receptor 1A gene (MTNR1A) linkage and association to type 2 diabetes in Italian families. Eur Rev Med Pharm Sci (In Press; 2023.
Amin M et al. The role of melatonin receptor 1B gene (MTNR1B) in the susceptibility to depression and type 2 diabetes comorbidity Genes & Diseases (In Press), 2023.
Galecka E, et al. Single nucleotide polymorphisms and mRNA expression for melatonin MT(2) receptor in depression. Psychiatry Res. 2011;189(3):472–4.
Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030.
Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertil Steril. 2010;93(7):2421–3.
Wang Y, Ni Z, Li K. The prevalence of anxiety and depression of different severity in women with polycystic ovary syndrome: a meta-analysis. Gynecol Endocrinol. 2021;37(12):1072–8.
Kolhe JV, et al. PCOS and depression: common links and potential targets. Reprod Sci. 2022;29(11):3106–23.
Xing L, et al. Depression in polycystic ovary syndrome: focusing on pathogenesis and treatment. Front Psychiatry. 2022;13:1001484.
Joham AE, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022;10(9):668–80.
Unluturk U, et al. The genetic basis of the polycystic ovary syndrome: a Literature Review including discussion of PPAR-gamma. PPAR Res. 2007;2007:p49109.
Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520.
Wang F, et al. Association between circadian rhythm disruption and polycystic ovary syndrome. Fertil Steril. 2021;115(3):771–81.
Seyed Abutorabi E, et al. Investigation of the FSHR, CYP11, and INSR mutations and polymorphisms in Iranian infertile women with polycystic ovary syndrome (PCOS). Rep Biochem Mol Biol. 2021;9(4):470–7.
Singh S, et al. Association analysis of LHCGR variants and polycystic ovary syndrome in Punjab: a case-control approach. BMC Endocr Disord. 2022;22(1):335.
Yi S et al. Association between melatonin receptor gene polymorphisms and polycystic ovarian syndrome: a systematic review and meta-analysis. Biosci Rep, 2020. 40(6).
Woo MM, et al. Direct action of melatonin in human granulosa-luteal cells. J Clin Endocrinol Metab. 2001;86(10):4789–97.
Rotterdam EA. .-S.P.c.w.g., revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7.
Hiekkalinna T, et al. PSEUDOMARKER: a powerful program for joint linkage and/or linkage disequilibrium analysis on mixtures of singletons and related individuals. Hum Hered. 2011;71(4):256–66.
Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75.
Xu Z, Taylor JA. SNPinfo: Integrating GWAS and candidate gene information into functional SNP selection for genetic association studies Nucleic Acids Research, 2009. 37(SUPPL. 2).
Liu C, et al. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics. 2012;2012 13(1):1–10.
Jaganathan K, et al. Predicting Splicing from primary sequence with deep learning. Cell. 2019;176(3):535–548e24.
Boyle AP, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22(9):1790–7.
Cao P, et al. Characterization of DNA methylation and screening of epigenetic markers in polycystic ovary syndrome. Front Cell Dev Biol. 2021;9:664843.
Stelzer G, et al. The GeneCards suite: from Gene Data Mining to Disease Genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:p1301–13033.
Song J, et al. Androgen upregulates NR4A1 via the TFAP2A and ETS signaling networks. Int J Biochem Cell Biol. 2019;113:1–7.
Wang C, et al. Inhibition of melatonin metabolism in humans induced by chemical components from herbs and effective prediction of this risk using a computational model. Br J Pharmacol. 2016;173(22):3261–75.
Acknowledgements
We thank the families who participated in the study, and we thank Bios Biotech Multi-Diagnostic Health Center, Rome, Italy, for data access and for financial, medical, and laboratory staff support. This publication was supported in part with the funds received under Nebraska Laws 2021, LB 380, Sect. 109 awarded to C.G. (PI), Creighton University School of Medicine, through the Nebraska Department of Health & Human Services (DHHS). Its contents represent the views of the authors and do not necessarily represent the official views of the State of Nebraska or DHHS.
Author information
Authors and Affiliations
Contributions
C.G. (https://orcid.org/0000-0002-3873-6617) conceived and performed the study and critically revised the manuscript. T.T.P. (https://orcid.org/0000-0001-6056-4244) helped with the data interpretation and manuscript critical revision. Q.M.A.T drafted the manuscript and helped with literature search.
Corresponding author
Ethics declarations
Ethics approval
Families were recruited following the Helsinki declaration guidelines, and individuals provided written informed consent prior to participation. The Bios Ethical Committee approved this study (Prot.PR/Mg/Cg/311,708).
Competing interests
The authors declare no competing interests.
Authors’ information
C.G. is Professor of Medicine and Chief of Endocrinology and Endowed Puller Chair at Creighton University School of Medicine, Omaha, NE, and Adjunct Professor of Public Health Sciences, Penn State University College of Medicine, Hershey, PA; Q.M.A.T. is an internal medicine resident at Creighton University School of Medicine, Omaha, NE. T.T.P. is a Professor of Psychiatry at the University of Maryland School of Medicine, Baltimore, MD, and a research investigator with the Rocky Mountain MIRECC, Aurora, CO, and the VISN 5 MIRECC, Baltimore, MD.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Postolache, T.T., Al Tinawi, Q.M. & Gragnoli, C. The melatonin receptor genes are linked and associated with the risk of polycystic ovary syndrome. J Ovarian Res 17, 17 (2024). https://doi.org/10.1186/s13048-024-01343-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-024-01343-1