Abstract
Background
The rescue in vitro mature(Rescue IVM) technique allows the use of immature oocytes collected in conventional COH to obtain more mature oocytes for fertilization through in vitro maturation. Some studies have shown that Rescue IVM could improve clinical outcomes in patients undergoing IVF/ICSI, but the effectiveness and the indications for the clinical application of this technique remain controversial. It remains to be studied whether Rescue IVM should be universally applied in all conventional IVF/ICSI cycles.
Method
This is a large retrospective cohort study that included a total of 22,135 female patients undergoing their first IVF treatment cycles. The effect of the number of mature oocytes(metaphaseII[MII]) on the cumulative live birth rate was investigated in a population with routine IVF/ICSI first. The receiver operating characteristic curve(ROC) analysis was used to explore the cut-off point of the number of MII affecting CLBR. Secondly, Patients undergoing ICSI with Rescue IVM were included in the analysis with those who underwent ICSI only during the same period, grouped according to the MII cut-off values. Multi-factor binary logistic regression and inverse probability weighting (IPW) were used to investigate whether Rescue IVM influenced the final cumulative live birth rate(CLBR).
Results
The CLBR increased with the number of MIIoocytes (P < 0.001). The ROC analysis showed the cut-off point for the number of MIIoocytes to have a significant effect on CLBR was 9 (sensitivity 0.715, specificity 0.656). Furthermore, 912 patients who underwent ICSI with Rescue IVM were included and compared to those who underwent ICSI only during the same period, and found Rescue IVM significantly increased the number of available MIIoocytes. For patients with MII numbers < 9, Rescue IVM significantly improves their clinical pregnancy rate(55.6% vs. 46.7%, P = 0.001) and CLBR(65.4% vs. 48.1%, P < 0.001), but not for those patients with MII numbers ≥ 9.
Conclusion
This study further clarifies the candidates for the application of Rescue IVM technique: patients with an MII oocytes < 9 in a conventional IVF/ICSI cycle. In contrast, it is not necessary for patients who already have sufficient mature oocytes(≥ 9), to avoid over-medication.
Similar content being viewed by others
Introduction
In 1978, Robert Edwards et al. collected oocytes by the natural cycle, performed in vitro fertilization and embryo transfer (IVF-ET) and successfully delivered a normal, healthy baby weighing 2700 g [1], in subsequent decades, more and more infertile couples were benefiting from various types of assisted reproductive technology (ART). In China, the total number of babies born through ART was 311,309 in 2016, accounting for 1.69% of all live babies [2]. IVF has become an important routine treatment for infertility. During the development of ART, controlled ovarian hyperstimulation (COH) is an important technology to improve the IVF success rate. COH induces multiple follicle development, and it also greatly improves the probability of pregnancy in each cycle [3]. In addition to conventional IVF/ICSI cycles after ovarian stimulation, Rescue IVM is increasingly becoming a method of increasing the number of available embryos, especially in patients with higher than expected immature oocytes [4]. With the significant progress in cryopreservation techniques such as vitrification [5], the number of euploid embryos for embryo transfer increases, which further affects the overall cumulative live birth rates(CLBR) [6].
Classic IVM is often defined as the in vitro maturation of oocytes retieved from follicles at the GV or MI stage after exposure to exogenous FSH and/or hCG [7]. While the Rescue IVM refers to the collection of immature oocytes from conventional IVF/ICSI cycles and culturing them in vitro to the metaphase II(MII) stage for subsequent fertilization and embryo culture [8]. Rescue IVM can help patients make the most of the immature oocytes that are often discarded in conventional IVF/ICSI cycles to obtain more embryos available for transfer, and to increase live birth rates [9], especially for patients with low functional ovarian reserve, every additional mature oocyte or embryo has considerable potential clinical significance [10]. The reproductive potential and safety of oocytes obtained by Rescue IVM remains controversial [11,12,13,14].
The most obvious effect of Rescue IVM is to increase the number of mature oocytes available. However, whether more oocytes means better reproductive outcomes is still debated. In IVF/ICSI treatment, it has been agreed that the number of oocytes retrieved are predictors of pregnancy [15]. In most studies, it is generally accepted that 12–18 is the optimal oocytes range for the highest live birth rate in the fresh IVF/ICSI cycles(fLBR) [16,17,18]. In terms of CLBR, the common view is that cumulative live birth rates steadily increased with the number of oocytes [19,20,21,22], but some studies have also shown that there is a plateau in CLBR growth as the number of oocytes increases, meaning that excess oocytes do not lead to a better CLBR [23, 24]. Meanwhile, studies have shown that the increasing estradiol levels after COH cause the damage of endometrial receptivity and affect the embryo quality, leading to a decrease in CLBR [25, 26].
However, previous studies mainly concentrated on the relationship between the total number of oocytes retrieved and the fresh live birth rate(fLBR) or CLBR, as the total number of oocytes retrieved always reflects the ovarian response [27]. Nevertheless, in the routine IVF/ICSI treatment regimens of most medical institutions, MII oocytes are directly used for fertilization and subsequent embryo culture due to their better fertilization and embryonic development ability, while the immature oocytes(GV or MI) which could have been used by Rescue IVM are often sacrificed. In the application of Rescue IVM, there is no consensus on whether patients who undergo conventional IVF/ICSI can benefit from Rescue IVM, some studies have concluded that the clinical value of IVM is not as good as conventional IVF/ICSI at present [28, 29], but there are also studies suggesting that Rescue IVM improved reproductive outcomes in women undergoing ICSI [30]. These conflicting conclusions bring up a question: for those patients who already have enough mature oocytes, is it necessary for them to undergo Rescue IVM to obtain additional MII oocytes? We conducted this study to investigate the association between the number of MII oocytes and the CLBR in patients undergoing conventional IVF/ICSI, to find the MII oocytes count threshold affecting CLBR, and verify whether Rescue IVM could help these “mature oocyte deficient” patients to achieve better live birth outcomes.
Materials and methods
Study Population
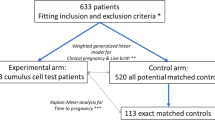
This retrospective cohort study collected data from the patients at the Reproductive Center of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, from January 2015 to December 2019 who underwent their first IVF or ICSI cycle, and patients undergoing Rescue IVM from January 2016 to December 2019. The study was approved by the institutional review board of Tongji Hospital. Individual patient data were collected from medical records database of Tongji hospital, including patient demographic characteristics, examination results, treatment process and outcomes. The clinical information had been fully anonymized before analysis.
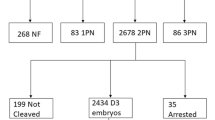
The exclusion criteria for the study: (1) patients with oocyte donated or oocyte cryopreserved, (2) patients who underwent pre-implantation genetic testing (PGT), (3) patients with remaining frozen embryos without obtaining a live birth, (4) patients with natural cycle, (5) patients who failed to obtain MIIoocytes. Overall, 22,135 female patients were eventually enrolled, 912 of whom were treated with Rescue IVM.
Clinical protocols
Populations using three main COH regimens were included in the study, including (1) the Depot GnRH-agonist regimen, (2) the Daily GnRH-agonist regimen, and (3) the GnRH-antagonist regimen. The down-regulation methods and ovarian stimulation protocols were performed as described in previous literature [31]. Briefly, the depot GnRH-agonist regimen was administered with 3.75 mg Triptorelin on the first day of the menstrual cycle. 150–225 IU rFSH per day was started 28 days later until the human chorionic gonadotropin (hCG) trigger day. The daily GnRH-agonist regimen was 0.1 mg/d of GnRH-a (Decapeptyl, Ferring, Switzerland, or Diphereline, Ipsen, Australia) subcutaneously from mid-luteal phase until pituitary suppression was reached with gonadotropins (rFSH; Gonal-F, Seron, Switzerland, or Puregon, Organon, The Netherlands) to start ovarian stimulation, while the dose of GnRH-a was reduced to 0.05 mg/d until the administration date of hCG. As for the GnRH-antagonist regimen, recombinant follicle-stimulating hormone (r-FSH) was started on day 2 or 3 of the menstrual cycle when the leading follicle reached an average diameter of 12–14 mm with subcutaneous cetrotide acetate (Cetrotide; Merck Serono, Geneva, Switzerland) at a dose of 0.25 mg/d. When the mean diameter of the 2–3 follicles reached 18 mm, 10,000 IU rhCG (Ovidrel; Merck-Serono, Geneva, Switzerland) was injected to trigger ovulation. Oocytes were obtained by transvaginal ultrasound-guided puncture 36–38 h after hCG injection. The granulosa cells surrounding the oocytes were removed using hyaluronidase (Vitrolife, Sweden) 2 to 4 h after egg collection, and the period of time the oocytes were in was assessed and recorded. ICSI or Rescue IVM combined with ICSI was then performed. For patients undergoing ICSI with Rescue IVM, their immature oocytes were collected and continued to be cultured in G1-plus medium (Vitrolife, Sweden) and checked for oocyte maturity every 6 h until 24 h. MII stage oocytes were used for ICSI fertilization.
IVF and embryos culture
Depending on the quality of the sperm, fertilization can be performed by IVF or intracytoplasmic sperm injection (ICSI). For rescued IVM-derived MII eggs, ICSI was the preferred method of fertilization in our center. Oocytes were incubated in G-IVF medium (Vitrolife, Sweden) and fertilized 3–4 h after oocyte retieved. Oocytes were checked for fertilization 16–18 h after fertilization and were considered successfully inseminated if zygotes with two pronuclei (2PN) were visible. On day 3 after retrieval of the oocytes, one or two embryos of the best quality are transferred. Embryos are scored according to the number of blastomeres, the homogeneity of the blastomeres and the number of embryonic fragments at the speed of embryonic development on day 3 [32]. Blastocysts were also evaluated with 3 main morphological parameters: the stage of development, inner cell mass(ICM), andtrophoectoderm(TE) [33].
Embryo vitrification and frozen-embryo transfer
The remaining high-quality day 3 embryos or blastocysts were cryopreserved for subsequent FET cycles. Details of embryo cryopreservation and frozen-thawed embryo transfer protocols have been described previously [34]. Our center used endometrial preparation for FET cycles with natural cycles (NC) or artificial cycles (AC). FET is performed 3 or 5 days after endometrial transformation, followed by luteal phase support until the 10th week of gestation.
Main outcome measures
The primary outcome was the CLBR defined as at least one live birth either by fresh embryo transfer or subsequent frozen-thawed cycle in a complete oocyte retrieval cycle. Meanwhile live birth means newborns delivered with at least one of four vital signs: heartbeat, respiration, umbilical cord pulsation, and random muscle contraction. The secondary outcome was live birth rate after fresh cycle.
Statistical analysis
Patient demographic characteristics, ovarian response, IVF/ICSI treatment characteristics, and pregnancy outcomes were entered into spreadsheets and used for statistical analysis. Continuous variables were expressed as mean ± SD or median (IQR), and categorical variables were expressed as percentages.
The characteristics of the live-birth and non-live-birth populations were described separately. Bar graphs were used to describe the overall trend of mature oocytes count with changes in CLBR and fLBR. Patients were divided into four groups according to mature oocyte count: 1–3(Group A), 4–8 (Group B), 9–14 (Group C) or ≥ 15 (Group D), one-way analysis of variance (ANOVA), Kruskal-Wallis test and chi-square test were performed appropriately for different type of data. Multiple logistic regression models with CLBR as the dependent variable were developed to analyze the relationship between different numbers of MII oocytes and CLBR after inclusion of confounding factors(Logistic regression model results are presented as ORs with SEs and 95% CIs.). The above data were analyzed using SPSS(SPSS Inc, version 23, Chicago, IL, USA) for data analysis. The ROC curve analysis was constructed using MedCalc statistical software (MedCalc version 20.0.26, Mariakerke, Belgium) and the Youden index was used to determine the cut-off value of the total number of eggs retrieved and the number of MII oocytes with respect to the CLBR.
Patients undergoing ICSI with Rescue IVM were included in the analysis with those who underwent ICSI only during the same period, grouped according to the MII cut-off values. The characteristics and treatment outcomes were compared between different treatment groups. When further investigating whether Rescue IVM influenced the final CLBR, we used two probability-adjusted models, multi-factor binary logistic regression and inverse probability weighting (IPW), to eliminate the effect of confounding factors. The results of the models are expressed as aOR with SEs and 95% CIs. All statistics were considered statistically significant when the value of P < 0.05.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (IRB ID: TJ-C20211013; 2021.10.23).
Results
Patient characteristics
Of the final 22,135 female patients included in the analysis, Table 1 shows the patients’ characteristics grouped by ART outcome. 14,694 (66.38%) patients achieved live births in fresh embryo transfer or frozen-thawed cycles while 7,441 (33.62%) patients did not after all embryos were used. In the total population, the average oocyte maturation rate was 88.12% and the patients’ average age was 30.9 years, tubal factors were the main cause of infertility (50.1%), and most patients underwent IVF (68.2%) for fertilization. Women in the live-birth group were younger than the other group on average (29.97 vs. 32.71) and had a lower mean basal FSH (7.37 vs. 8.36). Among the causes of infertility, a higher proportion of the non-live birth group was diagnosed with DOR (3.8% vs. 8.1%). Regarding the number of MII obtained, a higher proportion of the non-live birth group was in group A (1–3) (24.4% vs. 3.5%) and a lower proportion in group D (≥ 15) (8.7% vs. 30.7%).
Association between the number of MII oocytes and LBR/CLBR
Figure 1 shows the fLBR and CLBR outcomes according to the number of MII oocytes. The fLBR initially increased with the increase in the number of MII oocytes until the number of MII reached 8, with the LBR 43.7%, and then reached a plateau (42.6-49.1%) when the number of MII was 9–14, followed by a decrease. While the CLBR increased steadily with the increase in the number of MIIoocytes beyond 25, when the cumulative live birth rate reached 93.0% and there was no obvious plateau period. The folded line represents the number of patients who obtained that number of MII.
To examine the relationship between the number of MII and CLBR in different age groups, we grouped age (< 30, 30–35, > 35 years). The results (Supplemental Fig. 1) showed that although the overall CLBR decreased with increasing age, the CLBR showed a positive trend in relation to the number of MII in the different age subgroups.
The patients were divided into four groups according to the number of MIIoocytes retrieved, Table 2 presents the treatment outcome indicators such as oocyte maturation rate, fertilization rate, blastocyst rate, fLBR and CLBR. The group of patients with more mature oocytes obtained were younger and with a higher CLBR. Interestingly, the oocyte maturation rate, fertilization rate, and blastocyst formation rate were also increased with the number of MIIoocytes, as well as more transferable embryos (p < 0.001). However, there was no significant difference in Cleavage rate(p = 0.994). That seemed to mean that the patients with high MII numbers also had better oocyte quality. Whereas the fLBR was significantly higher in Group C (9–14) than the other three groups (p < 0.001).
Using Multivariable logistic regression to adjust for confounding factors, Fig. 2 demonstrates the effect of increasing MII number group on LBR after fresh embryo transfer and CLBR after adjustment. For CLBR, the ORs of the MII number 4–8, 9–14, and ≥ 15 groups were 3.734 (2.896–4.813), 6.808 (5.277–8.783), and 11.644 (8.934–15.177) respectively (p < 0.001) compare to the control group(1–3). As for LBR, the ORs of the MII numbers 4–8, 9–14 remained significant compared to the control group, but did not show a statistically significant difference when MII number ≥ 15(P = 0.071).
The cut-off point of mature oocyte count regarding CLBR
Figure 3 shows the ROC curve analysis of the number of MIIoocytes and the total number of oocytes with respect to the CLBR, and calculated the cut-off values by Youden index (Supplementary Table 1). The cut-off value for MIIoocytes was 9 (sensitivity 0.7155, specificity 0.6561) and the total number of oocytes was 11 (sensitivity 0.6707, specificity 0.6793). Supplementary Fig. 2 shows the ROC results on fLBR, however, both the total number of eggs retrieved(AUC = 0.522) and the MIIoocytes(AUC = 0.530) are poor predictors of overall fLBR. Moreover, we compare the predictive ability of the number of MIIoocytes and the total number of oocytes in predicting CLBR, we compared their AUC and found that the AUC of MII was greater than the AUC of the total number of eggs retrieved (7.52 vs. 7.36, P < 0.0001) (Supplementary Table 2), suggesting that using MII to predict CLBR has greater sensitivity and accuracy.
In order to verify whether patients with less than 9 MII oocytes could increase the cumulative live birth rate by Rescue IVM, patients were divided by cut-off values into two groups: MII oocytes < 9 and ≥ 9 (before Rescue IVM), and basic patient information (Table 3) and cumulative treatment outcomes (Table 4) were counted separately. We found that in both groups, performing Rescue IVM resulted in a significant increase in the number of MII, and in patients with MII<9, it also resulted in a significant reduction in the proportion of patients whose cycles were canceled due to lack of available embryos compared to conventional ICSI (3.9% vs. 12.5%, P < 0.001). In terms of treatment outcomes, Rescue IVM did not appear to significantly contribute to fresh transfer cycles. However, after considering FET, patients with Rescue IVM had significantly higher rates of implantation, clinical pregnancy and cumulative live births than controls in the < 9 group. However, in patients with MII numbers ≥ 9, Rescue IVM did not significantly contribute to clinical outcomes.
To exclude the effect of other confounding factors, we performed binary logistic regression and inverse probability weighting (IPW) analysis (Table 5). The two models have similar conclusions. Rescue IVM had no significant effect on the live birth rate of fresh cycles in either group. However, performing Rescue IVM was a significantly positive factor in improving CLBR in patient with MII<9(aOR = 2.325 95%CI: 1.698–3.187, P < 0.001), but not in MII ≥ 9 group (aOR = 2.291 95%CI:0.922–5.693, P = 0.074).
Discussion
Our results show a significant positive relationship between the number of mature oocytes and cumulative live birth outcomes in first IVF/ICSI cycle patients, and find 9 and 11 as the appropriate cut-off point of the number of MII and total eggs for the CLBR respectively. Besides, based on a controlled analysis of patients with and without Rescue IVM, we conclude that Rescue IVM is effective in helping patients with a mature oocyte less than 9 to obtain more available mature oocytes, and resulting in better clinical outcomes.
Quality and quantity of the whole embryos are the two most important predictors of the cumulative outcome in IVF/ICSI [35]. With the total number of oocytes retrieved after COH, reproductive clinicians can describe the patients’ ovarian function accurately [27] and thus predict their IVF/ICSI live birth outcome. However, the role of immature oocytes is still very limited, the source of available embryos is mainly from mature oocytes. Moreover, for a subset of patients with oocyte maturation failure [36], the total number of eggs retrieved is more difficult to accurately describe their pregnancy outcome. Compared to previous studies, therefore, our study paid more attention to the effect of MII oocytes quantity on IVF/ICSI outcome, as reflected by the CLBR. Our result is generally similar to the overall tendency in previous studies about the total number of oocytes retrieved regarding fLBR or CLBR [16,17,18, 20,21,22]. Based on our data, the fLBR plateaued when the number of MII reached 8 and decreased after > 14. As for the CLBR per started cycle, the adjusted ORs for CLBR continued to increase with increasing MII without a plateau period. There are some differences in our study: firstly, when MII oocytes was used as the dependent variable instead of the total number of oocytes retrieved, the fLBR plateau appeared earlier (plateau in previous studies was mostly 12–18), it is a reasonable difference considering the average oocyte maturation rate of the total population in this study (88.12%). Another results in contrast with 2 single-center studies is that when using MII data as a basis for grouping, the group with higher numbers of mature oocytes had significantly higher oocyte fertilization rates, rather than decreasing with increasing number of oocytes retrieved [20, 22], a potential explanation for this difference is that after removing the effect of immature oocytes, the MIIoocytes retrieved from the patients with more MIIoocytes are of better quality. That result also explains why the number of mature oocytes would have a more significant effect on CLBR. On the one hand, patients with more MIIoocytes have better oocytes, and on the other hand, more quantities of MIIoocytes and embryos bring more opportunities to choose the better embryos for transfer. Results of the ROC curves also verify this result, that MIIoocytes has a stronger sensitivity and accuracy for CLBR.
Increasing the number of mature oocytes is the most obvious effect of Rescue IVM. Based on ROC results in the conventional IVF/ICSI patient, the cut-off point of mature oocytes for CLBR is 9. A previous retrospective study that included 737 cycles concluded that the minimum MII yield to predict live birth after ART was 6, and more than six mature oocytes did not result in a significant benefit in the take-home baby [37]. A reasonable explanation for the difference in cut-off values is due to the fact that this study only studied live birth rates in fresh embryo transfer and did not consider the situation in FETs. To verify our result, we further compared the treatment outcomes of patients who underwent ICSI only and ICSI with Rescue IVM, and found that for patients with MII counts < 9, performing Rescue IVM may help them achieve better clinical pregnancy rates and CLBR, whereas for patients who already have a high number of mature oocytes (≥ 9), Rescue IVM will not significantly improve their treatment outcome.
The large sample size and strict exclusion criteria are the strengths of this study. Despite the various methods we have used to reduce the generation of bias, we still need to acknowledge that this retrospective study has some limitations. Firstly, the strength of evidence from retrospective studies is weaker than other forms of research, such as prospective studies or randomized controlled trials. Secondly, the data is from a single center and considering the heterogeneity of reproductive hospitals in different regions, the conclusions may lack evidence of general application. Nevertheless, the conclusions of this study are still of guidance for clinical application as well as for further experimental investigations: from the aspect of clinical application, the number of MII oocytes is critical to achieving a satisfactory CLBR, suggesting that reproductive scientists should pay more attention to increase the oocyte maturation rate rather than simply increasing the number of oocytes retrieved through higher doses of gonadotropins. In addition, Rescue IVM is a potential rescue method for patients with a higher than expected percentage of immature oocytes after routine COH [4]. This study further clarifies the candidates for the application of Rescue IVM technique: patients with MII oocytes < 9 in a conventional IVF/ICSI cycle. In contrast, it is not necessary for patients who already have sufficient mature oocytes, thus avoiding over-medication. However, due to the limitations of the type of study, we hope that future large prospective or randomized controlled trials will further validate our conclusion.
In conclusion, the number of mature oocytes is an independent influence of CLBR in infertile patients undergoing IVF/ICSI treatment. After taking into account the frozen-thawed cycles, a higher number of MII tends to result in better cumulative live birth outcomes, and the appropriate cut-off point for MII oocyte count is 9. For the patients with less than 9 MII oocyte retrieved, the additional mature oocytes obtained by Rescue IVM contributed to better clinical outcome, in contrast, patients with MII oocyte ≥ 9 are not necessary for Rescue IVM.
Data Availability
The data generated or analyzed during this study is available from the corresponding author upon reasonable request.
References
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366.
Bai F, Wang DY, Fan YJ, Qiu J, Wang L, Dai Y, Song L. Assisted reproductive technology service availability, efficacy and safety in mainland China: 2016. Hum Reprod. 2020;35(2):446–52.
Fauser BC, Devroey P, Macklon NS. Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet. 2005 May;21–27(9473):1807–16.
Escrich L, Pellicer A, Meseguer M. Let’s rescue oocytes: in vitro maturation 2.0 is coming. Fertil Steril. 2018;110(4):638–9.
Blockeel C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye H. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod. 2016;31(3):491–7.
Labarta E, Bosch E, Mercader A, Alamá P, Mateu E, Pellicer A. A higher ovarian response after Stimulation for IVF is related to a higher number of Euploid embryos. Biomed Res Int. 2017;2017:5637923.
Practice Committees of the American Society for Reproductive Medicine. The Society of Reproductive biologists and technologists, and the society for assisted Reproductive Technology. Electronic address: jgoldstein@asrm.org. In vitro maturation: a committee opinion. Fertil Steril. 2021;115(2):298–304.
Jie H, Zhao M, Alqawasmeh OAM, Chan CPS, Lee TL, Li T, Chan DYL. In vitro rescue immature oocytes - a literature review. Hum Fertil (Camb). 2022;25(4):640–50.
Martin-Palomino Olid N, García D, Rodríguez A, Vassena R. Could fertility clinics offer a sizable improvement of live birth rates by maturing post-GVBD oocytes in vitro? J Assist Reprod Genet. 2019;36(9):1927–34.
Lee HJ, Barad DH, Kushnir VA, Shohat-Tal A, Lazzaroni-Tealdi E, Wu YG, Gleicher N. Rescue in vitro maturation (IVM) of immature oocytes in stimulated cycles in women with low functional ovarian reserve (LFOR). Endocrine. 2016;52(1):165–71.
Walls ML, Hunter T, Ryan JP, Keelan JA, Nathan E, Hart RJ. In vitro maturation as an alternative to standard in vitro fertilization for patients diagnosed with polycystic ovaries: a comparative analysis of fresh, frozen and cumulative cycle outcomes. Hum Reprod. 2015;30(1):88–96.
Shani AK, Haham LM, Balakier H, Kuznyetsova I, Bashar S, Day EN, Librach CL, THE DEVELOPMENTAL POTENTIAL OF MATURE OOCYTES DERIVED FROM RESCUE IN VITRO MATURATION (IVM). Fertil Steril 2023 May 29:S0015-0282(23)00539-3.
Fadini R, Mignini Renzini M, Guarnieri T, Dal Canto M, De Ponti E, Sutcliffe A, Shevlin M, Comi R, Coticchio G. Comparison of the obstetric and perinatal outcomes of children conceived from in vitro or in vivo matured oocytes in in vitro maturation treatments with births from conventional ICSI cycles. Hum Reprod. 2012;27(12):3601–8.
Mostinckx L, Segers I, Belva F, Buyl R, Santos-Ribeiro S, Blockeel C, Smitz J, Anckaert E, Tournaye H, De Vos M. Obstetric and neonatal outcome of ART in patients with polycystic ovary syndrome: IVM of oocytes versus controlled ovarian stimulation. Hum Reprod. 2019;34(8):1595–607.
van Loendersloot LL, van Wely M, Limpens J, Bossuyt PM, Repping S, van der Veen F. Predictive factors in in vitro fertilization (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010 Nov-Dec;16(6):577–89.
Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26(7):1768–74.
Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, Muasher SJ. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014;101(4):967–73.
Briggs R, Kovacs G, MacLachlan V, Motteram C, Baker HW. Can you ever collect too many oocytes? Hum Reprod. 2015;30(1):81–7.
Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, Bosch E, Garcia-Velasco J. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ~ 15,000 women. Fertil Steril. 2018;110(4):661–670e1.
Ji J, Liu Y, Tong XH, Luo L, Ma J, Chen Z. The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod. 2013;28(10):2728–34.
Vaughan DA, Leung A, Resetkova N, Ruthazer R, Penzias AS, Sakkas D, Alper MM. How many oocytes are optimal to achieve multiple live births with one stimulation cycle? The one-and-done approach. Fertil Steril. 2017;107(2):397–404e3.
Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, Polyzos NP. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;31(2):370–6.
Chen YH, Wang Q, Zhang YN, Han X, Li DH, Zhang CL. Cumulative live birth and surplus embryo incidence after frozen-thaw cycles in PCOS: how many oocytes do we need? J Assist Reprod Genet. 2017;34(9):1153–9.
Magnusson Ã, Källen K, Thurin-Kjellberg A, Bergh C. The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod. 2018;33(1):58–64.
Pellicer A, Valbuena D, Cano F, Remohí J, Simón C. Lower implantation rates in high responders: evidence for an altered endocrine milieu during the preimplantation period. Fertil Steril. 1996;65(6):1190–5.
Valbuena D, Martin J, de Pablo JL, Remohí J, Pellicer A, Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962–8.
Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. 2015;30(9):2005–8.
Ho VNA, Braam SC, Pham TD, Mol BW, Vuong LN. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum Reprod. 2019;34(6):1055–64.
Vuong LN, Ho VNA, Ho TM, Dang VQ, Phung TH, Giang NH, Le AH, Pham TD, Wang R, Smitz J, Gilchrist RB, Norman RJ, Mol BW. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum Reprod. 2020;35(11):2537–47.
Qin DY, Jiang HH, Yao QY, Yao W, Yuan XQ, Wang Y, Deng TR, Du YY, Ren XL, Guo N, Li YF. Rescue in vitro maturation may increase the pregnancy outcomes among women undergoing intracytoplasmic sperm injection. Front Endocrinol (Lausanne). 2022;13:1047571.
Guo Y, Jiang H, Hu S, Liu S, Li F, Jin L. Efficacy of three COS protocols and predictability of AMH and AFC in women with discordant ovarian reserve markers: a retrospective study on 19,239 patients. J Ovarian Res. 2021;14(1):111.
Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A, BFS. Elective single embryo transfer: guidelines for practice british Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb). 2008;11(3):131–46.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270-83.
Ai J, Jin L, Zheng Y, Yang P, Huang B, Dong X. The morphology of Inner Cell Mass is the strongest predictor of live birth after a frozen-thawed single embryo transfer. Front Endocrinol (Lausanne). 2021;12:621221.
Cai QF, Wan F, Huang R, Zhang HW. Factors predicting the cumulative outcome of IVF/ICSI treatment: a multivariable analysis of 2450 patients. Hum Reprod. 2011;26(9):2532–40.
Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril. 2010;94(7):2507–13.
McAvey B, Zapantis A, Jindal SK, Lieman HJ, Polotsky AJ. How many eggs are needed to produce an assisted reproductive technology baby: is more always better? Fertil Steril. 2011;96(2):332–5.
Acknowledgements
We sincerely thank the staff who contributed their efforts to collect and organize the data and participants that provided all help to the study.
Funding
This work was supported by the National Key Technology Research and Development Program of China (2022YFC2702504).
Author information
Authors and Affiliations
Contributions
Conception and design: JW, JA, XD. Data collection: JW, HJ, LZ. Analysis and interpretation of data: HJ, JW, ZL. Manuscript writing: JW, XD. All authors were involved in the editing and approval of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, J., Luo, Z., Dong, X. et al. Cut-off point of mature oocyte for routine clinical application of rescue IVM: a retrospective cohort study. J Ovarian Res 16, 226 (2023). https://doi.org/10.1186/s13048-023-01294-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-023-01294-z