Abstract
Background
Tumor-to-tumor metastasis (TTM) is a rare but well-established phenomenon where histologically distinct tumors metastasize within each other. Here we report the first “known” case of follicular lymphoma that metastasized and extended to a mature ovarian teratoma.
Case presentation
A 59-year-old Japanese postmenopausal woman visited our hospital for a detailed examination of an ovarian tumor. Clinical imaging suggested it to be either teratoma-associated ovarian cancer with multiple lymph node metastases, or tumor-to-tumor metastasis from malignant lymphoma to ovarian teratoma. A bilateral adnexectomy and retroperitoneal lymph node biopsy were performed. Lined with squamous epithelium, the cyst constituted a mature ovarian teratoma, and the solid part showed diffuse proliferation of abnormal lymphoid cells. Immunohistochemically, the abnormal lymphoid cells were negative for CD5, MUM1, and CyclinD1, and positive for CD10, CD20, CD21, BCL2, and BCL6. Genetic analysis using G-banding and fluorescence in situ hybridization identified a translocation of t(14;18) (q32;q21), and we diagnosed tumor-to-tumor metastasis from nodal follicular lymphoma to mature ovarian teratoma. Twelve months after surgery, the patient showed no progression without adjuvant therapy.
Conclusions
The present case suggests that molecular approaches are useful in the diagnosis of TTM in mature ovarian teratomas when morphologic and immunohistochemical findings alone are insufficient for diagnoses.
Similar content being viewed by others
Background
Ovarian mature teratomas (OMTs), which are composed of cells derived from two or three germ cell layers, are among the most common benign ovarian tumors. Malignant transformation of OMTs causes various cancers, such as squamous cell carcinoma (SCC), adenocarcinoma, neural tumor, malignant melanoma, and lymphoma [1,2,3]. The ovaries can also receive cancer metastasis from other organs [4]. Therefore, when cancers are found in OMTs, it is difficult to determine whether they are due to malignant transformation (primary) or metastasis.
Tumor-to-tumor metastasis (TTM) is a rare but well-established phenomenon defined as metastasis in histologically distinct tumors [5]. To date, three cases of TTM to OMTs have been reported: appendiceal adenocarcinoma [5], cervical adenocarcinoma [6], and breast cancer [3]. Ten cases of primary lymphoma derived from OMTs [7, 8] and only one cases of lymphoma-led invasions into OMTs are reported [9]. Here, we report a case of coexisting ovarian maturing teratoma and follicular lymphoma, diagnosed as TTM, using morphological, immunohistochemical, and molecular approaches.
Case presentation
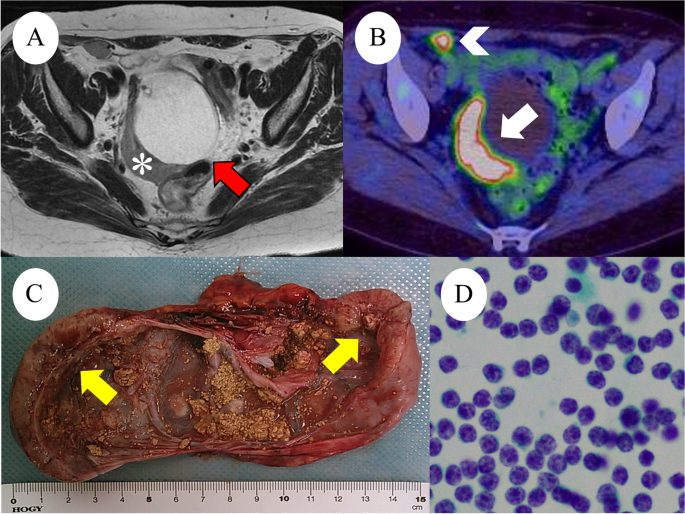
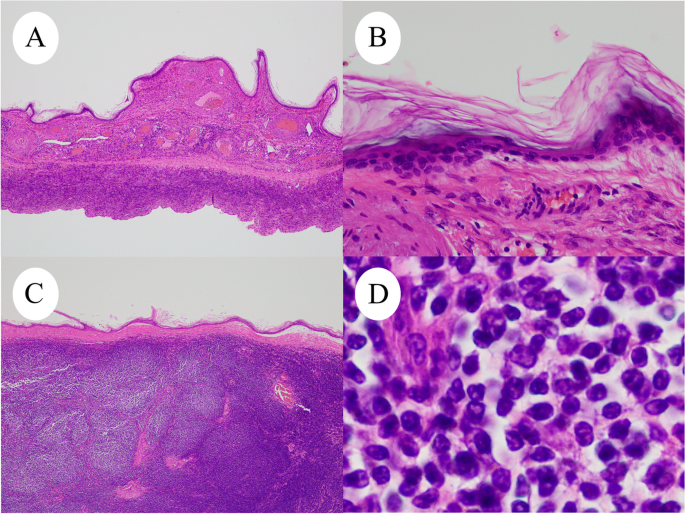
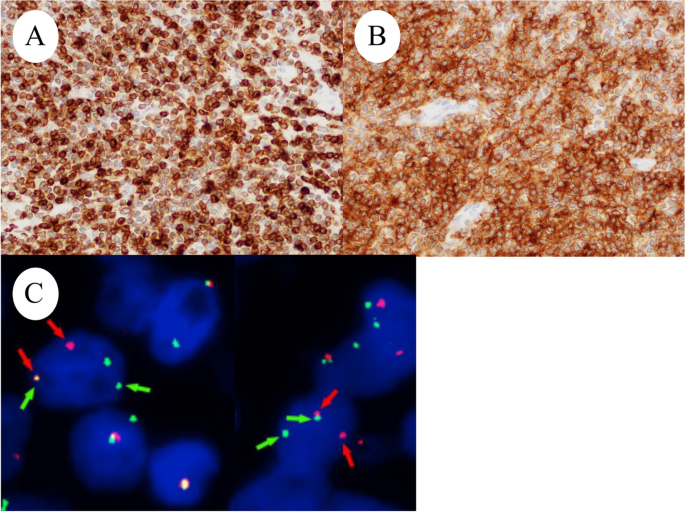
A 59-year-old Japanese postmenopausal woman (gravida 2, para 2) with no history of being immunocompromised, visited our hospital for a detailed examination of an ovarian tumor found during a medical checkup. She had a family history of prostate and pharyngeal cancer in her father and mother, respectively. Transvaginal ultrasonography showed a 70-mm cystic tumor with solid parts in the right ovary. Contrast-enhanced computed tomography and plain magnetic resonance imaging (MRI) showed a right ovarian teratoma and multiple enlarged retroperitoneal lymph nodes (Fig. 1A). 18 F-fluorodeoxyglucose-positron emission, tomography-computed tomography revealed increased fluorodeoxyglucose accumulation in the right ovarian tumor and pelvic to axillary lymph nodes (Fig. 1B). These clinical images suggested OMT-associated ovarian cancer with multiple lymph node metastases or TTM from malignant lymphoma to OMT. Carbohydrate antigen 125 level of 32.9 U/mL (normal range, ≤ 35.0 U/mL), carbohydrate antigen 19 − 9 level of 3.0 U/mL (normal range, ≤ 37.0 U/mL), and soluble Interleukin-2 receptors level of 432 U/mL (normal range, 204–587 U/mL) were normal. Soluble interleukin-2 receptors are one of the useful serum markers for lymphoma. A bilateral adnexectomy and retroperitoneal lymph node biopsy were performed, revealing a moderate amount of milky ascites. Macroscopically, the right ovarian tumor was a cystic mass with fat and solid parts (Fig. 1C). Ascitic cytology revealed no neoplastic (Fig. 1D). The cyst was lined by squamous epithelium constituting an OMT, and the solid part showed diffuse proliferation of abnormal lymphoid cells (Fig. 2A-D). Immunohistochemically, abnormal lymphoid cells of the ovarian tumor tested negative for CD5, MUM1, and CyclinD1, and positive for CD10, CD20, CD21, BCL2, and BCL6 (Fig. 3A-B). The abnormal lymphoid cells of the retroperitoneal lymph nodes were negative for CD5, CMYC, MUM1, and CyclinD1, and positive for CD10, CD20, CD21, CD79a, BCL2, and BCL6. Genetic analysis of the lymphoma using G-banding and fluorescence in situ hybridization (FISH) identified a translocation of t(14;18) (q32;q21) (Fig. 3C) and diagnosed TTM from nodal follicular lymphoma (histological grade 1) to OMT. Twelve months after the surgery, the patient showed no recurrence without adjuvant therapy.
A: Axial T2-weighted magnetic resonance image reveals a 70-mm right ovarian mass (red arrows) with a 20-mm crescent solid part at the dorsal side (white asterisks). B: Positron emission tomography-CT shows FDG accumulation in the solid component (white arrows), measuring SUV max od 8.3 and retroperitoneal lymph node (white arrowhead), measuring SUV max of 7.8. C: Right ovarian tumor, macroscopic examination shows cystic tumor filled with fatty and yellow sebaceous component and solid nodule (yellow arrows). D: Ascites cytology was no neoplastic findings
Discussion
TTM is defined as metastasis in distinct tumors of the same body, and 15 cases of TTM in ovarian tumors have been reported to date [5]. OMTs causes various cancers via malignant transformation; therefore, the coexistence of OMT and cancers requires the differentiation between primary and metastatic cancers.
Ten cases of primary malignant lymphoma derived from OMT have been reported [7, 8]. TTM of malignant lymphoma to OMT is the only existing case of plasmablastic lymphoma [9]. TTM to OMT is summarized in Table 1 [3, 5, 6, 9] and shows that the present case is the first recorded case of nodal follicular lymphoma metastasizing and extending to the OMT. Historically, the most common method for diagnosing TTM to OMT was immunohistochemical analysis (4/5 cases), followed by virological examination and FISH, which were performed in two cases each. This is the first molecular analysis report that combines G-banding and FISH. These results recommend molecular diagnostic approaches for TTM and OMT when morphological and immunohistochemical findings alone are insufficient for diagnoses.
Ozsan et al. divided 16 follicular lymphomas initially diagnosed in the ovary into two groups based on clinical, morphological, immunophenotypic, and genetic features [10]. Ozsan et al. reported that ovarian extension of nodal follicular lymphoma was characterized as low histologic grade, BCL2 protein positivity, and presence of t(14;18)(q32;q21), while true primary ovarian follicular lymphoma was characterized as higher histologic grade, CD10 and BCL2 negativity, and absence of t(14;18)(q32;q21) [10]. We diagnosed the present case with TTM from nodal follicular lymphoma to OMT based on the distribution of lymph node lesion (pelvic to axillary), genetic analysis of t(14;18)(q32;q21), immunohistochemical expression of BCL2 and CD10, and histologic grades (grade 1). However, this study included only one case of OMT [10]. It is unclear whether OMT-derived follicular lymphoma has the same biology as that of a conventional follicular lymphoma. Tamura et al. reported that SCCs derived from OMTs share genetic characteristics with pulmonary SCCs rather than cutaneous SCCs, which is the most common component of OMTs [11]. It is expected that more cases will be accumulated in the future.
Conclusion
To the best of our knowledge, the present case is the first report of follicular lymphoma metastasizing and extending to the OMT. To make this differential diagnosis of TTM and primary ovarian follicular lymphoma, we used immunohistochemical and molecular analyses (G-banding and FISH) for the first time.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008;9:1173–80. https://doi.org/10.1016/S1470-2045(08)70306-1.
Yano M, Nasu K, Yasuda M, Katoh T, Kagabu M, Kobara H, et al. Clinicopathological features and programmed death-ligand 1 immunohistochemical expression in a multicenter cohort of uterine and ovarian melanomas: a retrospective study in Japan (KCOG-G1701s). Melanoma Res. 2022;32:150–8. https://doi.org/10.1097/CMR.0000000000000811.
Kirova YM, Feuilhade F, de Baecque-Fontaine C, Le Bourgeois JP. Metastasis of a breast carcinoma in a mature teratoma of the ovary. Eur J Gynaecol Oncol. 1999;20:223–5.
De Waal YR, Thomas CM, Oei AL, Sweep FC, Massuger LF. Secondary ovarian malignancies: frequency, origin, and characteristics. Int J Gynecol Cancer. 2009;19:1160–5. https://doi.org/10.1111/IGC.0b013e3181b33cce.
Yano M, Katoh T, Hamaguchi T, Kozawa E, Hamada M, Nagata K, et al. Tumor-to-tumor metastasis from appendiceal adenocarcinoma to an ovarian mature teratoma, mimicking malignant transformation of a teratoma: a case report. Diagn Pathol. 2019;14:88. https://doi.org/10.1186/s13000-019-0865-6.
Santos F, Oliveira C, Caldeira JP, Coelho A, Félix A. Metastatic endocervical adenocarcinoma in a mature cystic teratoma: a case of a tumor-to-tumor metastasis. Int J Gynecol Pathol. 2018;37:559–63. https://doi.org/10.1097/PGP.0000000000000457.
Hutspardol S, Li Y, Dube V, Delabie J. Concurrent primary follicular lymphoma and a mature cystic teratoma of the ovary: a Case report and review of literature. Case Rep Pathol. 2022;2022:5896696. https://doi.org/10.1155/2022/5896696.
Tandon N, Sultana S, Sun H, Zhang S. Follicular lymphoma arising in a mature cystic teratoma in a 26 year old female. Ann Clin Lab Sci. 2016;46:298–301.
Hadžisejdić I, Babarović E, Vranić L, Duletić Načinović A, Lučin K, Krašević M, et al. Unusual presentation of plasmablastic lymphoma involving ovarian mature cystic teratoma: a case report. Diagn Pathol. 2017;12:83. https://doi.org/10.1186/s13000-017-0672-x.
Ozsan N, Bedke BJ, Law ME, Inwards DJ, Ketterling RP, Knudson RA, et al. Clinicopathologic and genetic characterization of follicular lymphomas presenting in the ovary reveals 2 distinct subgroups. Am J Surg Pathol. 2011;35:1691–9. https://doi.org/10.1097/PAS.0b013e31822bd8a8.
Tamura R, Yoshihara K, Nakaoka H, Yachida N, Yamaguchi M, Suda K, et al. XCL1 expression correlates with CD8-positive T cells infiltration and PD-L1 expression in squamous cell carcinoma arising from mature cystic teratoma of the ovary. Oncogene. 2020;39:3541–54. https://doi.org/10.1038/s41388-020-1237-0.
Acknowledgements
We would like to thank Editage (www.editage.jp) for the English language editing.
Funding
This research was supported by Grants-in-Aid for Scientific Research (22K15409) from the Ministry of Education, Culture, Sports, Science, and Technology, and The Seiichi Imai Memorial Foundation.
Author information
Authors and Affiliations
Contributions
YS and MY: conception, pathological diagnosis, immunohistochemical analysis, molecular analysis, writing of the manuscript. SE: collection of clinical data. KT: Immunohistochemical and molecular analyses. KN: Collection of clinical data and revision of the manuscript. All authors read and approved the final manuscript prior to submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sato, Y., Yano, M., Eto, S. et al. Metastasis from follicular lymphoma to an ovarian mature teratoma: a case report of tumor-to-tumor metastasis. J Ovarian Res 16, 106 (2023). https://doi.org/10.1186/s13048-023-01188-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-023-01188-0