Abstract
Background
The insulin receptor substrate 1 (IRS1), phosphoinositide 3-kinase (Pi3k), protein kinase B (Akt1), Forkhead Box O3a (FOXO3a) pathway is directly involved in aging and ovarian activation of follicle growth. Therefore, the aim of this work was to measure the expression of genes related to the ovarian pathway for activation of primordial follicles and FOXO3a protein phosphorylation between young and old female Ames dwarf (df/df) and normal (N) mice.
Methods
For this study ovaries from N (n = 10) and df/df (n = 10) female mice were collected at 5–6 months of age and at 21–22 months of age. For immunohistochemistry ovaries from 12 month-old and df/df mice were used.
Results
The expression of Irs1, Pi3k, Akt1, mammalian target of rapamycin (Mtor), suppressor of cytokine signaling −2 (Socs2), Socs3 was lower (P < 0.05) in older than younger N mice and not different (P > 0.05) between young and old df/df mice. The expression of Foxo3a was also lower (P < 0.05) in old than younger N and df/df mice and was higher (P < 0.05) in old df/df than N mice. Expression of Amh was lower (P < 0.05) in old than young N and df/df mice and was higher (P = 0.0009) in df/df than N mice. Imunnostaining for p-FOXO3 was lower in df/df than N mice (P < 0.001), although FOXO3 immunostaining was not different (P > 0.05) between df/df and N mice.
Conclusions
In sum, the present study indicates that lower expression of Irs1, Socs2, Socs3, Akt1, Pi3k, Mtor and Foxo3a mRNA in the ovaries of older mice of both genotypes is associated to a reduced ovarian activity revealed by lower expression of Amh mRNA. At the same time, ovaries of old df/df mice maintained higher expression of Foxo3a mRNA, which was associated to higher ovarian activity. We have shown that df/df females have a lower level of p-FOXO3 in oocytes from primordial/primary follicles, an important activator of follicular growth. Therefore, this study strongly indicates that Prop1df mutation causes delayed ovarian aging.
Similar content being viewed by others
Background
The Ames dwarf mice (df/df) carry a mutation at the Prop1 (Prophet of Pit1) locus that impairs the development of the anterior pituitary gland [1], resulting in deficiency of growth hormone (GH), thyroid-stimulating hormone (TSH), and prolactin [2]. As the result of the GH deficiency these mice are characterized by severely low circulating insulin-like growth factor I (IGF-I) and reduced adult body size [3]. Importantly, regardless of these hormonal deficiencies, df/df mice live 35-75% longer than their normal littermates [4]. Similarly to the df/df mice, the knockout mice with a disruption of the GH receptor gene/GH binding protein (GHRKO) and normal mice (N) subjected to mild calorie restriction have reduced serum IGF-I concentrations and extended lifespan [5],[6]. At the same time transgenic mice over-expressing GH are characterized by significant reduction of their lifespan when comparing to their normal controls [7]. Subjecting GH-deficient df/df mice to 6 weeks of GH replacement therapy early in life decreased the longevity of these long-living mutants to the lifespan maintained by N mice [8]. All these data indicate that the GH/IGF-I axis and its intracellular mediators play a major role in the process of aging and longevity [9]. However, regardless of severe reduction of IGF-I in circulation it was shown that there is compensatory, GH independent production of IGF-I in the hippocampus and ovaries [10],[11].
It is well established in reproductive studies that a functional GH/IGF-I axis is important for normal ovarian function [12]. Although df/df female mice are infertile, they have normal estrous cycles and are able to ovulate [2]. The infertility probably arises from the inability to maintain the gestation, since normal pregnancy can be achieved after prolactin treatment, with an average 2.6 live offspring/pregnancy [13]. Pituitary production and circulating concentrations of follicle stimulating hormone (FSH) and luteinizing hormone (LH) are present but reduced in df/df mice [14]. GHRKO mice and N mice subjected to calorie restriction share several physiological characteristics with df/df mice; however, they are able to reproduce naturally, despite a reduced litter size, mainly due to a reduction in the number of large follicles and the ovulation rate [15],[16]. One noteworthy point is the prolonged reproductive lifespan of GHRKO mice, as indicated by the presence of ovarian activity at a later age, when normal mice have already depleted the ovarian follicular reserves [17]. This condition seems to occur due to the reduction in the progression of follicles from the primordial to the primary stage in both GHRKO and calorie restriction mice [16],[18], although no reports for df/df mice were found in the literature.
The ovarian primordial follicle reserve is established during fetal development [19]. After birth there is a continuous activation of the primordial follicle pool which leads to growth of individual follicles, while the rest of the pool remain quiescent for months or even years, until the reserve is depleted in a event known as menopause. Several local factors are believed to be involved in this activation process [19]. One of them is the activation of the phosphoinositide 3-kinase (Pi3k)/protein kinase B (Akt1) signaling pathway, an important regulator of cell proliferation and survival, that has been implicated in the activation of primordial follicles [20],[21]. It is known that activation of the Pi3k/Akt1 pathway and the downstream transcription factor Forkhead Box O3a (FOXO3a) induced by the oocyte is a key regulator of the activation of primordial follicles [22]. The hyperactivation of Akt1 results in hyperphosphorylation and nuclear export of FOXO3a, culminating with the global activation of primordial follicles and premature ovarian failure [23]. The mammalian target of rapamycin (mTOR) pathway also has a direct relationship with activation of the Pi3k/Akt1 pathway [24]. mTOR activation accelerates primordial follicle activation in a synergistic way with the Pi3k/Akt1 pathway [25]. The IRS-Pi3k-Akt1-FOXO pathway is stimulated by both insulin and IGF-I and its reduced activation seems to be involved in the extended longevity observed in the mouse models mentioned before [26]. Therefore, it is possible that the extended reproductive lifespan in these mice can be also related to the components of this pathway.
Based on these evidences the aim of this work was to measure the expression of genes related to the ovarian pathway for activation of primordial follicles between young (6 months old) and old (22 months old) female df/df and N mice and also measure the level of FOXO3 and p-FOXO3 protein in the ovaries of 12-month old N and Ames dwarf mice.
Methods
Normal (N; n = 10) and Ames dwarf mice (df/df; n = 10) (all females) were bred and maintained under temperature- and light-controlled conditions (22 ± 2°C, 12 hour light/12 hour dark cycle) [27]. The animals were anesthetized and euthanized after overnight fasting and the pair of ovaries was collected and stored at −80°C. The ovarian collection was performed at 5–6 months of age (young; 5 N and 5 df/df mice) and at 21–22 months of age (old; 5 N and 5 df/df mice).
The pair of ovaries was removed from the −80°C and homogenized with 400 μL of Qiazol (Qiagen, Valencia, CA, USA) using 0.5 mm zirconium oxide beads in the Bullet Blender 24 (Next Advance, Averill Park, NY, USA) for 4 minutes at speed 10. After that, 300 μL of Qiazol were added to the samples and incubated for 5 min at room temperature. Next 170 μL of chloroform was added, the samples were homogenized and incubated for 3 min at room temperature. Samples were then centrifuged at 4°C at 12000 g for 15 min and the clear upper phase transferred to a new tube with 525 μL of cold ethanol. The solution was transferred for a commercial column purification system (miRNeasy Mini Kit, Qiagen) and on-column DNase treatment (RNase-free DNase Set, Qiagen) following manufacturer's instructions was performed. The quantity of RNA was determined using a spectrophotometer (Epoch Microplate Spectrophotometer, Biotek, Winooski, VT, USA) and diluted to 200 ng/μL. Reverse transcription reactions were performed with 1 μg of RNA (5 μL) using iScript Synthesis Kit (Biorad, Hercules, CA, USA) in a 20 μL volume incubated at 5 min at 25°C, 30 min at 42°C and 5 min at 85°C (MJ Mini Personal Thermal Cycler, Biorad). The final cDNA solution was diluted to 10 ng/μL before use.
Real-time PCR using SYBR Green dye was used to evaluate gene expression. β2 microglobulin expression was used as an internal control. The primer sequences are listed in Table 1. The PCR reactions were performed in duplicate in a 20 μL volume using 5 μL of Fast SYBR Green Mastermix (Applied Biosystems, Foster City, CA, USA), 0.4 μL of each primer (10 μM stock) and 2 μL of cDNA. Fluorescence was quantified with the ABI Prism 7500 Fast Real Time PCR System (Applied Biosystems). For each assay 45 PCR cycles were run (95°C for 3 sec and 60°C for 30 sec) and a dissociation curve was included at the end of the reaction to verify the amplification of a single PCR product. Analyses of amplification plots were performed with the 7500 Software (Applied Biosystems). Each assay plate included a negative control. The coefficient of variation was below 5% for all the primer pairs used. Relative expression was calculated from the equation 2A−B/2C−D (where A is the cycle threshold [Ct] number for the gene of interest in the first control sample; B, the Ct number for the gene of interest in the analyzed sample; C, the Ct number for β2 microglobulin in the first control sample; D, the Ct number for β2 microglobulin in the analyzed sample). The first control sample was expressed as 1.00 by this equation, and all other samples were calculated in relation to this value. Afterward, the results in the control group (normal young mice) were averaged, and all other outputs were divided by the mean value of the relative expression in the control group to yield the fold change of the genes of interest expression compared to the control group [28].
For the immunohistochemistry (IHC) analysis, ovaries from 12-month old N (n = 4) and Ames dwarf (n = 4) were collected and embedded in paraffin. After that, 3 μm slices obtained in an automatic microtome were adhered in slides impregnated by 3% organosilane (Sigma Chemical Company®, St. Louis, MO, USA) in ethanol. The samples were deparaffinized with xylene and rehydrated with graded alcohols. The primary polyclonal antibodies were: anti-Foxo3 phosphorylated (p-Foxo3) [p-FKHRL1 antibody; Ser 253, sc-101683-rabbit (IgG)] and anti-Foxo3 [FKHRL1 antibody; N16, sc-101683-goat (IgG)], both diluted 1:50. The primary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and diluted in 1.5% BSA solution. Blockage of the endogenous peroxidase activity was conducted with hydrogen peroxide block solution (Spring Bioscience, Pleasanton, CA, USA), whereas the antigenic recovery was performed in humid heat, during 3 min after the boiling point, in citrate solution pH 6.0. Non-specific background staining was reduced by covering the tissue sections that received protein block (Spring Bioscience, Pleasanton, CA, USA). Thereafter, slides were incubated overnight in a humid chamber at 4°C. Slides with p-Foxo3 antibody were instilled with Reveal Polyvalente HRP® Kit (Spring Bioscience, Pleasanton, CA, USA) and slides with anti-Foxo3 were instilled with the Histofine® kit (Nichirei Bioscience, Tokyo, Japan). Slides were incubated at room temperature, with 3,3’ diaminobenzidine (DAB-K3468, Dako Corporation, Carpinteria, CA, USA), counterstained with Mayer’s hemalum solution (Merck, Darmstadt, Germany) and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, MO, USA). The same technician conducted all procedures for IHC.
Images of ovarian sections were captured with a digital camera (Olympus DP72) attached to a compound light microscope (Olympus BX 51, Tokyo, Japan), using the 40 X objective. Only oocytes included in primordial, primary and secondary follicles were marked for image analysis and the intensity of labeling was captured. The oocytes were classified as: included in primordial/primary follicles (OIPF), when surrounded by one layer of flat or cubic granulosa cells and included in secondary follicles (OISF), when surrounded by two or more layers of cubic granulosa cells [29]. For the Foxo3 antibody a total of 28 OIPF (14 N and 14 df/df) and 8 OISF (4 N and 4 df/df) were analyzed; and for the evaluation of p-Foxo3 immunostaining 30 OIPF (15 N and 15 df/df) and 9 OISF (4 N and 5 df/df) were used. Protein quantification was performed by software image analysis [30]-[33]. The most common value (the mode) of each area was registered by the 32-bit histogram application of the Image J® software, using a scale ranging from 0 (the greatest staining intensity) to 255 (no staining) that was converted to a percentual scale from 0% (no staining) to 100% (greatest staining).
The results are presented as mean ± standard error of the mean (SEM). All statistical analyses were performed using Graphpad Prism 5 (Graphpad Software Inc., La Jolla, CA, USA). One-way ANOVA was performed to estimate between groups difference, followed by Tukey’s multiple comparison test. Immunostaining intensity for FOXO3 and p-FOXO3 was compared between strains by t-test. A P value lower than 0.05 was considered statistically significant.
All animal procedures employed in our presented work were approved by the Laboratory Animal Care and Use Committee (LACUC) at the Southern Illinois University School of Medicine (Springfield, IL).
Results
The expression of insulin receptor substrate 1 (Irs1), Pi3k, Akt1, Mtor, suppressor of cytokine signaling −2 (Socs2), Socs3 was lower (P < 0.05) in older than younger N mice (Figures 1 and 2). Despite that, the expression of Irs1, Pi3k, Akt1, Mtor, Socs2, Socs3 was not different (P > 0.05) between young and old df/df mice (Figures 1 and 2). The expression of Foxo3a was also lower (P < 0.05) in old than younger N and df/df mice. In addition, Foxo3a expression was higher (P < 0.05) in old df/df than N mice. The expression of Ghr and Igf was lower (P < 0.05) in old than young df/df mice. Despite that, expression Ghr and Igf was not different (P > 0.05) between young and old N mice. Expression of Amh was lower (P < 0.05) in old than young N and df/df mice. In addition Amh expression was higher (P = 0.0009) in df/df than N mice. Expression of Bmp15 was lower (P < 0.05) in old than young N mice, but not different (P > 0.05) between old and young df/df mice. Expression of Gdf9 was lower (P < 0.05) in old than young df/df mice, but not different (P > 0.05) between old and young N mice (Figures 3).
Ovarian gene expression for genes in the insulin signaling pathway (IRS1, Pi3k, Akt1 and FOXO3a) in young (6 months–black bar) and old (22 months–grey bar) normal (N) and Ames dwarf (df/df) mice. Different letter indicate significant differences (P<0.05). Expression of all genes showed was lower in old than younger mice (P<0.05).
Ovarian gene expression for genes indicating ovarian activity (AMH, GDF9 and BMP15) in young (6 months–black bar) and old (22 months–grey bar) normal (N) and Ames dwarf (df/df) mice. Different letter indicate significant differences (P<0.05). Expression of all genes showed was lower in old than younger mice (P<0.05).
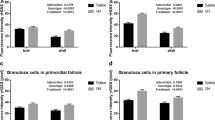
Foxo3 and p-Foxo3 immunostaining for OIPF and OISF in df/df and N mice is shown in Figures 4 and 5. It was observed that there was no difference between df/df and N mice for Foxo3 immunostaining in OIPF and OISF (P > 0.05). However, immunostaining for p-Foxo3 was higher in N vs df/df mice in OIPF (P = 0.0008), and there was no difference (P = 0.09) when comparing N vs Ames dwarf mice in OISF.
Immunostaining (arrows) for FOXO3 (A-D) and p-FOXO3 (E-H) in oocytes included in primordial/primary follicles (OIPF) and oocytes included in secondary follicles (OISF) in N mice (A, C, E and G) and df/df mice (B, D, F and H). Negative controls are represented in small Figures a-h. OIPF immunostained with FOXO3 in N and df/df mice, respectively. (Photomicrograph 100 X). (C and D) OISF immunostained with FOXO3 in N and df/df mice, respectively (photomicrograph 10 X). (E and F) OIPF immunostained with pFOXO3 in N and df/df mice, respectively. (Photomicrograph 100 X). (G and H) OISF immunostained with pFOXO3 in N and df/df mice, respectively. (Photomicrograph 10 X).
Discussion
Expression of mRNA for Irs1, Socs2, Socs3, Akt1, Pi3k, Mtor and Foxo3a was lower in the ovaries of older mice of both genotypes. As mentioned earlier, activation of this pathway by growth factors is involved in the activation of primordial follicle growth by ultimately promoting phosphorylation and nuclear export of FOXO3a [20],[22]. The lower expression of these genes in older mice points to this pathway as associated with the process of ovarian aging and follicle depletion. In fact, a recent study indicate that down-regulation of oocyte FOXO3a expression is essential for follicle progression from the primordial to later stages of development [34]. Older mice also had lower mRNA expression for Amh, Gdf9 and Bmp15. The AMH is a member of the transforming growth factor β family (TGFβ) exclusively produced by granulosa cells (GC) of developing preantral and small antral follicles [35]. Serum AMH levels are widely used to estimate the size of the ovarian reserve [36]. GDF9 and BMP15 are also members of the TGFβ family and produced exclusively by growing oocytes in the mice ovary [37],[38]. In the GDF9 null mice folliculogenesis does not progress beyond the primary stage [39], while BMP15 null mice had impaired oocyte maturation leading to infertility [40]. Therefore, both GDF9 and BMP15, in addition to AMH, are indicating lower ovarian activity in 22-month-old in relation to 6-month-old mice of both genotypes as we would expect during aging of the ovaries. Together, these results indicate that reduced ovarian activity with increasing age is associated with reduced expression of the members of the IRS1-Pi3k-Akt1-FOXO3 pathway.
Interestingly, expression of Foxo3a mRNA was 10-fold higher in the ovaries of old df/df mice when comparing to old N mice. FOXO3a is crucial in the maintenance of follicles in the primordial quiescent stage while not phosphorylated [22]. Therefore, the higher expression of Foxo3a indicates that follicles could be trapped in the primordial stage. This was confirmed by the observation that oocytes from primordial/primary follicles from Ames dwarf mice had a lower level of p-FOXO3 compared to N mice, although no difference in the level of FOXO3 was detected, indicating a lower level of activation of primordial follicles. It is known that phosphorylation of FOXO3a by the IRS-Pi3k-Akt pathway is what indicates its activation, therefore higher gene expression from members of the IRS-Pi3k-Akt pathway does not necessarily means more FOXO3a activation, because it depends on the level of activation of this pathway by insulin/IGF. Since Ames dwarf mice have strongly reduced insulin/IGF signaling [3], it is possible that this is the cause of the reduced level of p-FOXO3 in oocytes from Ames dwarf mice. This hypothesis agree with histological observations in long-living GH-resistant GHRKO mice and mice subjected to calorie restriction where a higher number of follicles in the primordial stage is observed concomitantly with less follicles in the primary, secondary and tertiary stages [16],[18]. A recent paper has demonstrated that oocyte expression of a constitutively active form of FOXO3 in a transgenic mouse model is associated with a younger ovarian phenotype [41]. In this way FOXO3a is been referred as a potential determinant of the onset of menopause, as well as a potential agonist intervention to reduce the physiological decline in female fertility at later ages [41].
Therefore our study, in agreement with previous observations [41], indicates that higher expression of Foxo3a mRNA and decreased level of p-FOXO3 in oocytes from primordial/primary follicles, together with other genes in the pathway of insulin signaling as observed in df/df mice, is associated to increased expression of genes related to follicle recruitment and growth (Amh, Gdf9 and Bmp15). Amh mRNA expression was about 80 times higher in old df/df when compared to old N mice. This result indicates that although normal old mice had severely depleted the ovarian follicular reserve (Amh mRNA expression about 900 times lower than young N mice), df/df mice still have some follicular activity (Amh mRNA expression about 20 times lower than young df/df mice). More studies are necessary to identify if the same pattern will be observed in GHRKO and calorie restricted N mice, but already points that modulation of the IRS-Pi3k-Akt pathway can modulate ovarian FOXO3a expression and therefore help in preventing follicle loss in older females.
Even though the liver is the main source of circulating IGF-I, many local tissues are able to produce IGF-I [42]. The importance of the endocrine versus the paracrine/autocrine ovarian IGF-I has been debated since the demonstration that GHRKO mice [15] and mice with liver-specific knockout of IGF-I [43] are able to reproduce, while the IGF-I null mice are infertile and fail to ovulate [44]. In this way, the data of the present study further support these findings by showing that df/df mice, that have no detectable levels of circulating GH and severely reduced IGF-I, are able to produce ovarian Igf1 mRNA. The results indicate that ovarian Igf1 mRNA expression is largely independent of GH as demonstrated in the df/df mice hippocampus [10] and in the ovaries of GHRKO mice [11].
Conclusions
In sum, the present study indicates that lower expression of Irs1, Socs2, Socs3, Akt1, Pi3k, Mtor and Foxo3a mRNA in the ovaries of older mice of both genotypes is associated to a reduced ovarian activity revealed by lower expression of Amh mRNA. At the same time, ovaries of old df/df mice maintained higher expression of Foxo3a mRNA, which was associated to higher ovarian activity. We have shown that df/df females have a lower level of p-FOXO3 in oocytes from primordial/primary follicles, an important activator of follicular growth. Therefore, our study strongly indicates that Prop1df mutation causes delayed ovarian aging.
References
Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG: Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 1996, 384: 327–333. 10.1038/384327a0
Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C: Prolonged longevity of hypopituitary dwarf mice. Exp Gerontol 2001, 36: 21–28. 10.1016/S0531-5565(00)00205-9
Chandrashekar V, Bartke A: Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod 1993, 48: 544–551. 10.1095/biolreprod48.3.544
Brown-Borg HM, Borg KE, Meliska CJ, Bartke A: Dwarf mice and the ageing process. Nature 1996, 384: 33. 10.1038/384033a0
Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ: Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 2003, 144: 3799–3810. 10.1210/en.2003-0374
Masoro EJ: Overview of caloric restriction and ageing. Mech Ageing Dev 2005, 126: 913–922. 10.1016/j.mad.2005.03.012
Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D: Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides 2002, 36: 201–208. 10.1054/npep.2002.0889
Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM: Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J 2010, 24: 5073–5079. 10.1096/fj.10-163253
Barbieri M, Bonafe M, Franceschi C, Paolisso G: Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab 2003, 285: E1064–1071. 10.1152/ajpendo.00296.2003
Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A: Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging 2005, 26: 929–937. 10.1016/j.neurobiolaging.2004.07.010
Schneider A, Zhi X, Bartke A, Kopchick JJ, Masternak MM: Effect of growth hormone receptor gene disruption and PMA treatment on the expression of genes involved in primordial follicle activation in mice ovaries. Age 2014, 36: 9701. 10.1007/s11357-014-9701-9
Chandrashekar V, Zaczek D, Bartke A: The consequences of altered somatotropic system on reproduction. Biol Reprod 2004, 71: 17–27. 10.1095/biolreprod.103.027060
Bartke A: Reproduction of female dwarf mice treated with prolactin. J Reprod Fertil 1966, 11: 203–206. 10.1530/jrf.0.0110203
Tang K, Bartke A, Gardiner CS, Wagner TE, Yun JS: Gonadotropin secretion, synthesis, and gene expression in human growth hormone transgenic mice and in Ames dwarf mice. Endocrinology 1993, 132: 2518–2524.
Bachelot A: Growth Hormone Is Required for Ovarian Follicular Growth. Endocrinology 2002, 143: 4104–4112. 10.1210/en.2002-220087
Li L, Fu YC, Xu JJ, Chen XC, Lin XH, Luo LL: Caloric restriction promotes the reproductive capacity of female rats via modulating the level of insulin-like growth factor-1 (IGF-1). Gen Comp Endocrinol 2011, 174: 232–237. 10.1016/j.ygcen.2011.09.005
Sluczanowska-Glabowska S, Laszczynska M, Piotrowska K, Glabowski W, Kopchick JJ, Bartke A, Kucia M, Ratajczak MZ: Morphology of ovaries in laron dwarf mice, with low circulating plasma levels of insulin-like growth factor-1 (IGF-1), and in bovine GH-transgenic mice, with high circulating plasma levels of IGF-1. J Ovarian Res 2012, 5: 18. 10.1186/1757-2215-5-18
Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ: Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction 2006, 131: 525–532. 10.1530/rep.1.00946
McGee EA, Hsueh AJ: Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000, 21: 200–214.
Brown C, LaRocca J, Pietruska J, Ota M, Anderson L, Smith SD, Weston P, Rasoulpour T, Hixon ML: Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKB alpha/Akt1. Biol Reprod 2010, 82: 246–256. 10.1095/biolreprod.109.077925
Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K: Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008, 319: 611–613. 10.1126/science.1152257
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA: Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003, 301: 215–218. 10.1126/science.1086336
Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D: Cyclophosphamide triggers follicle activation and "burnout"; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 2013, 5: 185ra-162.
Caron E, Ghosh S, Matsuoka Y, Ashton-Beaucage D, Therrien M, Lemieux S, Perreault C, Roux PP, Kitano H: A comprehensive map of the mTOR signaling network. Mol Syst Biol 2010, 6: 453. 10.1038/msb.2010.108
Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K: Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 2010, 19: 397–410. 10.1093/hmg/ddp483
Bartke A: Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell 2008, 7: 285–290. 10.1111/j.1474-9726.2008.00387.x
Masternak MM, Al-Regaiey K, Bonkowski MS, Panici J, Sun L, Wang J, Przybylski GK, Bartke A: Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci 2004, 59: 784–788. 10.1093/gerona/59.8.B784
Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Bonkowski MS, Panici JA, Przybylski GK, Bartke A: Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci 2005, 60: 1238–1245. 10.1093/gerona/60.10.1238
Silva RC, Bao SN, Jivago JL, Lucci CM: Ultrastructural characterization of porcine oocytes and adjacent follicular cells during follicle development: lipid component evolution. Theriogenology 2011, 76: 1647–1657. 10.1016/j.theriogenology.2011.06.029
Atkin G, Barber PR, Vojnovic B, Daley FM, Glynne-Jones R, Wilson GD: Correlation of spectral imaging and visual grading for the quantification of thymidylate synthase protein expression in rectal cancer. Hum Pathol 2005, 36: 1302–1308. 10.1016/j.humpath.2005.08.016
Di Cataldo S, Ficarra E, Macii E: Computer-aided techniques for chromogenic immunohistochemistry: status and directions. Comput Biol Med 2012, 42: 1012–1025. 10.1016/j.compbiomed.2012.08.004
Melendez-Ferro M, Rice MW, Roberts RC, Perez-Costas E: Dual use of immunohistochemistry for film densitometry and light microscopy. J Neurosci Methods 2012, 208: 86–91. 10.1016/j.jneumeth.2012.05.003
Moreira F, Corcini CD, Mondadori RG, Gevehr-Fernandes C, Mendes FF, Araujo EG, Lucia T Jr: Leptin and mitogen-activated protein kinase (MAPK) in oocytes of sows and gilts. Anim Reprod Sci 2013, 139: 89–94. 10.1016/j.anireprosci.2013.03.011
Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K: Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development 2007, 134: 199–209. 10.1242/dev.02667
Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N, Campbell BK, Yding Andersen C: Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod 2013, 19: 519–527. 10.1093/molehr/gat024
Visser JA, de Jong FH, Laven JS, Themmen AP: Anti-Mullerian hormone: a new marker for ovarian function. Reproduction 2006, 131: 1–9. 10.1530/rep.1.00529
McGrath SA, Esquela AF, Lee SJ: Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol 1995, 9: 131–136.
Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM: The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol 1998, 12: 1809–1817. 10.1210/mend.12.12.0206
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM: Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996, 383: 531–535. 10.1038/383531a0
Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM: Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 2001, 15: 854–866. 10.1210/mend.15.6.0662
Pelosi E, Omari S, Michel M, Ding J, Amano T, Forabosco A, Schlessinger D, Ottolenghi C: Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat Commun 2013, 4: 1843. 10.1038/ncomms2861
Le Roith D, Bondy C, Yakar S, Liu JL, Butler A: The somatomedin hypothesis: 2001. Endocr Rev 2001, 22: 53–74. 10.1210/edrv.22.1.0419
Liu JL, Yakar S, LeRoith D: Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proc Soc Exp Biol Med 2000, 223: 344–351. 10.1046/j.1525-1373.2000.22349.x
Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A: Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 1996, 10: 903–918.
Acknowledgments
Research reported in this publication was supported by National Institute on Aging of the National Institutes of Health under award number R01AG032290 and P01AG031736. A Schneider was supported by Federal University of Pelotas during the period of the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AS performed RNA extraction, gene expression and data analysis and drafted the manuscript. MMM and XZ designed the study, performed the experiment and data analysis and revised the manuscript. FM, TLJ and RGM performed protein analysis and revised the manuscript. All of the authors have read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Schneider, A., Zhi, X., Moreira, F. et al. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res 7, 120 (2014). https://doi.org/10.1186/s13048-014-0120-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13048-014-0120-4