Abstract
Fibroblast growth factors (FGFs) and their receptors (FGFRs) play critical roles in many biological processes and developmental functions. Chromosomal translocation of FGFRs result in the formation of chimeric FGFR fusion proteins, which often cause aberrant signaling leading to the development and progression of human cancer. Due to the high recurrence rate and carcinogenicity, oncogenic FGFR gene fusions have been identified as promising therapeutic targets. Erdafitinib and pemigatinib, two FGFR selective inhibitors targeting FGFR fusions, have been approved by the U.S. Food and Drug Administration (FDA) to treat patients with urothelial cancer and cholangiocarcinoma, respectively. Futibatinib, a third-generation FGFR inhibitor, is under phase III clinical trials in patients with FGFR gene rearrangements. Herein, we review the current understanding of the FGF/FGFRs system and the oncogenic effect of FGFR fusions, summarize promising inhibitors under clinical development for patients with FGFR fusions, and highlight the challenges in this field.
Similar content being viewed by others
Background
Structural chromosome rearrangements between two genes may lead to the deregulation of genes originating from translocation, insertion, inversion, or deletion. Such gene fusions are mostly pathogenic and have offered important insights into carcinogenesis [1]. Enforced dimerization/oligomerization and inactivation of autoinhibition mechanisms are the two key mechanisms that lead to aberrant kinase activity [2]. Growing numbers of fusion proteins encoded by the hybrid genes have been novel targets for personalized cancer therapy that significantly improved patient survival [3, 4]. Imatinib was the first U.S. Food and Drug Administration (FDA) approved drug that was designed to inhibit the oncogenic fusion protein BCR–ABL [5]. The success of imatinib in chronic myeloid leukemia (CML) patients has triggered great interest in developing novel drugs targeting the chimeric proteins, including ALK [6], ROS1 [7], RET [8], MET [9] and NTRK [10] fusions. To date, more than 10 kinase inhibitors have been approved by the FDA for the treatment of fusion-positive cancers.
Fibroblast growth factor (FGF) receptors (FGFRs) are highly conserved single transmembrane receptor tyrosine kinases (RTKs), consisting of an extracellular ligand-binding domain and a cytoplasmic conserved tyrosine kinase domain. FGFR signals play important roles in cellular proliferation and survival, embryonic development, fetal organogenesis, metabolism homeostasis, and tissue repair [11, 12]. Dysregulation of FGFR signaling contributes to oncogenesis and tumour progression, drug resistance to anticancer therapy, as well as the occurrence of immune evasion and angiogenesis in the tumour microenvironment (TME). Aberrant activation of the oncogenic FGFR signaling pathway is mainly caused by the deregulation of FGF ligand and FGFR genetic alterations, including amplification, activating mutations, and gene fusions [12,13,14].
FGFR fusions occur when the kinase domain of FGFR1–4 fuses with a partner containing a constitutive dimerization/oligomerization motif, thereby activating the signaling in a ligand-independent manner [15,16,17]. With the advances in deep-sequencing technology, cases of FGFR-related fusion genes are growing exponentially. The dominant oncogenic fusion partners drive malignant initiation and progression, especially in urothelial cancer, cholangiocarcinoma, and glioblastoma [3, 18]. Therefore, certain FGFR fusions have emerged as biomarkers and rational druggable targets.
FGF–FGFR-HS system
The human FGF family consists of 18 functional glycoproteins (FGF1–10 and FGF16–23). They are grouped into six subfamilies according to their sequence similarity and phylogeny [11, 19]. FGFs belonging to the FGF1/4/7/8/9 subfamilies act as paracrine ligands, whereas FGF19/21/23 belonging to FGF19 subfamily are endocrine hormones (Fig. 1A) [20]. FGFs exert their function through binding to and dimerizing their cognate receptors. The extracellular ligand-binding segment of FGFR1–4 comprises three immunoglobulin-like domains (D1–D3) [21]. The C-terminal half of D3 domains of FGFR1–3 are encoded by two alternative exons (termed exon IIIb and IIIc), yielding “b” and “c” isoforms. These cell and tissue-specific alternative splicing events converse the sequence of crucial residues in the pocket of the ligands-binding D3 domain of FGFR1–3, thus governing FGF binding specificity and varying signaling patterns [22]. The “c” isoforms of FGFRs are primarily expressed in mesenchymal cells and favorably recognize epithelial-derived FGF1/4/8/9/19 subfamilies, whereas the “b” isoforms are mostly expressed in epithelial tissues, showing rigorous binding specificity for the mesenchymal-expressed FGF7 subfamily [23].
FGF–FGFR-HS system. A 18 functional mammalian FGFs sorted into six subfamilies. Each founding members are colored in orange. B Left: paracrine FGFs bind to the D2-D3 domains of FGFRs and HS to form 2:2:2 FGF-FGFR-HS complex (PDB: 1FQ9). Right: Endocrine FGF-FGFR-Klotho complex PDB ID: 5 W21). Alternatively-spliced D3 domain of FGFR is highlighted in purple
Besides ligands, receptors dimerization is assisted by a cofactor named heparan sulfate proteoglycans (HSPGs) ubiquitously present on the cell surface and in the extracellular matrix. Sulfate group-rich HSPGs interact with the lysine/arginine-rich surface termed “heparan binding site (HBS)” of both FGFs and receptors to stabilize the FGF–FGFR binding interface, thereby promoting the formation of a 2:2:2 quaternary complex of FGF, FGFR, and HS (Fig. 1B) [24]. HSPGs are obligatory cofactors for both paracrine and endocrine FGFs. The endocrine FGF19 subfamily (FGF19/21/23) has less surface-exposed lysine/arginine residues on the HBS than the paracrine FGFs, leading to intrinsically reduced affinity to HSPG binding. Thereby, the FGF19 subfamily acts as hormones released from the expression site into the body circulation system. For endocrine FGFs, a secondary cofactor is necessary to stabilize FGF–FGFR complex (Fig. 1B), i.e., FGF19/21 requires β-Klotho as a secondary cofactor to promote signaling, whereas FGF23 utilizes α-Klotho to form FGF23–FGFR–αKlotho–heparan sulfate quaternary complex [25, 26].
FGF signaling pathways in cancer
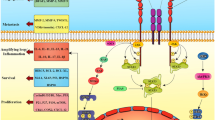
FGF ligands-induced receptor dimerization and tyrosine trans-phosphorylation ultimately generate docking sites for intracellular effector molecules. FGFR substrates 2α (FRS2α) and phospholipase Cγ (PLCγ) are the two substrates that bind directly to the kinase domain [27]. Following phosphorylation, FRS2α and PLCγ will trigger multiple signaling pathways, including RAS–mitogen-activated protein kinase (MAPK), phosphoinositide 3- kinase (PI3K)–protein kinase B (AKT), protein kinase C (PKC), and STAT-dependent signaling, thereby contributing to carcinogenesis by stimulating cancer cell proliferation and survival, neoangiogenesis, and drug resistance (Fig. 2).
FGFR signaling in cancers. FGF, HSPG, and FGFR form 2:2:2 ternary complex, followed by receptor dimerization and kinase transphosphorylation. FGFR downstream adaptor protein FRS2 interacts with SHP2 and GRB2 complex, leading to subsequent activation of PI3K-AKT and RAS-MEK-ERK signaling pathways. Another FGFR substrate, PLC-g, binds to phosphotyrosine and hydrolyzes PIP2 to generate IP3 and DAG, which in turn activate PKC and MAPK pathway, resulting in cell migration, proliferation, and differentiation. Depending on the cellular context, FGFRs have the capability to activate the JAK-STAT3 signalling pathway. Aberrant FGFR signaling may be induced by (i) increased expression of FGFs (ligand-dependent), or (ii) FGFR alteration, including mutation, amplification or translocation (ligand-independent)
FRS2α is a non-enzymatic adaptor protein of FGFR signaling, which functions as the central node for the assembly of various signaling complexes [28, 29]. FRS2α has six tyrosine residues in the long flexible C-terminal tail that can be phosphorylated by activated FGFRs. Specifically, phosphorylation at the four tyrosine residues (Tyr-196, Tyr-306, Tyr-349 and Tyr-392) will enable FRS2α to recruit two preformed growth factor receptor-bound protein 2 (GRB2) containing binary complexes, i.e., GRB2–son of Sevenless (SOS) and GRB2–GRB2-associated-binding protein 1 (GAB1). FRS2α assists the translocation of GRB2–SOS complex to downstream substrate RAS and activates RAS through GTP exchange, followed by activation of MAPK signaling. Upon the recruitment of the GRB2–GAB1 complex, PI3K will be recruited to FRS2α, leading to the translocation and transphosphorylation of AKT [30, 31]. In addition, FRS2α phosphorylation at C-terminal tail (pTyr-436 and pTyr-471) offers a docking site for SH2-containing tyrosine phosphatase (SHP2) and further diversifies FGFR signaling [32, 33].
PLCγ is a hydrolase docking to the phosphorylated tyrosine (Tyr-769 in FGFR2) in the C- terminal tail of FGFR through its cSH2 domain. PLCγ stimulates the release of Ca2+ from the endoplasmic reticulum into the cytosol and activates the Ser/Thr kinase protein kinase C (PKC), resulting in cell migration, proliferation and differentiation [34]. The crystal structure of PLCγ cSH2 domain and C-terminal phosphorylated FGFR2 kinase complex has shown the engagement of cSH2 of PLCγ with activated FGFR2 kinase, demonstrating that the recruitment of PLCγ is followed by FGFR dimerization. One activated FGFR kinase functions as the recruiter of PLCγ, whereas the other is responsible for PLCγ phosphorylation at Tyr-771 and Tyr-783 [35].
Mechanisms of FGFR fusion oncoprotein
Autoinhibition modes of FGFR
Receptor tyrosine kinase (RTK) signaling is tightly regulated by protein allostery from the extracellular domain or the intracellular tyrosine kinase domains (Fig. 3A) [36]. The first line of FGFR autoinhibition is mediated by the extracellular D1 domain and the acid box subregion between the D1 and D2 domain that prevents inadvertent ligand activation [21]. As in cytoplasmic FGFRs kinase domains, unphosphorylated kinase are more precisely controlled to minimize undesired signaling [37]. Binding of extracellular ligand induces receptor dimerization, thereby forcing the kinase domains into appropriate proximity and orientation for transautophosphorylation on specific tyrosine sites. Accumulated biochemical and structural evidences indicate that autophosphorylation of FGFR kinase occurs in a sequentially and accurately ordered reaction that can be separated into three phases [38]. During the first phase, the tyrosine residues positioned at the activation loop (A-loop) accomplish phosphorylation, which in turn induces the active conformation of the kinase, leading to 50–100-fold upregulation of kinase activity. The second phase includes the phosphorylation of tyrosine residues located in the kinase insert, JM segment, and the carboxy-tail region. In the last phase, FGFR kinase activity is increased by an additional 10-fold via the phosphorylation of the second tyrosine residue within the A-loop (Y654 in FGFR1). Thus, A-loop transphosphorylation is an obligatory step for kinase activation, and therefore is the key mechanism for FGFR kinase autoinhibition [39].
FGFR autoinhibition mechanisms. A Schematic representations of FGFR autoinhibition modes consisting of acid box regulation, molecular brake, DFG latch, and the repulsion between enzyme and substrate kinases B overall view of the asymmetric FGFR kinase A-loop transphosphorylation complex (PDB: 6PNX). Enzyme- and substrate-acting FGFR kinases are colored in green, blue and wheat, respectively. C FGFR3 K659 and R669 form enzyme-substrate electrostatic clash. Sequence alignment of the kinase domains of FGFR1–4 shows the conservation of autoinhibition mechanism. D Hydrogen bonding pattern of the autoinhibitory molecular brake in FGFR1 (left, PDB ID: 1fgk) and disengaged brake (right, PDB ID: 3gqi). The dashed lines denote hydrogen bonds
In 2020, the structure of an asymmetric complex of two FGFR3 kinases caught in the act of transphosphorylation was successfully solved [40]. In this complex, one kinase serves as an enzyme, whereas the other is the substrate (Fig. 3B). This is distinct from the activator-receiver relationship of EGFR asymmetric homodimer. The substrate acting kinase offers its first A-loop tyrosine residue (Tyr647 in FGFR3) for the initiation of A-loop tyrosine transphosphorylation reaction. Notably, the FGFR asymmetric kinase dimer is thermodynamically disadvantaged owing to the electrostatic repulsion between their C-lobes, which is mainly caused by the two conserved residues within the kinase domain, i.e., Lys659 in enzyme and Arg669 in substrate kinases, thereby governing the kinase A-loop transphosphorylation event (Fig. 3C) [40]. In addition, an autoinhibitory network of hydrogen bond interactions named ‘molecular brake’ controls the kinase activity to a low-level state. These include Glu562 residue (FGFR1 numbering) in the kinase hinge, Asn546 in the αC-β4 loop, and Lys638 in the β8 strand (Fig. 3D) [41]. Another cluster of hydrophobic interactions centered on the Asp-Phe-Gly (DFG) motif called the ‘DFG latch’ is also involved in FGFR autoinhibition by affecting the conformation of the A-loop as well as the N-lobe rotation [36, 42].
The activation of FGFRs is normally down-regulated by those autoinhibitory mechanisms, and the disruption of autoinhibition promotes various cancers. It is important to find the underlying mechanism of gene fusion-induced autoinhibition release. Firstly, the oncogenic potential of fusions has been attributed to the loss of the critical D1 domain and acid box region of FGFRs [43]. Besides, the energy gain from fusion-induced receptor dimerization may overcome the repulsion between two kinases, thereby promoting the A-loop transphosphorylation and leading to kinase activation [38, 40]. Indeed, analysis of phosphopeptides from human astrocytes expressing FGFR3-TACC3 fusions shows the tyrosine Tyr647 (first A-loop tyrosine) in FGFR3 exhibited the highest enrichment in phosphorylation [17].
Oncogenic mechanisms of FGFR chimeric proteins
Upon ligand-induced receptor dimerization and intracellular kinase A-loop phosphorylation, these self-regulatory switches are subsequently released to turn on the kinase activity. Genetic alterations of FGFRs, including FGFR fusions that eliminate molecular brake or enzyme-substrate repulsion, are proved to be oncogenic. Around 8% of FGFR genetic alterations-related cancers are driven by FGFR gene fusion, which can be classified into type I or type II fusions (Fig. 4A) [18]. In type I fusions, the extracellular and the transmembrane part of the receptors are replaced by the fusion partners. The FGFR kinase domains are forced to dimerization, facilitated by the 5′ fusion partner. In type II fusions, kinase activity is triggered by the fusion at C-terminal regions with the whole receptors remaining intact [44].
FGFR fusions. A Schematic representations of FGFR type I/II fusions. Fusions of FGFR with genes that encode other signaling proteins at N- terminal (type I) or C- terminal (type II) result in release of autoinhibition state and followed by aberrant kinase activation. B Potential oncogenic mechanisms of FGFR fusions. Left: fusions produce elevated oncogenic signaling through promoter exchange and FGFR overexpression. Middle: ligand-independent FGFR oligomerization lead to constitutively activation of FGFR kinase mediated by the PPIs through the oligomerization domain (OD) within the fusion partners. Right: FGFR fusion oncoproteins may undergo a higher-order assembly to produce membraneless cytoplasmic protein granules that promote local RAS activation and induce MAPK signaling activation in cancer. TM, transmembrane region; TK, tyrosine kinase domain
A few fusion partners, such as SLC45A3 identified in patients with prostate cancer, can drive overexpression of FGFR2 through promoter exchange (Fig. 4B) [45]. The SLC45A3–FGFR2 fusion contains most of the promoter region of SLC45A3 and only the non-coding region of exon 1, which has the similar oncogenic mechanism to the most famous TMPRSS2–ERG fusion gene existing in more than 50% of prostate cancers [45, 46]. For most cases, the fusion domains provide particular self-association domains that induce ligand-independent dimerization/oligomerization through specific protein-protein interactions (PPIs). These result in constitutive autophosphorylation of the FGFR and aberrant activation of multiple downstream oncogenic signaling cascades in a ligand-independent manner [45]. Those fusion partners include coiled-coil (e.g. FGFR3-TACC3, FGFR2–CCDC6 and FGFR2–CIT), LIS1-homologous (LisH, e.g. FGFR2–OFD1), sterile alpha motif (SAM, e.g. FGFR2–BICC1), IRSp53/MIM homology domain (IMD, FGFR3–BAIAP2L1), and caspase domains (e.g. FGFR2–CASP7) (Fig. 4B) [4, 45].
Besides, Tulpule et al. [47] demonstrated that RTK fusion oncoproteins can form membraneless intracellular protein granules. An array of RTK adaptor and effector molecules and RAS activating proteins are identified at the biomolecular condensates, including GAB1, GRAB2, SHP2, and SOS1. This higher-order protein assembly is crucial for the activation of oncogenic RAS-MAPK signaling (Fig. 4B). Interestingly, the cytoplasmic granule formation may be a general mechanism for oncogenic RTK-mediated signaling activation by FGFR [47]. Thus, drugs to disrupt the nucleation of FGFR membraneless cytoplasmic protein granules may provide opportunities for the treatment of FGFR oncoprotein-driven cancers.
FGFR gene fusions in cancers
The use of next-generation sequencing approaches for clinical diagnostics greatly promoted the discovery of FGFR molecular alterations in cancers [48, 49]. Fusion events were reported between FGFRs and numerous partners correlated to various cancer progressions (Table 1). FGFR1 fusions correlate to aggressive haematological malignancies and solid tumors, including breast cancer and lung cancer. FGFR2 fusions mainly correlate to cholangiocarcinoma [13, 50]. FGFR3 functions corrupted by translocations are frequently observed in urothelial carcinoma and glioblastoma. FGFR4 fusions are rare, and only some cases were reported in non-small cell lung cancer (NSCLC) [48]. In addition, FGFRs-containing gene fusions are emerging targets for FGFR-targeted cancer therapies.
FGFR1 fusions in haematological malignancies
Myeloid and lymphoid neoplasms with aberrant FGFR1 activities have been classified into a distinct disease group in haematological neoplasms by the World Health Organization in 2008 [51]. This kind of rare but aggressive haematological malignancies are associated with chromosomal translocation of FGFR1 on chromosome 8p11–12, and later are described as 8p11 myeloproliferative syndrome, also known as stem cell leukemia/lymphoma (SCLL). The symptoms include eosinophilia, lymphadenopathy, and lymphoma with subsequent progress to B-cell lymphoma and acute myeloid leukemia [51].
To date, more than 14 FGFR1 fusion partners in hematopoietic neoplasm have been described, and the zinc-finger domain ZNF198 (also known as ZMYM2) on chromosome 13q12 is the most typical partner gene [52,53,54]. Other neoplasms with chromosomal abnormalities include t(8;22)(p11;q11), t(6;8)(q27;p11) and t(8;9)(p11;q33). These chromosomal abnormalities result in FGFR1 fusion with BCR (breakpoint cluster region) [55], FOP1(FGFR1 oncogenic partner 1) [56], and CNTRL (centrosomal Protein 1) [57], respectively. All these fusion proteins related to haematological malignancies are type I FGFR fusions. These fusion proteins do not have extracellular FGF binding domains, so the dimerization/oligomerization and transphosphorylation of FGFR kinase occur in a ligand-independent manner.
BCR–FGFR1 occurs in stem cell leukemia/lymphoma, which can progress to atypical chronic myeloid leukemia, acute myeloid leukemia, or B-cell lymphoma. In BCR–FGFR1 fusion, BCR functions as a coiled-coil oligomerization domain and promotes oncogenic transformation. Recently, Peiris et al. [55] demonstrated the formation of three interhelical salt bridges by BCR domain contributing to the cellular transforming ability of BCR–FGFR1 fusion. Furthermore, BCR–FGFR1 is a heat shock protein 90 (Hsp90) addicted fusion to evade ubiquitination and proteasomal degradation. Thus, in addition to the kinase domain inhibitors, targeting BCR oligomerization and chaperonin Hsp90 complex can be alternative therapeutic strategies to combat BCR–FGFR1 fusion-positive SCLL.
FGFR2 fusions in cholangiocarcinoma
Cholangiocarcinoma is a fatal biliary tract cancer. The five-year survival rate of cholangiocarcinoma patients was less than 10%, owing to the limited therapeutic options [58]. Genomic analysis has shown that FGFR2 fusions were identified in 13% ~ 50% of intrahepatic cholangiocarcinoma (iCCA) patients. FGFR2 fusion harboring iCCA shows unique pathologic characteristics, including growing with a tubular anastomosing or intraductal pattern, and lack of stem-like cell markers (CD56 and KIT) [50, 59]. Notably, FGFR2-fusions were rarely observed in perihilar cholangiocarcinoma (PHC) or distal cholangiocarcinoma (DC) [60].
Currently, more than a hundred different FGFR2 fusion protein chimeras have been reported in iCCA. FGFR2–PPHLN1 (Periphilin) is a particularly common type II FGFR fusion chimera in iCCA, resulting from the t(10;12)(q26,q12) translocation. It was identified in nearly 16% of iCCA patients. The C-terminal coiled-coil region derived from PPHLN1 was found to mediate dimerization/oligomerization and favor the oncogenic capability [50, 61]. Indeed, expression of FGFR2-PPHLN1 in HEK293T cells showed robust FGFR phosphorylation and activation of downstream MAPK signaling. NIH3T3 cells transfected with FGFR2–PPHLN1 displayed increased transforming activity in a soft agar assay. In addition, the HUCCT1 cell line overexpressing FGFR2–PPHLN1 obtained increased viability and migratory capacity [50]. Other coiled-coil or sterile alpha motif (SAM) domains containing FGFR2 fusion partners, including coiled-coil domain containing 6 (CCDC6) [62], BicC family RNA-binding protein 1 (BICC1) [63] and adenosylhomocysteinase like 1 (AHCYL) [50] are also found in iCCA patients with frequencies of 3, 6 and 11%, respectively [59]. FGFR2–CCDC6 fusion significantly enhanced tumor cell proliferation and tumorigenesis in an iCCA patient-derived xenograft (PDX) mouse model [62].
FGFR3 fusions in urothelial carcinoma and glioblastoma
Compared to the high frequency of FGFR2 fusions in cholangiocarcinoma, FGFR3 fusions are more commonly detected in urothelial carcinoma and glioblastoma multiforme (GBM), with fewer cases detected in lung cancer [4, 13]. For instance, FGFR3 genetic alterations are detected in 20–50% of bladder cancer patients, particularly with a high frequency of oncogenic gene fusion FGFR3–TACC3 (the transforming acidic coiled-coil containing protein gene-3) [44, 64]. FGFR3–TACC3 was first described in human glioblastoma (3% cases) and was subsequently found in many other cancers like urothelial carcinoma [65]. The unique feature of oncogenic TACC proteins is a prominent coiled-coil domain at the C-terminus, facilitating kinase transphosphorylation and localization of FGFR–TACC3 to the mitotic spindle leading to chromosomal segregation defects in cancer cells [65]. Of interest, this type II FGFR–TACC3 chimera can activate MAPK and JAK-STAT signaling pathways but not PLCγ-dependent signaling because of the lack of PLCγ docking site at Tyr760 [66].
Although two direct intracellular substrates of FGFRs (FRS2 and PLCγ) have been known for decades, the downstream effectors of FGFR fusions have not been clearly elucidated. Recently, Frattini and colleagues [17] identified PIN4, as a novel substrate of the FGFR3–TACC3 fusions, was required for reactive oxygen species (ROS)-mediated induction of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) and tumor growth. Compared to its kinase-dead form (K508M mutant), FGFR3–TACC3 increased PIN4 phosphorylation at Tyr122, thereby promoting mitochondrial respiration and ATP production and tumor progression [17]. This finding highlights the downstream substrate as a therapeutic opportunity for the treatment of tumors with FGFR fusions. However, due to the diversity of FGFR gene fusion types, it should be further studied whether those effectors such as PIN4 work as a common node.
FGFR-targeted inhibitors for FGFR fusion-harboring cancer therapy
Chromosomal translocations that generate in-frame oncogenic gene fusions are remarkable examples of the success of targeted cancer therapies. Although FGFR fusions are relatively rare, they have become novel druggable targets. According to the records on the ClinicalTrials.gov website, there are currently 27 FGFR-targeted inhibitors in clinical trials for cancer therapy. Those trials have included FGFR fusion-addicted cancers in the subsets of patients with cholangiocarcinoma, urothelial carcinoma, glioma, breast cancer, lung cancer or lymphoma (Table 2).
First-generation FGFR inhibitors
The first-generation FGFR inhibitors (e.g., derazantinib, ponatinib, lucitanib, dovitinib, lenvatinib and nintedanib) were non-selective inhibitors against multiple tyrosine kinases (e.g., PDGFRs, VEGFRs, KIT, and RET) owing to the high similarity at the ATP binding site of the intracellular kinase domains among RTK family. There are few ongoing clinical trials to use first-generation inhibitors to treat cancer patients with FGFR fusions.
Derazantinib (ARQ 087) is a 5,6 dihydrobenzo[h]quinazolin-2-amine derivative inhibitor against FGFR2 (1.8 nM), FGFR1 (4.5 nM), and FGFR3 (4.5 nM) kinases (Fig. 5). The crystal structure of the FGFR2-ARQ069 complex shows that the aminopyrimidine scaffold contributes to hinge interaction, and the hydrophobic part of the compound stabilizes the G-loop conformation through non-polar interactions [67]. In FGFR2 transfected Ba/F3 cell lines, derazantinib displayed potent inhibition of FGFR2 fusions, including FGFR2-CCDC6, FGFR2-BICC1, TEL-FGFR2, FGFR3-BAIAP2L1 and FGFR2-AAF3 with GI50 values between 39.9 nM and 1121 nM. Derazantinib also showed strong tumor inhibition in FGFR2 fusion-driven tumor xenograft models [68]. In the phase I/II study (NCT01752920), derazantinib exhibited promising antitumor activity in subjects with progressed iCCA harboring FGFR2 gene fusions after systemic chemotherapy. The overall response rate (ORR), disease control rate (DCR) and estimated median progression-free survival (PFS) were 20.7, 82.8% and 5.7 months, respectively. A following larger pivotal trial of derazantinib in iCCA is under recruitment (NCT03230318) [69, 70].
FGFR inhibitors. A Chemical structures of selected FGFR inhibitors and PROTAC. The hinge binding region and FGFR hydrophobic pocket binding group are highlighted. B Structures of second- and third-generation drug-FGFR complexes, including erdafitinib (5ew8), infigratinib (3tt0), debio-1347 (5b7v), and TAS120 (6mzw) in complex with FGFR1 kinase domain
Second-generation FGFR inhibitors
Multi-kinase FGFR inhibitors can result in a series of adverse effects due to low specificity and potency, such as cardiovascular and liver toxicities, proteinuria, and hypertension [71, 72]. Thus, second-generation FGFR selective inhibitors (e.g., erdafitinib, pemigatinib, infigratinib, Debio1347, rogaratinib, and AZD4547) are emerging to lower the risk of adverse effects and improve clinical outcomes (Fig. 5). Two selective FGFR-TKIs (erdafitinib and pemigatinib) have been approved by the U.S. FDA for the treatment of FGFR-driven cancers.
Erdafitinib is a pan-FGFR inhibitor with a quinoxaline core which was granted accelerated approval by the FDA for the first-line treatment of urinary bladder tumors with FGFR2/3 mutant or FGFR2/3 fusion and the second-line treatment of metastatic or unresectable urothelial carcinoma. In the phase II study (NCT02365597) that enrolled 99 patients with advanced urinary bladder cancers harboring FGFR3 point mutation or FGFR2/3-containing fusions receiving erdafitinib, the ORR was 40% with 3% of the patients getting a complete response, and the patients had a median PFS duration of 5.5 months and median overall survival (OS) duration of 13.8 months. In the subgroup of 25 patients with FGFR fusions, the ORR was 16%. Particularly, 36% (4/11) of FGFR3–TACC3 fusion carrying patients had a response to erdafitinib treatment [73, 74].
Pemigatinib is a tetra-azatricyclotridecatetraene derivative with a highly selective inhibition against FGFR1–3. FGFR2 fusions have been considered a promising therapeutic target for cholangiocarcinoma in clinical practice after the FDA-accelerated approval of pemigatinib to treat cholangiocarcinoma patients carrying FGFR2 fusions or rearrangements. The efficacy of pemigatinib was evaluated in a phase II study (NCT02924376) among 107 cholangiocarcinoma patients harboring chimeric FGFR2 proteins. The ORR was 35.5% (38/107) with a 2.8% (3/107) complete response rate. Notably, no complete or partial responses were observed in patients with other types of FGFR alterations or without FGFR alterations [75, 76]. These encouraging data demonstrate the potential benefit of pemigatinib in cholangiocarcinoma patients with FGFR2 fusions or rearrangements. A phase III FIGHT-302 study (NCT03656536) is ongoing to compare the efficacy of pemigatinib versus chemotherapy as first-line treatment for unresectable or metastatic cholangiocarcinoma with FGFR2 alterations.
Infigratinib (BGJ398) is a dianimopyrimidine derivative that selectively targets FGFR1–3. In a phase II study (NCT02150967) of infigratinib in advanced refractory or metastatic cholangiocarcinoma with chimeric FGFR2 fusions or other FGFR alterations, all responsive tumors carry FGFR2 fusions. The ORR and the disease control rate (DCR) in patients with FGFR2 fusions were 18.8% (9/48) and 83.3% (40/48), respectively. Reduced target lesion size was observed in 75% (36/48) of FGFR2 fusion-positive patients. Currently, infigratinib is in a phase III study (NCT03773302) as first-line treatment for patients with cholangiocarcinoma harboring FGFR2 gene translocations, and in a phase I study (NCT04424966) in patients with high-grade glioma carrying FGFR3–TACC3 fusions [77]. Another clinical trial (NCT02824133) evaluating the efficacy of AZD4547 (FGFR1–3 inhibitor) in glioma patients with FGFR3–TACC3 fusion is under recruitment [78]. A phase I dose-escalation trial (NCT03834220) using another ATP-competitive FGFR1–3 inhibitor, Debio 1347 (CH5183284), also reported preliminary evidence of antitumor activity in several tumor types, including iCCA [79].
Third-generation FGFR inhibitors
Although second-generation FGFR inhibitors showed promising antitumor activity in patients with FGFR fusions, acquired resistance occurred due to the emergence of secondary mutations in the FGFR kinase domain [80, 81]. Specifically, clinically observed mutations including N550K, V565F, L618V, and K660M are resistant to infigratinib treatment, whereas N550K, L618V, and K660M mutations confer principal resistance to Debio 1347 [82].
Irreversible kinase inhibitors (e.g., osimertinib, ibrutinib, and neratinib) have been proven feasible in multiple cancers and approved by the FDA for the treatment of EGFR-driven NSCLC, lymphomas, and HER2-positive breast cancer [83]. Futibatinib, a pyrazolo[3,4-d]pyrimidine derivative, is designed to covalently bind to a highly conserved cysteine residue (Cys488 in FGFR1c) within the P-loop of FGFR kinase, thus prolonging the pharmacodynamic duration (Fig. 5). Preliminary results from a phase I trial (NCT02052778) of futibatinib in advanced refractory tumors determined an ORR of 25% (7/28) and a DCR of 78.6% in patients with ICC carrying chimeric FGFR2 proteins, including selected patients who had experienced prior therapy with second-generation FGFR inhibitors.
Recently, Goyal et al. [82] reported the results of futibatinib in iCCA patients with FGFR2 translocation and disease progression upon infigratinib or Debio 1347 treatment. Futibatinib effectively overcame multiple secondary FGFR2 resistance mutations and showed clinical benefits in infigratinib or Debio 1347 resistant iCCA patients. These data support that strategically sequencing therapies with anti-FGFR molecules could benefit iCCA patients with FGFR2 fusion. Currently, futibatinib is in a multinational, randomized phase III clinical study (NCT04093362) to assess the efficiency and safety of futibatinib as first-line therapy for advanced or recurrent unresectable iCCA patients with FGFR2 gene rearrangements.
FGFRs degraders
Despite major progress in the discovery of selective and potent FGFR inhibitors over the past decade, the long-term value of these drugs in cancer treatment has been hindered by the quick onset of acquired resistance. While the third-generation covalent inhibitor futibatinib is effective against certain FGFR mutants, it fails to overcome the gatekeeper mutation [82]. Tumor cells may acquire resistance to irreversible inhibitors like futibatinib by mutating the cysteine residue, a common resistance mechanism previously described for EGFR [84] and BTK [85] covalent inhibitors. Small molecule-induced protein degradation is an emerging strategy in the field of drug discovery. Event-driven proteolysis targeting chimeras (PROTACs) can avoid mutation-related resistance based on the unique degradation mechanism [86]. To test whether FGFRs and their fusion variants are degradable targets, Du et al. [87] developed a low nanomolar PROTAC for FGFRs degradation, DGY-09-192 (Fig. 5). This heterobifunctional molecule showed dose-dependent degradation of FGFR fusion proteins in both CCLP-1-FP and ICC13–7 cells, expressing the FGFR2-PHGDH and FGFR2-OPTN fusion, respectively. In the CCLP1-FGFR2-PHGDH xenograft model, DGY-09-192 at 20 or 40 mg/kg can reduce both FGFR2-PHGDH protein levels and phosphorylation of downstream molecules in vivo. However, DGY-09-192 still has some limitations, i.e., it degrades all FGFR isoforms. Further optimization will be necessary to improve selectivity for a particular FGFR or fusion before reaching the clinic. Besides, we envision that the discovery of degraders towards the non-FGFR part of the oncogenic fusions, such as TACC3 and PHGDH, should be also interesting and promising.
Conclusion and perspective
Based on the results from clinical trials, tumors with genetic alterations of FGFRs would respond to FGFR inhibitor therapies. In this regard, targeted therapies for FGFR fusion-driven tumors offer an efficient therapeutic strategy in these cancer types. The benefits of FGFR targeting therapy in subsets of fusion-positive patients with haematological malignancies, iCCA, lung cancer, urothelial carcinoma and glioblastoma have been widely proved in clinical trials. Currently, most FGFR fusion-related clinical studies are focused on FGFR2, while there are relatively few clinical studies on FGFR1 and FGFR3 gene fusion. In this April, FDA has granted a breakthrough therapy designation to futibatinib to treat iCCA patients that harbor FGFR2 gene rearrangements or fusions. However, some challenges still exist, such as patient selection, molecule basis study of FGFR fusions, acquired resistance of FGFR inhibitors, and management of adverse events.
FGFR fusions are relatively rare genetic alterations. To improve the clinical benefits of FGFR inhibitor treatment, a more refined patient selection strategy is required. Although many methods effectively detect single nucleotide variants and copy numbers, few methods are accurate for FGFR gene fusion detections due to the complex nature of fusions [88]. Developing diagnostic tests such as whole-genome sequencing (WGS) and immunohistochemical (IHC) staining for the detection of FGFR genetic alterations have the potential to perform clinical trials specifically for patients with rare fusions.
Oncogenic partners contain multimerization motifs, which are generally assumed to constitutively increase their kinase activity by promoting kinase transphosphorylation. However, the structural basis whereby the N/C-terminal fusion partners drive kinase oligomerization is poorly understood. With the recent revolution in structural study tools such as cryoEM, unraveling higher-order FGFR fusion protein assemblies and the cytoplasmic protein granules is becoming more feasible [89]. The better understanding of the molecule basis of FGFR fusion-induced molecule assembly will, in turn, aid in the drug discovery for FGFR fusion-positive cancers.
Despite the promising results of FGFR inhibitors in clinical trials, acquired resistance limits the response duration to anti-FGFR agents. In particular, gatekeeper mutations (e.g., V564F in FGFR2) arise as the common mechanism of acquired resistance after pemigatinib and infigratinib treatment. Although irreversible inhibitors such as futibatinib can overcome those resistant mutations, the cysteine mutation might still occur in the clinical trial [84]. Alternatively, developing FGFR kinase allosteric inhibitor or specific fusion partner inhibitors may avoid acquired mutations within the kinase domain. Currently, several TACC3-targeting inhibitors (e.g., KHS101, BO-264) have been considered as novel anticancer drug candidates [90, 91]. In addition, developing FGFR-targeting PROTACs can directly degrade FGFR and fusion partners, which may avoid inhibitor-induced acquired mutation. Anyway, the important role of FGFR fusions should be taken as a key consideration in the drug design and development of FGFR-TKI molecules for clinical studies.
Inhibitors disrupting the physiological functions of FGF/FGFR signaling lead to the unique spectrum of on-target side-effects. Notably, these inhibitors block the FGF23-FGFR1-αKlotho endocrine signaling, leading to phosphate homeostasis disorders such as hyperphosphataemia, which occur in most patients after drug treatment. Strategies such as co-administration with phosphate binders or intermittent dosing have been taken in the clinical trials to control serum phosphate elevation and avoid dysregulation of the endocrine system [92]. Other common FGFR inhibition-related toxic events include asthenia, alopecia, hyponatraemia, skin and eye dryness, and nail toxicities [93]. Thus, more efforts should be spurred to constantly refine and enhance the clinical management of FGFR inhibition-associated adverse effects.
In conclusion, the awareness of the important role of FGFR fusions has significantly boosted the development of FGFR inhibitors. A comprehensive and deep understanding of FGFR fusion proteins would definitely contribute to the FGFR-targeting drug discovery and benefit cancer patients carrying FGFR fusions.
Availability of data and materials
Data are available upon reasonable request to the corresponding author.
Abbreviations
- A-loop:
-
Activation loop
- AHCYL:
-
Adenosylhomocysteinase like 1
- AKT:
-
Phosphoinositide 3- kinase (PI3K)–protein kinase B
- BICC1:
-
BicC family RNA-binding protein 1
- CCDC6:
-
Coiled-coil domain containing 6
- CML:
-
Chronic myeloid leukemia
- DCR:
-
Disease control rate
- FGFRs:
-
Fibroblast growth factor receptors
- FRS2α:
-
FGFR substrate 2α
- GRB2:
-
Growth factor receptor-bound protein 2
- HBS:
-
Heparan binding site
- HSPGs:
-
Heparan sulfate proteoglycans
- iCCA:
-
Intrahepatic cholangiocarcinoma
- JM:
-
Juxtamembrane
- MAPK:
-
Mitogen-activated protein kinase
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Overall response rate
- PGC1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PLCγ:
-
Phospholipase Cγ
- PKC:
-
Protein kinase C
- PROTACs:
-
Proteolysis targeting chimeras
- ROS:
-
Reactive oxygen species
- RTKs:
-
Receptor tyrosine kinases
- SAM:
-
Sterile alpha motif
- SCLL:
-
Stem cell leukemia/lymphoma
- SHP2:
-
SH2-containing tyrosine phosphatase
References
Mertens F, Johansson B, Fioretos T, Mitelman F. The emerging complexity of gene fusions in cancer. Nat Rev Cancer. 2015;15:371–81.
Medves S, Demoulin JB. Tyrosine kinase gene fusions in cancer: translating mechanisms into targeted therapies. J Cell Mol Med. 2012;16:237–48.
De Luca A, Esposito Abate R, Rachiglio AM, Maiello MR, Esposito C, Schettino C, et al. FGFR fusions in cancer: from diagnostic approaches to therapeutic intervention. Int J Mol Sci. 2020;21(18):6856.
Wu YM, Su F, Kalyana-Sundaram S, Khazanov N, Ateeq B, Cao X, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–47.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of Imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–27.
Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer. 2018;9:423–30.
Drilon A, Siena S, Dziadziuszko R, Barlesi F, Krebs MG, Shaw AT, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:261–70.
Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–76.
Guo R, Luo J, Chang J, Rekhtman N, Arcila M, Drilon A. MET-dependent solid tumours - molecular diagnosis and targeted therapy. Nat Rev Clin Oncol. 2020;17:569–87.
Marcus L, Donoghue M, Aungst S, Myers CE, Helms WS, Shen G, et al. FDA approval summary: entrectinib for the treatment of NTRK gene fusion solid tumors. Clin Cancer Res. 2021;27:928–32.
Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53.
Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34:280–300.
Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17:318–32.
Katoh M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol Sci. 2016;37:1081–96.
Guasch G, Delaval B, Arnoulet C, Xie MJ, Xerri L, Sainty D, et al. FOP-FGFR1 tyrosine kinase, the product of a t(6;8) translocation, induces a fatal myeloproliferative disease in mice. Blood. 2004;103:309–12.
Nelson KN, Meyer AN, Siari A, Campos AR, Motamedchaboki K, Donoghue DJ. Oncogenic gene fusion FGFR3-TACC3 is regulated by tyrosine phosphorylation. Mol Cancer Res. 2016;14:458–69.
Frattini V, Pagnotta SM, Tala, Fan JJ, Russo MV, Lee SB, et al. A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature. 2018;553:222–7.
Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22:259–67.
Xie Y, Zinkle A, Chen L, Mohammadi M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat Rev Rheumatol. 2020;16:547–64.
Belov AA, Mohammadi M. Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb Perspect Biol. 2013;5(6):a015958.
Kalinina J, Dutta K, Ilghari D, Beenken A, Goetz R, Eliseenkova AV, et al. The alternatively spliced acid box region plays a key role in FGF receptor autoinhibition. Structure. 2012;20:77–88.
Yeh BK, Igarashi M, Eliseenkova AV, Plotnikov AN, Sher I, Ron D, et al. Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors. Proc Natl Acad Sci U S A. 2003;100:2266–71.
Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700.
Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6:743–50.
Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, et al. alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–6.
Lee S, Choi J, Mohanty J, Sousa LP, Tome F, Pardon E, et al. Structures of beta-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature. 2018;553:501–5.
Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49.
Dhalluin C, Yan KS, Plotnikova O, Lee KW, Zeng L, Kuti M, et al. Structural basis of SNT PTB domain interactions with distinct neurotrophic receptors. Mol Cell. 2000;6:921–9.
Ong SH, Guy GR, Hadari YR, Laks S, Gotoh N, Schlessinger J, et al. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol Cell Biol. 2000;20:979–89.
Chardin P, Camonis JH, Gale NW, van Aelst L, Schlessinger J, Wigler MH, et al. Human Sos1: a guanine nucleotide exchange factor for Ras that binds to GRB2. Science. 1993;260:1338–43.
Touat M, Ileana E, Postel-Vinay S, Andre F, Soria JC. Targeting FGFR signaling in cancer. Clin Cancer Res. 2015;21:2684–94.
Hanafusa H, Torii S, Yasunaga T, Matsumoto K, Nishida E. Shp2, an SH2-containing protein-tyrosine phosphatase, positively regulates receptor tyrosine kinase signaling by dephosphorylating and inactivating the inhibitor Sprouty. J Biol Chem. 2004;279:22992–5.
Nichols RJ, Haderk F, Stahlhut C, Schulze CJ, Hemmati G, Wildes D, et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol. 2018;20:1064–73.
Bunney TD, Esposito D, Mas-Droux C, Lamber E, Baxendale RW, Martins M, et al. Structural and functional integration of the PLCgamma interaction domains critical for regulatory mechanisms and signaling deregulation. Structure. 2012;20:2062–75.
Huang Z, Marsiglia WM, Basu Roy U, Rahimi N, Ilghari D, Wang H, et al. Two FGF receptor kinase molecules act in concert to recruit and transphosphorylate phospholipase Cgamma. Mol Cell. 2016;61:98–110.
Chen H, Marsiglia WM, Cho MK, Huang Z, Deng J, Blais SP, et al. Elucidation of a four-site allosteric network in fibroblast growth factor receptor tyrosine kinases. Elife. 2017;6:e21137.
Schlessinger J. Signal transduction. Autoinhibition control. Science. 2003;300:750–2.
Furdui CM, Lew ED, Schlessinger J, Anderson KS. Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol Cell. 2006;21:711–7.
Bae JH, Boggon TJ, Tome F, Mandiyan V, Lax I, Schlessinger J. Asymmetric receptor contact is required for tyrosine autophosphorylation of fibroblast growth factor receptor in living cells. Proc Natl Acad Sci U S A. 2010;107:2866–71.
Chen L, Marsiglia WM, Chen H, Katigbak J, Erdjument-Bromage H, Kemble DJ, et al. Molecular basis for receptor tyrosine kinase A-loop tyrosine transphosphorylation. Nat Chem Biol. 2020;16:267–77.
Chen H, Ma J, Li W, Eliseenkova AV, Xu C, Neubert TA, et al. A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases. Mol Cell. 2007;27:717–30.
Marsiglia WM, Katigbak J, Zheng S, Mohammadi M, Zhang Y, Traaseth NJ. A conserved allosteric pathway in tyrosine kinase regulation. Structure. 2019;27:1308–1315. e1303.
Olsen SK, Ibrahimi OA, Raucci A, Zhang F, Eliseenkova AV, Yayon A, et al. Insights into the molecular basis for fibroblast growth factor receptor autoinhibition and ligand-binding promiscuity. Proc Natl Acad Sci U S A. 2004;101:935–40.
Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol. 2019;16:105–22.
Parker BC, Engels M, Annala M, Zhang W. Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J Pathol. 2014;232:4–15.
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8.
Tulpule A, Guan J, Neel DS, Allegakoen HR, Lin YP, Brown D, et al. Kinase-mediated RAS signaling via membraneless cytoplasmic protein granules. Cell. 2021;184(10):2649–64.
Qin A, Johnson A, Ross JS, Miller VA, Ali SM, Schrock AB, et al. Detection of known and novel FGFR fusions in non-small cell lung cancer by comprehensive genomic profiling. J Thorac Oncol. 2019;14:54–62.
Ross JS, Wang K, Gay L, Al-Rohil R, Rand JV, Jones DM, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19:235–42.
Sia D, Losic B, Moeini A, Cabellos L, Hao K, Revill K, et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087.
Kumar KR, Chen W, Koduru PR, Luu HS. Myeloid and lymphoid neoplasm with abnormalities of FGFR1 presenting with trilineage blasts and RUNX1 rearrangement: a case report and review of literature. Am J Clin Pathol. 2015;143:738–48.
Jackson CC, Medeiros LJ, Miranda RN. 8p11 myeloproliferative syndrome: a review. Hum Pathol. 2010;41:461–76.
Xiao S, Nalabolu SR, Aster JC, Ma J, Abruzzo L, Jaffe ES, et al. FGFR1 is fused with a novel zinc-finger gene, ZNF198, in the t(8;13) leukaemia/lymphoma syndrome. Nat Genet. 1998;18:84–7.
Xiao S, McCarthy JG, Aster JC, Fletcher JA. ZNF198-FGFR1 transforming activity depends on a novel proline-rich ZNF198 oligomerization domain. Blood. 2000;96:699–704.
Peiris MN, Meyer AN, Nelson KN, Bisom-Rapp EW, Donoghue DJ. Oncogenic fusion protein BCR-FGFR1 requires the breakpoint cluster region-mediated oligomerization and chaperonin Hsp90 for activation. Haematologica. 2020;105:1262–73.
Popovici C, Zhang B, Gregoire MJ, Jonveaux P, Lafage-Pochitaloff M, Birnbaum D, et al. The t(6;8)(q27;p11) translocation in a stem cell myeloproliferative disorder fuses a novel gene, FOP, to fibroblast growth factor receptor 1. Blood. 1999;93:1381–9.
Ren M, Qin H, Kitamura E, Cowell JK. Dysregulated signaling pathways in the development of CNTRL-FGFR1-induced myeloid and lymphoid malignancies associated with FGFR1 in human and mouse models. Blood. 2013;122:1007–16.
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79.
Arai Y, Totoki Y, Hosoda F, Shirota T, Hama N, Nakamura H, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–34.
Graham RP, Barr Fritcher EG, Pestova E, Schulz J, Sitailo LA, Vasmatzis G, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1630–8.
Kazerounian S, Aho S. Characterization of periphilin, a widespread, highly insoluble nuclear protein and potential constituent of the keratinocyte cornified envelope. J Biol Chem. 2003;278:36707–17.
Wang Y, Ding X, Wang S, Moser CD, Shaleh HM, Mohamed EA, et al. Antitumor effect of FGFR inhibitors on a novel cholangiocarcinoma patient derived xenograft mouse model endogenously expressing an FGFR2-CCDC6 fusion protein. Cancer Lett. 2016;380:163–73.
Ying X, Tu J, Wang W, Li X, Xu C, Ji J. FGFR2-BICC1: a subtype of FGFR2 oncogenic fusion variant in cholangiocarcinoma and the response to Sorafenib. Onco Targets Ther. 2019;12:9303–7.
Glaser AP, Fantini D, Shilatifard A, Schaeffer EM, Meeks JJ. The evolving genomic landscape of urothelial carcinoma. Nat Rev Urol. 2017;14:215–29.
Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–5.
Williams SV, Hurst CD, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer. Hum Mol Genet. 2013;22:795–803.
Eathiraj S, Palma R, Hirschi M, Volckova E, Nakuci E, Castro J, et al. A novel mode of protein kinase inhibition exploiting hydrophobic motifs of autoinhibited kinases: discovery of ATP-independent inhibitors of fibroblast growth factor receptor. J Biol Chem. 2011;286:20677–87.
Hall TG, Yu Y, Eathiraj S, Wang Y, Savage RE, Lapierre JM, et al. Preclinical activity of ARQ 087, a novel inhibitor targeting FGFR dysregulation. PLoS One. 2016;11:e0162594.
Mazzaferro V, El-Rayes BF, Droz Dit Busset M, Cotsoglou C, Harris WP, Damjanov N, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120:165–71.
Papadopoulos KP, El-Rayes BF, Tolcher AW, Patnaik A, Rasco DW, Harvey RD, et al. A phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br J Cancer. 2017;117:1592–9.
Soria JC, DeBraud F, Bahleda R, Adamo B, Andre F, Dientsmann R, et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol. 2014;25:2244–51.
Hahn NM, Bivalacqua TJ, Ross AE, Netto GJ, Baras A, Park JC, et al. A phase II trial of Dovitinib in BCG-unresponsive urothelial carcinoma with FGFR3 mutations or overexpression: Hoosier Cancer Research Network trial HCRN 12-157. Clin Cancer Res. 2017;23:3003–11.
Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381:338–48.
Patani H, Bunney TD, Thiyagarajan N, Norman RA, Ogg D, Breed J, et al. Landscape of activating cancer mutations in FGFR kinases and their differential responses to inhibitors in clinical use. Oncotarget. 2016;7:24252–68.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–84.
Romero D. Benefit from pemigatinib in cholangiocarcinoma. Nat Rev Clin Oncol. 2020;17:337.
Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36:276–82.
Van Cutsem E, Bang YJ, Mansoor W, Petty RD, Chao Y, Cunningham D, et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann Oncol. 2017;28:1316–24.
Nakanishi Y, Akiyama N, Tsukaguchi T, Fujii T, Sakata K, Sase H, et al. The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol Cancer Ther. 2014;13:2547–58.
Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7:252–63.
Saborowski A, Lehmann U, Vogel A. FGFR inhibitors in cholangiocarcinoma: what's now and what’s next? Ther Adv Med Oncol. 2020;12:1758835920953293.
Goyal L, Shi L, Liu LY, Fece de la Cruz F, Lennerz JK, Raghavan S, et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9:1064–79.
Zhao Z, Bourne PE. Progress with covalent small-molecule kinase inhibitors. Drug Discov Today. 2018;23:727–35.
Chen L, Fu W, Zheng L, Liu Z, Liang G. Recent progress of small-molecule epidermal growth factor receptor (EGFR) inhibitors against C797S resistance in non-small-cell lung cancer. J Med Chem. 2018;61:4290–300.
Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94.
Burslem GM, Crews CM. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell. 2020;181:102–14.
Du G, Jiang J, Wu Q, Henning NJ, Donovan KA, Yue H, et al. Discovery of a potent degrader for fibroblast growth factor receptor 1/2. Angew Chem Int Ed Engl. 2021;60:15905–11.
Krook MA, Reeser JW, Ernst G, Barker H, Wilberding M, Li G, et al. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 2021;124:880–92.
Nakane T, Kotecha A, Sente A, McMullan G, Masiulis S, Brown P, et al. Single-particle cryo-EM at atomic resolution. Nature. 2020;587:152–6.
Campo L, Breuer EK. Inhibition of TACC3 by a small molecule inhibitor in breast cancer. Biochem Biophys Res Commun. 2018;498:1085–92.
Akbulut O, Lengerli D, Saatci O, Duman E, Seker UOS, Isik A, et al. A highly potent TACC3 inhibitor as a novel anticancer drug candidate. Mol Cancer Ther. 2020;19:1243–54.
Katoh M. FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole-body homeostasis (review). Int J Mol Med. 2016;38:3–15.
Yanochko GM, Vitsky A, Heyen JR, Hirakawa B, Lam JL, May J, et al. Pan-FGFR inhibition leads to blockade of FGF23 signaling, soft tissue mineralization, and cardiovascular dysfunction. Toxicol Sci. 2013;135:451–64.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFA0506000 to GL), the National Natural Science Foundation of China (81930108 to GL and 82103999 to LC), and Zhejiang Provincial Key Scientific Project (2021C03041 to GL).
Author information
Authors and Affiliations
Contributions
LC and GL conceived the review. YZ, LY, and BC undertook the initial research. LC, PH, XL and GL participated in writing and reviewing the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, L., Zhang, Y., Yin, L. et al. Fibroblast growth factor receptor fusions in cancer: opportunities and challenges. J Exp Clin Cancer Res 40, 345 (2021). https://doi.org/10.1186/s13046-021-02156-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-021-02156-6