Abstract
Background
Myelofibrosis (MF) is a clonal disorder of hemopoietic stem/progenitor cells (HSPCs) with high prevalence in elderly patients and mutations in three driver genes (JAK2, MPL, or CALR). Around 10–15% of patients are triple-negative (TN) for the three driver mutations and display significantly worse survival. Circulating extracellular vesicles (EVs) play a role in intercellular signaling and are increased in inflammation and cancer. To identify a biomolecular signature of TN patients, we comparatively evaluated the circulating HSPCs and their functional interplay with the microenvironment focusing on EV analysis.
Methods
Peripheral blood was collected from MF patients (n = 29; JAK2V617F mutation, n = 23; TN, n = 6) and healthy donors (HD, n = 10). Immunomagnetically isolated CD34+ cells were characterized by gene expression profiling analysis (GEP), survival, migration, and clonogenic ability. EVs were purified from platelet-poor plasma by ultracentrifugation, quantified using the Nanosight technology and phenotypically characterized by flow cytometry together with microRNA expression. Migration and survival of CD34+ cells from patients were also analyzed after in vitro treatments with selected inflammatory factors, i.e. (Interleukin (IL)-1β, Tumor Necrosis Factor (TNF)-α, IL6) or after co-culture with EVs from MF patients/HD.
Results
The absolute numbers of circulating CD34+ cells were massively increased in TN patients. We found that TN CD34+ cells show in vitro defective functions and are unresponsive to the inflammatory microenvironment. Of note, the plasma levels of crucial inflammatory cytokines are mostly within the normal range in TN patients. Compared to JAK2V617F-mutated patients, the GEP of TN CD34+ cells revealed distinct signatures in key pathways such as survival, cell adhesion, and inflammation. Importantly, we observed the presence of mitochondrial components within plasma EVs and a distinct phenotype in TN-derived EVs compared to the JAK2V617F-mutated MF patients and HD counterparts. Notably, TN EVs promoted the survival of TN CD34+ cells. Along with a specific microRNA signature, the circulating EVs from TN patients are enriched with miR-361-5p.
Conclusions
Distinct EV-driven signals from the microenvironment are capable to promote the TN malignant hemopoiesis and their further investigation paves the way toward novel therapeutic approaches for rare MF.
Similar content being viewed by others
Background
Myelofibrosis (MF) is a chronic myeloproliferative neoplasm (MPN) that can be diagnosed as a primary disease (PMF) or secondary to polycythemia vera or essential thrombocythemia (PPV/PET-MF, also known as SMF). MF predominantly affects elderly patients with more than 65% of diagnosis occurring after 65 years of age [1] and is clinically characterized by debilitating systemic symptoms, progressive splenomegaly, cytopenias, and overall reduced survival, mainly due to disease progression and leukemic transformation [2,3,4].
The molecular pathogenesis of MF relates to mutations in three “driver” genes (namely: JAK2, CALR, MPL) with around 60% of patients showing the JAK2V617F mutation. Interestingly, 10% of patients are unmutated for the JAK2, CALR, and MPL genes and are classified as triple-negative (TN) [4]. Irrespective of “driver” mutations, a hyper-activation of the JAK-STAT pathway is observed in all MF patients [5]. The molecular basis of TN remains mostly unknown, although a high molecular complexity has been previously described [6] and rare, alternative, somatic mutations in both JAK2 exon 14 and MPL exon 10 have been previously annotated [7, 8]. TN MF is associated with an aggressive clinical behavior characterized by a higher risk of developing anemia and thrombocytopenia, poorer outcomes in comparison with patients affected by the other MF molecular subtypes and a high rate of leukemic transformation [9, 10].
Besides molecular alterations, a state of chronic inflammation involving the malignant hemopoietic stem/progenitor cells (HSPCs) and the non-malignant/malignant microenvironment has been indicated as the main contributor in MF initiation/clonal evolution [11, 12]. Abnormal expression and activity of several cytokines involved in inflammation and immunoregulation are described in MF [13, 14] and correlate with more severe marrow fibrosis [15], worsening systemic symptoms [16], and decreased survival [17]. Importantly, this chronic inflammation may allow the neoplastic clone to gain a selective advantage [18, 19] and the potential role of the interaction between the inflammaging process [20] and niche effect has recently been raised up [21].
Extracellular vesicles (EVs) have emerged as crucial actors in intercellular communication. Involved in a myriad of biological processes, including regulation of immunity and inflammation [22, 23], they are released from a variety of cell types exerting pleiotropic effects. EVs present antigens and contain constituents from the cell of origin including microRNAs (miRNAs), transcription factors, mitochondria, nucleic acid, and microenvironmental signals that may contribute to the propagation of inflammation [24,25,26]. Of interest, both damaged and functional mitochondria may be carried by EVs and they exert a role in immune regulation [27,28,29]. Various hematological malignancies including MPN have been associated with increased numbers of circulating EVs [30,31,32,33]. To date, the role of circulating EVs is still elusive in overall MF patients and their characterization remains fully obscure in the TN subclass.
In particular, the aggressive clinical behavior of TN MF patients is currently without any biological explanation and it is currently unclear the role of the HSPCs and/or the inflammatory microenvironment of TN MF patients. Here, we aim to identify a distinct signature of circulating CD34+ cells, cytokines, and EVs isolated from TN MF patients by comparatively evaluating the JAK2V617F-mutated counterparts.
Materials and methods
Patients
Peripheral blood (PB) was obtained from and MF patients (n = 29) and age-matched healthy donors (HD; n = 10). The clinical/laboratory characteristics of the patient cohorts are shown in Additional file 1: Table S1. In 15 pts., who received previous treatment (Hydroxyurea/Ruxolitinib), therapies had been discontinued for at least 3 months before sample collection.
CD34+ cells isolation
PB, anticoagulated with ethylenediaminetetraacetic acid (EDTA), was obtained from patients/controls. Mononuclear cells (MNC) were separated from MF and cord blood (CB) samples by stratification on Lympholyte-H 1.077 g/cm3 gradient (Gibco-Invitrogen, Milan, Italy), followed by red blood cell lysis for 15 min at 4 °C. MNCs were then processed on magnetic columns for CD34+ cell isolation (mean purity 94% ± 5%) (MACS CD34 Isolation kit; Miltenyi Biotech, Bologna, Italy), as previously described [19].
Functional characterization of CD34+ cells
Immunomagnetically isolated CD34+ cells from MF patients or CB units were maintained in RPMI 1640 with 10% fetal bovine serum (FBS) with or without IL-1β (10 ng/mL, Thermo Scientific Pierce Biotechnology, Rockford, IL, USA), TNF-α (100 ng/mL, Thermo Scientific), IL-6 (10 ng/mL, Thermo Scientific), alone or in combination, for 24 h. In vitro survival was analyzed as previously described by apoptotic assay [19, 34].
Clonogenic assay of CD34+ cells
MF/CB-derived CD34+ cells were cultured in vitro to achieve hematopoietic cell differentiation and the formation of multi-lineage colony-forming units (CFU-Cs), including colony forming unit-granulocyte macrophage (CFU-GM) and Burst Forming Unit-erythroid (BFU-E) in the presence or the absence of IL-1β (1 ng/mL), TNF-α (100 ng/mL), IL-6 (10 ng/mL), alone or in combination, as previously described [19, 34].
Migration assay of CD34+ cells
Migration of MF/CB purified CD34+ cells were assayed towards a CXCL12 gradient (150 ng/ml, R&D) in transwell chambers (diameter 6.5 mm, pore size 8 μm; Costar; Corning), as previously described [19, 34]. Specifically, 50 μl of RPMI 1640 plus 10% FBS containing 0,5 × 105 cells were added to the upper chamber and 150 μl of medium with or without CXCL12 ± IL-1β (1 ng/mL], TNF-α (100 ng/mL), IL-6 (10 ng/mL) (alone or in combination) were added to the bottom chamber.
The phenotype of circulating CD34+ cells
The phenotype of circulating CD34+ cells was evaluated in PB from MF patients and in CB samples by conventional immunofluorescence, as previously described [19, 34]. A minimum of 1 × 104 CD34+ cells was acquired by flow cytometer BD Accuri C6 (Becton Dickinson). The analysis was performed excluding cellular debris in an SSC/FSC dot plot. The percentage of positive cells was calculated by subtracting the value of the appropriate isotype controls. The absolute number of positive cells/mL was calculated as follows: the percentage of positive cells × White Blood Cell count/100.
Gene expression profiling (GEP) of circulating CD34+ cells
GEP was performed on RNA samples of immunomagnetically isolated CD34+ cells from MF patients using GeneChip Human Transcriptome Array 2.0 (Thermo Fisher Scientific), according to the manufacturer’s recommendations. Data quality control and normalization (signal space transformation robust multiple-array average, sst-RMA) and supervised analysis were carried out Transcriptome Analysis Console v4.0 software (Thermo Fisher Scientific). Fold-change absolute value ≥2 and p ≤ 0.05 were used as a cut-off. The resulting genes were selected for functional annotation clustering, that was performed using David Bioinformatics Resources v6.8 (National Institute of Allergy and Infectious Diseases, NIH) [35]. Gene set enrichment analysis (GSEA) was performed with GSEA v3.0 software (Broad Institute) [36].
Collection of blood samples and isolation/enumeration of circulating EVs
Blood samples were collected into K2EDTA-containing collection tubes (Vacutainer® tubes, Becton Dickinson), plasma and EVs were prepared as previously described with minor modifications [30, 37]. Briefly, platelet-poor plasma (PPP) was obtained (within 2 h from blood collection) after two consecutive centrifugations at 2500 g for 15 min at room temperature. The supernatant was subsequently ultracentrifuged at 100,000×g for 2 h at 4 °C using Optima L-90 K ultracentrifuge (Beckman Coulter) equipped with Type 50.2 Ti rotor. After centrifugation, pelleted EVs were resuspended and washed with Dulbecco’s PBS (DPBS; Sigma Aldrich). Finally, EVs were resuspended in saline buffer solution with 1% DMSO and stored at − 80 °C and/or used for further experiments. Isolated EVs were analyzed by nanoparticle tracking analysis (NTA), using the NanoSight LM10 system (NanoSight Ltd., Amesbury, UK), equipped with a 405 nm laser and with the NTA 2.3 analytic software, to define their dimension and profile.
The phenotype of the isolated EVs
To phenotype EVs isolated from patients/HD, the MACSPlex Exosome Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) was utilized. It provides the detection of 37 surface epitopes plus 2 isotype controls as for manufacturer’s instructions. This approach allows semi-quantitative analysis of differential surface epitopes. The proportion of megakaryocyte (MK)- and platelets (PLT)-EVs in the isolated EVs samples was analyzed by flow cytometry as previously described [30] using CytoFLEX (Beckman Coulter).
Where indicated, MitoTracker™ Red CMXRos (Thermo Fisher Scientific, Waltham, MA, USA) was used to stain mitochondria/mitochondria components in plasma EVs following the manufacturer’s instructions and the geometric mean of fluorescence intensity (MFI) was determined by flow cytometry.
Appropriate controls were used: single color stains, sample dilution of EVs in double-filtered PBS or their buffer, unstained EVs were used to determine the fluorescence background as well as the buffer with antibody/dye. Antibody/dye filtration was performed before staining using Ultrafree®-MC/Durapore®-PVDF centrifugal filter units.
Transmission electron microscopy (TEM) of the isolated EVs
TEM was performed on EVs isolated by ultracentrifugation, placed on 200 mesh nickel formvar carbon-coated grids (Electron Microscopy Science, Hatfield, PA, USA) and left to adhere for 20 min. The grids were then incubated with 2.5% glutaraldehyde containing 2% sucrose and after washings in distilled water the EVs were negatively stained with NanoVan (Nanoprobes, Yaphank, NK, USA) and observed using a Jeol JEM 1010 electron microscope (Jeol, Tokyo, Japan).
Western blot analysis
EV protein extracts were separated by Sodium Dodecyl Sulphate-PolyAcrylamide Gel Electrophoresis (SDS-PAGE, Bio-Rad) and transferred onto nitrocellulose membranes.
Membranes were incubated overnight with the following antibodies: rabbit anti-TOMM20 (AbCam, Cambridge, UK; ab56783) and goat anti-ß-tubulin (Santa Cruz Biotechnology) as control. Horseradish peroxidase (HRP) conjugated anti-rabbit Immunoglobuluin (Ig) G (GE Healthcare) and anti-goat IgG (Santa Cruz) were used as secondary antibodies. Enhanced chemiluminescence Prime (ECL™) reaction kit (GE Healthcare, Amersham, U.K.) was utilized for detection by ChemiDoc XRS+ System (Bio Rad) and Image J software was applied to perform signal quantification.
Analysis of the miRNAs cargo of the isolated EVs
miRNA expression of isolated EVs (109) from patients/HD was investigated after RNA extraction with the miRNeasy Micro kit (Qiagen, Milan, Italy) and TaqManTM Array Human MicroRNA A and B Cards (Applied Biosystems by Life Technologies, NY, USA) according to manufacturer’s protocol. This discovery phase was assessed on 3 HD and 6 MF. In the latter case, patients were equally divided in JAK2V617F-mutated and TN. To discover significantly different expressed miRNAs, RT-qPCR validation assay was performed, as previously described, in the same samples used for the profiling and in an enlarged cohort of 10 HD, 6 TN and 10 JAK2V617F-mutated patients in total. Data were normalized with cel-miR-39 spiked-in at lysis step of RNA extraction. MiRNAs’ targets were investigated via KEGG analysis, using the mirPath software with DIANA tools [38]. This analysis is based on the already validated targets reported in the Tarbase database.
EV co-culture studies
Circulating CD34+ cells isolated from CB or MF patients were seeded in 96 wells plate and co-cultured over-night with plasma-derived EVs from HD or MF patients, respectively. EV concentration was calculated using Nanosight (Malvern) and EV concentration for co-cultures was determined by dose titration. Cells were then collected, counted, and stained with Annexin V and PI to detect the apoptotic rate by flow cytometry, as above described.
Plasma levels measurement of selected circulating cytokines
We measured the cytokine plasma levels of patients/HD by ELISA, according to the manufacturer’s instructions. PPP was obtained (within 2 h from blood collection) after two consecutive centrifugations at 2500 g for 15 min at room temperature. The plasma was then collected and stored at − 80 °C until quantification. In particular, the Human Thrombopoietin Quantikine ELISA Kit was provided from R&D Systems (Minneapolis, Minnesota, USA) and CXCL12 ELISA kit from Krishgen ByoSistems (Ashley CT, Whittier, CA, USA). The CiraplexTM immunoassay kit / Human 9-Plex Array (Aushon BioSystems, Billerica, MA, USA) was used for the measurement of various cytokines including IL-1β and TNF-α.
Mutation analysis
JAK2V617F allele-burden was assessed in granulocyte DNA with ipsogen JAK2 MutaQuant Kit (Qiagen, Marseille, France) 505 on 7900 HT Fast Real-Time PCR System (Applied Biosystem, Monza, Italy). CALR exon 9 sequencing was performed by Next Generation Sequencing (NGS) approach with GS Junior (Roche-454 platform; Roche Diagnostics, Monza, Italy); analysis was performed with AVA Software (GRCh38 as referenced). Rare CALR mutations identified by NGS were confirmed by Sanger sequencing. MPL mutations were investigated by ipsogen MPLW515K/L MutaScreen Kit (Qiagen) and by Sanger sequencing (for MPLS505N and other secondary exons 10 mutations).
Statistical analysis
Numerical variables have been summarized by their median and range, and categorical variables by count and relative frequency (%) of each category. Comparisons of quantitative variables between groups of patients were carried out by the nonparametric Wilcoxon rank-sum test. The differences between the groups were analyzed with Mann Whitney, Kruskal Wallis, one-way ANOVA tests as appropriate. Since miRNA fold change values and cytokines values were not normally distributed as computed by Shapiro-Wilk test, we performed Mann Whitney and Kruskal Wallis as non-parametric tests. All p values were considered as statistically significant when ≤0.05. Statistical analyses were performed using Graphpad (Graphpad Software Inc., La Jolla, CA) and SPSS software (PASW Statistics for Windows, Version 18.0. Chicago, IL).
Results
Circulating CD34+ cells of TN patients are functionally defective
MF patients (n = 29) and their demographics and clinical-laboratocharacteristics are shown in Table S1. The median age of MF patients was 72 years (range 43–84), 45% were of the female gender. The JAK2V617F mutations were present in 23 (80%) patients and 6 (20%) were TN. Within the classes, TN patients had significantly higher lymphocyte count compared to JAK2V617F-positive patients (p = 0.04).
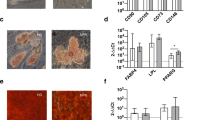
To investigate the ex vivo behavior of HSPCs, we firstly compared the number, phenotype, and function of CD34+ cells isolated from PB of TN and JAK2V617F mutated patients. We found a 5.5-fold increase in the absolute numbers of circulating CD34+ cells from TN (p = 0.01). In addition, CD34+ cells displayed a higher co-expression of markers such as CD184 (p = 0.03), CD133 (p = 0.04) and CD63 (p = 0.03; Fig. 1a).
Phenotype and function of circulating CD34+ cells. a The absolute number of MF patients subdivided into JAK2V617F-mutated (grey column, n = 18) or TN (blue column, n = 6) groups. CD34+ cells co-expressing the CD184, CD133, CD63 antigens are shown. Percentage of Annexin V/PI negative (survival) CD34+ cells (b), CFU-C counts (c), and migration as input (d) of cells cultured in vitro over-night. All data are presented as mean ± SEM (*p ≤ 0.05)
To investigate the functionality of circulating CD34+ cells, we performed in vitro experiments seeding the cells in overnight cultures in the absence of any stimuli. As shown in Fig. 1b, a marked decrease in the survival rate of TN-derived CD34+ cells was observed as compared to the JAK2V617F-mutated counterparts. Again, when we tested the colony-forming capacity (CFU-C), we found the reduced clonogenic output of TN-derived CD34+ cells, albeit not statistically significant (Fig. 1c). Conversely, no differences were observed between the two groups of patients when migration ability toward a CXCL12 gradient was tested (Fig. 1d).
Gene expression profiling (GEP) of PB CD34+ cells from MF patients reveals distinct signatures according to their mutational status
To elucidate whether the quanti-qualitative differences between PB CD34+ cells from TN and JAK2V617F-mutated patients are due to intrinsic factors, we performed the GEP on circulating CD34+ cells isolated from the two groups of patients. Overall, 151 genes were differentially expressed between the two cohorts (34 upregulated and 117 downregulated genes).
A signature of IL6-JAK-STAT3 signaling was significantly enriched in JAK2V617F-mutant compared with the TN CD34+ cells, indicating a preferential or enforced activation of the pathway in this cohort (Fig. 2a). Conversely, TN CD34+ cells were enriched for a signature of KRAS pathway activation, suggesting alternative oncogenic signaling supporting malignant cell growth (Fig. 2b).
The transcriptional program of CD34+ cells differs between TN and JAK2V617F MF patients. Signatures IL6/JAK/STAT3 signaling (a) and KRAS (b) enriched in JAK2V617F-mutated and TN patients, respectively (GSEA). c Gene expression differences in cell adhesion-, apoptosis-, immune response-, proliferation- and vesicle-related genes between JAK2V617F-mutated (n = 3) and TN (n = 3) patients. Each column represents a different patient. Data were standardized through a z-score transform; color changes within a row indicate expression levels relative to the mean and rescaled on the transcript standard deviation. Genes are ranked according to average linkage hierarchical clustering. d-e Cell adhesion-related signatures in TN, identified by GSEA and enriched in TN. f Apoptosis-related signatures enriched in the JAK2V617F cohorts. (NES: normalized enrichment score; TN: triple-negative)
Additional differentially expressed transcripts were enriched for genes involved in antigen processing/presentation, regulation of immune response, inflammation, and vesicles (Additional file 1: Table S2). Of interest, among the inflammation-related genes, MMP2, CD180, ROBO1, CD48, IL9-R, NLRP3 were significantly downregulated in TN patients as compared to the JAK2V617F counterparts (Fig. 2c).
Furthermore, 5 adhesion-related genes were upregulated (PCDHB14, PCDHB11, PCDHB12, PCDHB9, and GP5) and 9 were downregulated (CNTNAP2, ITGAL, LGALS3BP, MMP2, AMICA1, ROBO1, CDCP1, MLLT4, SERPINB8) in TN CD34+ cells (Fig. 2c). Among apoptosis-related genes, 2 pro-apoptotic genes (TNFSF10 and TP53INP1) were upregulated, and 4 antiapoptotic (TSPYL5, FCMR, PTPN13, TNFRSF1B) were downregulated in TN-derived CD34+ cells (Fig. 2c). Pathway analysis revealed that several altered genes were involved in cell adhesion, with enrichment of signatures of homophilic cell adhesion and calcium-dependent cell-cell adhesion via plasma membrane adhesion molecules in TN MF (Fig. 2d-e). However, CD34+ cells from JAK2V617F-mutated patients were enriched for gene signatures of apoptosis regulation (Fig. 2f). Genes involved in proliferation (HLF, DLK1, GFI-1, CECR1, NLRP3, PIK3R5, SUCNR1) were also downregulated.
The plasma levels of crucial inflammatory cytokines are mostly within the normal range in TN patients
To characterize the inflammatory microenvironment of TN patients, we analyzed the plasma concentration of key inflammatory cytokines and TPO. Compared to HD, only IL-1β and IL-12 plasma levels were significantly increased in TN patients (p < 0.05; Fig. 3b-g). Conversely, the plasma levels of TPO (p < 0.01), IL-1β (p < 0.01), TNF-α (p < 0.05), IFN-γ (p < 0.01), and IL-12 (p < 0.001) were significantly increased in the JAK2V617F-mutated patients compared to HD. The plasma levels of TPO were significantly decreased in TN patients compared to JAK2V617F-mutated patients (p < 0.05). Interestingly, TPO levels were 2-fold increased in overall female MF patients compared to males (mean: 242 ± 47.16 vs 118 ± 38.85, respectively; p = 0.02, data not shown).
Plasma levels of TPO and pro-inflammatory cytokines in MF patients according to mutational status. Violin plots of TPO (a), IL-1β (b), IL8 (c), TNF-α (d), IFN-γ (e), IL6 (f), and IL12 (g) plasma levels in MF patients (JAK2V617F, n = 14; TN, n = 6) and HD (n = 5-10) measured by ELISA. All data are presented as mean ± SEM (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001)
Circulating CD34+ cells from TN patients are functionally insensitive to pro-inflammatory stimuli
We then sought to assess whether the in vitro functional defects of CD34+ cells from TN patients might be due to abnormal response to the inflammatory signals of the microenvironment. For this purpose, we tested the effects of selected inflammatory cytokines (alone or in combination) on the in vitro functional behavior of PB CD34+ cells from the two groups of patients. Interestingly, TN CD34+ cells were unaffected by treatment with the selected inflammatory cytokines (either alone or combined) (Fig. 4a). This unresponsive behavior was even more evident when we studied the migratory capacity of the cells (Fig. 4b). Specifically, various combinations of TNF-α, IL-1β, or IL6 significantly stimulated the migration ability of the CD34+ cells of JAK2V617F-mutated patients. Notably, no differences were observed between the two groups of patients when spontaneous migration and migration towards CXCL12 alone were tested.
Survival and migration of CD34+ cells from TN patients are not increased by pro-inflammatory cytokines. a CD34+ cells from JAK2V617F (n = 4) or TN (n = 4) patients were in vitro treated with combined pro-inflammatory factors such as IL-1β, TNF-α, and IL6 and the fold-change of cell viability was assessed after Annexin V/PI staining, as described in Methods. Dot lines were used to mark control samples without any treatment, as fold-change = 1. b Spontaneous migration and toward CXCL12, alone or in combination with pro-inflammatory cytokines, were observed in JAK2V617F-derived (n = 4) CD34+ cells as compared with the TN (n = 4) counterparts. Results are expressed as mean percentages ± SEM of input (*p ≤ 0.05; **p ≤ 0.01)
TN-derived EVs show distinct phenotype, mitochondrial components and pro-survival signals
To elucidate whether crucial factors of the inflammatory network such as EVs have the potential to regulate the CD34+ cell behavior of MF, we compared size, phenotype, and mitochondrial content and function of the isolated EVs from TN and JAK2V627F-mutated patients.
No differences were observed when morphology and size of the isolated EVs were analyzed by electron microscopy and nanoparticle tracking analysis (NTA) (Additional file 1: Figure S1a-b).
To systematically evaluate and explore the surface signature of the isolated circulating EVs from patients/HD, we used a multiplex bead-based flow cytometry assay as previously reported [39] (Additional file 1: Figure S2a-c). Exosomal markers such as CD9, CD63, and CD81 were detected at low levels in all isolated EVs. To validate this observation, we repeated these analyses with CD9, CD63- and CD81-antibodies (Additional file 1: Figure S2d).
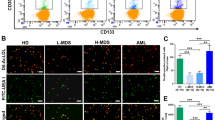
To further characterize the phenotype of the isolated circulating EVs, we specifically analyzed the proportion of the MK- and PLT-EVs by flow cytometry. As shown in Fig. 5b, the MK-EVs were significantly decreased in both patient groups (p < 0.01) as compared to the HD counterparts. Conversely, despite comparable platelet counts, the PLT-EVs were significantly increased (p < 0.05) in the JAK2V617F-mutated patients compared to both TN patients and HD (p < 0.05; Fig. 5c).
Plasma-derived EV characterization and effects on the survival of MF- or CB-derived CD34+ cells. a NTA size distribution profiles of the EVs collected from plasma of HD (n = 12), JAK2V617F-mutated (n = 13) and TN patients (n = 6). b Frequency of MK‐EVs (CD61+/CD62P‐; c PLT‐EVs (CD61+/CD62P+, d and Mitotracker-EVs (CD61+/Mito+); from HD (black, n = 3), JAK2V617F-mutated (grey, n = 3) or TN (blue, n = 3) patients. Accordingly, the MitoTracker Red CMXRos MFI in the same samples (e). f Survival rate as fold-change of cord blood-, JAK2V617F-, TN-derived CD34+ co-cultured with EVs isolated from plasma of HD (black, n=3), JAK2V617F-mutated (grey, n=3) or TN (blue, n = 3). Results are expressed as mean percentages ± SEM (*p ≤ 0.05; **p ≤ 0.01). CB: cord blood
We also tested the mitochondria content in plasma EVs. Despite an increase in the percentage of CD61+/MitoTracker+, EVs from the JAK2V617F-mutated patients (Fig. 5d), the MFI was higher in TN patients only as compared to the HD counterparts (p < 0.05, respectively; Fig. 5e). EVs isolated as above were also analyzed by western blot for the presence of the mitochondrial marker TOMM20, a subunit component of the outer mitochondrial membrane. TOMM20 was present in all EVs tested and no differences were reported between the groups at protein levels (Additional file 1: Suppl. S3 a-b).
To analyze the effect of EVs on the in vitro survival of the circulating CD34+ cells from patients and normal CD34+ from CB, we performed co-culture experiments. Specifically, circulating CD34+ cells from CB or JAK2V617F-mutated/TN patients were cultured in the presence of EVs isolated from allogeneic HD, JAK2V617F-mutated, and TN patients, respectively. As shown in Fig. 5f, the survival of CD34+ cells from the JAK2V617F-mutated patients was not influenced after co-cultures with any EVs. Conversely, CD34+ cells from TN patients were more responsive to EV treatments and showed a significantly increase in survival rate when co-cultured with EVs from TN patients (p < 0.05, Fig. 5f).
The circulating EVs from TN patients are enriched with miR-361-5p
To investigate whether the functional effects of TN EVs might be due to specific cargo, we analyzed the miRNA profile of the isolated EVs from MF patients/HD. After the profiling of 754 miRNAs, those with the highest or lowest fold change were selected for validation (the selected miRs and their FC are reported in Table S3).
Results of the validation analysis by RT-qPCR are shown in Fig. 6a-c. Comparing EVs from MF patients and HD, the expression of 3 miRNAs (miR-34a-5p, − 127-3p, − 212-3p) was significantly upregulated in MF patients. Specifically, miR-34a-5p was up-regulated in both TN (p = 0.02) and JAK2V617F-mutated (p = 0.005) patients. Conversely, miR-127-3p was significantly up-regulated in JAK2V617F-mutated patients only (p = 0.03; data not shown).
Expression of selected miRNAs in the plasma-derived EVs. Expression of miR-34a-5p (a), miR-127-3p (b), miR-212-3p (c), miR-222-3p (d), and miR-361-5p (e) in HD-derived EVs (n = 10), JAK2V617F-mutated (n = 10), and TN (n = 6) patients. f Expression of miR-361-5p in EVs from JAK2V617F-mutated, and TN patients, respectively. Results are expressed as mean ± SEM (*p ≤ 0.05; **p ≤ 0.01). g-h Linear regression analysis showing positive/negative correlation between JAK2V617F VAF and miR-34a or miR-212, respectively
Also, miR-222-3p and miR-361-5p showed increased levels in EVs from MF patients (p = 0.05, respectively; Fig. 6d-e). The discrepancies between RT-qPCR results and profiling data (in card-arrays) were likely due to the differences of methods and the associated variability of the enlarged cohort of the validation phase. Notably, KEGG analysis (mirPath) of these four miRNAs (miR-34a-5p, − 127-3p, − 212-3p, − 222-3p; genes intersection) revealed PABPC1 (Poly(A) Binding Protein Cytoplasmic 1) as common target gene.
When we compared the miRNA profile of the EVs isolated from the two groups of patients, we observed that only miR-361-5p was differentially expressed and exhibited 3.2-fold higher expression in TN-derived EVs (p = 0.04; Fig. 6f). Interestingly, GO category analysis of miR-361-5p showed significant pathways as ‘organelle’, ‘RNA binding’, ‘cellular protein modification process’, and ‘membrane organization’.
mportantly, the JAK2V617F variant allele frequency (VAF) was positively correlated with miR-34a-5p (r = 0.738; p = 0.02), as well as inversely correlated with miR-212-3p (r = − 0.72; p = 0.03; Fig. 6g-h).
Discussion
MF remains an incurable and critical disease [40] and the lack of studies on MF, particularly on the TN subtype, has not conferred advances in the treatment landscape in the last years. In the present study, through an in-depth analysis of HSPCs and key microenvironmental factors including cytokines and EVs, we compared TN and JAK2V617F-mutated patients providing novel insights in the pathogenesis of MF. As clearly reported by us [19] and others [41], the inflammatory microenvironment plays a crucial role in MF pathogenesis; however, few works deserved specific attention to the different molecular subgroups of MF patients.
Firstly, we demonstrated that the in vitro hemopoietic phenotype, survival, and function of circulating CD34+ cells are significantly altered in TN patients. Of note, we found an increase in the absolute number of circulating CD34+ cells in TN patients as a tool that may predict a more aggressive disease. These data were also consistent with the downregulation reported by GEP in selected genes involved in cell adhesion processes. Therefore, the enumeration of circulating CD34+ cells is highly relevant not only for improving diagnosis and risk assessment but also for evaluating response to potentially novel therapeutic approaches.
Specifically, TN patients show ex vivo/in vitro increased apoptosis within the CD34+ cell compartment with reduced hemopoietic function, revealing a stronger dependence on their microenvironment in comparison to the JAK2V617F-mutated CD34+ cells. Indeed, several genes involved in apoptotic and proliferation mechanisms are down-regulated in TN CD34+ cells, confirming the presence of deregulated survival pathways and an intrinsic disadvantage of TN CD34+ cells in terms of survival and proliferative intracellular signals. Among the differentially expressed genes, we observed an upregulation of tumor protein 53-induced nuclear protein 1 (TP53INP1) in TN CD34+, which is over-expressed during stress responses including inflammation [42]. TP53INP1 is a pro-apoptotic gene and its upregulation might partly explain the increased apoptotic rate of the TN CD34+ cells compartment in vitro. Consistent with this finding we observed KRAS signature enriched in TN CD34+ cells. It is noteworthy that KRAS overexpression confers an adverse prognosis in cytogenetically normal acute myeloid leukemia (AML) [43] and somatic activation of oncogenic KRAS in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder [44]. To sum up, in the present study, the GEP of TN HSPCs might highlight their vulnerability and might explain the more aggressive disease compared with the JAK2V617F MF molecular subtypes. Furtherly, we previously reported that pro-inflammatory cytokines (e.g. IL1-β, IL6, TNF-α) promoted the survival, clonogenic capacity, and migration of CD34+ isolated from MF patients, mostly JAK2V617F mutated [19]. By contrast, in the current study, we discovered that CD34+ cells from TN patients were insensitive to specific pro-inflammatory cytokines, suggesting their dependence on other factors from their microenvironment. Thus, it remains a matter of discussion whether the defective response to cytokines of TN CD34+ cells is related to altered apoptosis pathway, as reported by GEP analysis, “exhaustion” driven by previous inflammatory status, or to the dependence on “to be defined” factors from the microenvironment.
Notably, comparing the cytokine profiling between two subtypes of patients, TN patients show a milder inflammatory phenotype suggesting that therapies targeting the cytokine storm might be ineffective in this subset of MF patients. Moreover, only TPO plasma levels were lower in TN patients compared to the JAK2V617F-mutated patients, suggesting TPO as a biomarker of poor prognosis in MF. In accordance, Seiki Y et al. [45] reported relatively low TPO levels in Myelodysplastic syndromes (MDS) patients and that low levels of TPO were associated with poor prognosis and progression to AML. For the first time, we also reported higher TPO levels in female MF compared to males. Interestingly, Barraco et al. [46] observed that female MF patients had a better prognosis with slower disease progression. Consequently, all these data point out that an inflammatory microenvironment is closely associated with the JAK2V617F mutation but not to the TN counterparts. Whether this is due to the presence of a normally activated JAK/STAT pathway in TN patients remains a matter of speculation.
Based on our in vitro results, we then sought to determine whether the functional behavior of TN CD34+ cells might be influenced by circulating EVs as signals from the microenvironment. Here, for the first time, we analyzed the phenotype, the mitochondrial content, and the miRNA cargo profile of the isolated circulating EVs from MF patients/HD. Since most circulating EVs are of megakaryocyte and platelet origin, we demonstrated that the MK-EVs were significantly reduced in both JAK2V617F-mutated and TN patients. This finding confirms the occurrence of megakaryocyte abnormalities in MF; conversely, the PLT-EVs were decreased in TN patients. Of note, a previous study demonstrated that TPO may promote platelet aggregation [47]; therefore, it is likely that the increased level of circulating TPO in the JAK2V617F-mutated patients may partly contribute to the platelet activation and, as a consequence, to PLT-EVs release.
Recently, it has been described the existence and function of intact mitochondria or mitochondrial constituents transferred through EVs [48]. Consistently, platelet EVs may transport mitochondria as a mechanism to mediate inflammation [49] or immune cell regulation [28]. Conversely, mitochondria, either naked or encapsulated by EVs, may trigger inflammation, may interact with innate immune signaling pathways and may regulate cell metabolism, apoptosis, and various pathophysiological situations [50]. Here, we observed the presence of mitochondrial outer membrane protein TOMM20 in EVs from plasma and, interestingly, EVs from MF patients carry the highest level of MitoTracker MFI when compared with the healthy counterparts. These results, suggesting the presence of mitochondria in the EVs of our study, might be also in contrast to the TEM analysis that did not detect any intact mitochondria. However, although MitoTracker Red has been utilized as a membrane potential-sensitive dye, mitochondrial staining has several limitations [51, 52] and up to date has not been fully explored in EVs. Therefore, our data support the hypothesis that the MitoTracker dye might be associated with mitochondrial components rather than with respiring mitochondria in EVs. Despite the need for further studies, this finding might play a role in the Darwinian selection of cancer cells with the potential of fueling tumor cell maintenance and proliferation. As far as the increased prevalence in elderly patients is concerned, the role of inflammaging, i.e. the low chronic level of the inflammatory status associated with the aging process [20], could be another important risk factor for the change of microenvironment, making the cells more susceptible to undergo transformation, particularly for JAK2V617F-mutated patients. In this perspective, the additional role of mitochondria circulation, a phenomenon likely related to the PB increase of mtDNA with aging [53], and their possible transfer into other cells should be further addressed. To our knowledge, the mitochondria content of EVs is for the first time reported in MF patients and this might represent a novel clinically relevant therapeutic target that needs further investigations.
Recently, it has been also described that the miRNA cargo of EVs might have a prognostic role and can be used to evaluate disease progression [54, 55]. Comparing MF patients and HD, we showed that a distinct miRNA profiling characterizes the MF patients EVs with upregulation of miR-34a-5p, − 127-3p, and − 212-3p. In accordance, Bianchi et al. [56] found miR-34a-5p upregulation in PMF CD34+ hematopoietic progenitor cells, demonstrating that its overexpression favors the megakaryocyte and monocyte commitment of CD34+ cells. Therefore, our data suggest that the alteration in MF megakaryocytes might be also due to EV-driven signals and not only to intrinsic mechanisms encountered in CD34+ cells. It is therefore not surprising that miR-34a-5p expression is positively associated with the JAK2V617F allele burden. Of note, miR-212-3p expression was negatively associated with the JAK2V617F allele burden, in line with its role as tumor suppressor observed in AML [57]. Additionally, we also confirmed the upregulation of miR-127-3p, another miR highly expressed in MF CD34+ from MF patients as previously reported [58]. Specifically, we observed a high miR-127-3p expression in the EVs isolated from the JAK2V617F-mutated MF patients. Similarly to miR-34a [59], miR-127 has been associated with DNA damage response and with cellular senescence [60].
Notably, the common target of the identified miRNAs, i.e. PABPC1, plays a central role in mRNA processing binding the poly(A) tail of mRNA. PABPC1 deregulation by miRNAs interaction could contribute to making susceptible the cells of the microenvironment/niche to MF pathogenesis being known its role in carcinogenesis [61]. Thus, it is interesting the hypothesis of potential epigenetic modulators which, circulating in the blood via EVs, may also transport “potential cancer signals” far from the original site of cell development, accordingly to the theory of “inflammaging and garb-aging” [62].
Importantly, comparing the two groups of patients, only miR-361-5p expression was significantly upregulated in the TN EVs. Previous studies produced somewhat controversial results related to miR-361 [63]. Increased expression of miR-361 was detected in AML suggesting that miR-361 dysregulation might be required to impair differentiation program in hemopoiesis and leukemia [63]. It has also become apparent that miR-361 can downregulate the mRNA expression of IL-6 and IL-8 in endometrial cancer cells through targeting TWIST [64]. This finding suggests a potential anti-inflammatory role of miR-361-5p, which is in line with the mild pro-inflammatory profile observed in TN patients.
Conclusions
In conclusion, although the number of our patients is small and the study involved a rare MF subclass, the data presented here provide further insights into the pathogenesis of MF and highlight the biomolecular profile of TN CD34+ cells and circulating EVs within the inflammaging conceptualization. Our data demonstrate that in TN MF intrinsic HSPCs defects are associated with an impaired response to microenvironment signals. The results of this study may have diagnostic and prognostic implications in MF and suggest that TN patients deserve a personalized therapeutic approach targeting not only the stem cell compartment but also the EVs. Despite the specific role of EVs and the changes in their cargo remain to be further elucidated, we revealed crucial biomolecular vulnerabilities of TN patients providing the basis for novel and unexplored druggable pathways.
Availability of data and materials
The datasets used and analyzed during the current study are available upon reasonable request.
Abbreviations
- AML:
-
Acute myeloid leukemia
- BFU-E:
-
Burst Forming Unit-erythroid
- CB:
-
Cord blood
- CFU-C:
-
Colony-forming capacity
- CFU-GM:
-
Colony forming unit-granulocyte macrophage
- EVs:
-
Extracellular vesicles
- GEP:
-
Gene expression profiling
- HD:
-
Healthy donors
- HSPCs:
-
Hemopoietic stem/progenitor cells
- IL:
-
Interleukin
- MDS:
-
Myelodysplastic syndromes
- MF:
-
Myelofibrosis
- MFI:
-
Mean fluorescence intensity
- miRNA:
-
microRNAs
- MK:
-
Megakaryocyte
- MNC:
-
Mononuclear cells
- MPN:
-
Myeloproliferative neoplasm
- NTA:
-
Nanoparticle tracking analysis
- PABPC1:
-
Poly(A) Binding Protein Cytoplasmic 1
- PB:
-
Peripheral blood
- PET:
-
Post essential thrombocythemia
- PLT:
-
Platelets
- PMF:
-
Primary myelofibrosis
- PPP:
-
Platelet-poor plasma
- PPV:
-
Post polycythemia vera
- SMF:
-
Secondary myelofibrosis
- TEM:
-
Transmission electron microscopy
- TN:
-
Triple-negative
- TNF:
-
Tumor necrosis factor
- WB:
-
Western blot
References
Palandri F, Catani L, Bonifacio M, Benevolo G, Heidel F, Palumbo GA, et al. Ruxolitinib in elderly patients with myelofibrosis: impact of age and genotype. A multicentre study on 291 elderly patients. Br J Haematol. 2018;183(1):35–46.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15.
Gangat N, Tefferi A. Myelofibrosis biology and contemporary management. Br J Haematol. 2020. https://doi.org/10.1111/bjh.16576.
Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123(22):e123–e33.
Rotunno G, Guglielmelli P, Pacilli A, Fanelli T, Mannarelli C, Pancrazzi A, et al. Mutational Landscape of Patients with Myelofibrosis That Do Not Harbor Mutations in JAK2, MPL and Calreticulin Driver Genes. Blood. 2015;126(23):4091.
Jang MA, Choi CW. Recent insights regarding the molecular basis of myeloproliferative neoplasms. Korean J Intern Med. 2020;35(1):1–11.
Milosevic Feenstra JD, Nivarthi H, Gisslinger H, Leroy E, Rumi E, Chachoua I, et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple- negative myeloproliferative neoplasms. Blood. 2016;127(3):325–32.
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–13 quiz 615.
Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, Casetti I, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124(7):1062–9.
Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119(14):3219–25.
Sollazzo D, Forte D, Polverelli N, Perricone M, Romano M, Luatti S, et al. Circulating Calreticulin Is Increased in Myelofibrosis: Correlation with Interleukin-6 Plasma Levels, Bone Marrow Fibrosis, and Splenomegaly. Mediators Inflamm. 2016;2016:5860657.
Geyer HL, Dueck AC, Scherber RM, Mesa RA. Impact of Inflammation on Myeloproliferative Neoplasm Symptom Development. Mediators of Inflammation. 2015;2015:1–9.
Romano M, Sollazzo D, Trabanelli S, Barone M, Polverelli N, Perricone M, et al. Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. Oncoimmunology. 2017;6(10):e1345402.
Tefferi A. Myelofibrosis with Myeloid Metaplasia. New England Journal of Medicine. 2000;342(17):1255–65.
Squires M, Harrison CN, Barosi G, Vannucchi AM, Barbui T, Gisslinger H, et al. The Relationship Between Cytokine Levels and Symptoms in Patients (Pts) With Myelofibrosis (MF) From COMFORT-II, a Phase 3 Study of Ruxolitinib (RUX) Vs Best Available Therapy (BAT). Blood. 2013;122(21):4070.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–63.
Fleischman AG, Aichberger KJ, Luty SB, Bumm TG, Petersen CL, Doratotaj S, et al. TNF facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118(24):6392–8.
Sollazzo D, Forte D, Polverelli N, Romano M, Perricone M, Rossi L, et al. Crucial factors of the inflammatory microenvironment (IL-1beta/TNF- alpha/TIMP-1) promote the maintenance of the malignant hemopoietic clone of myelofibrosis: an in vitro study. Oncotarget. 2016;7(28):43974–88.
Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105.
Bonafe M, Storci G, Franceschi C. Inflamm-aging of the stem cell niche: breast cancer as a paradigmatic example: breakdown of the multi-shell cytokine network fuels cancer in aged people. Bioessays. 2012;34(1):40–9.
Oggero S, Austin-Williams S, Norling LV. The Contrasting Role of Extracellular Vesicles in Vascular Inflammation and Tissue Repair. Front Pharmacol. 2019;10:1479.
Hosseinkhani B, Kuypers S, van den Akker NMS, Molin DGM, Michiels L. Extracellular Vesicles Work as a Functional Inflammatory Mediator Between Vascular Endothelial Cells and Immune Cells. Front Immunol. 2018;9:1789.
Boilard E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J Lipid Res. 2018;59(11):2037–46.
Perez PS, Romaniuk MA, Duette GA, Zhao Z, Huang Y, Martin-Jaular L, et al. Extracellular vesicles and chronic inflammation during HIV infection. J Extracell Vesicles. 2019;8(1):1687275.
Han L, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol Cancer. 2019;18(1):59.
Marcoux G, Duchez AC, Cloutier N, Provost P, Nigrovic PA, Boilard E. Revealing the diversity of extracellular vesicles using high-dimensional flow cytometry analyses. Sci Rep. 2016;6:35928.
Hough KP, Trevor JL, Strenkowski JG, Wang Y, Chacko BK, Tousif S, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018;18:54–64.
Zhang X, Hubal MJ, Kraus VB. Immune cell extracellular vesicles and their mitochondrial content decline with ageing. Immun Ageing. 2020;17:1.
Barone M, Ricci F, Sollazzo D, Ottaviani E, Romano M, Auteri G, et al. Circulating megakaryocyte and platelet microvesicles correlate with response to ruxolitinib and distinct disease severity in patients with myelofibrosis. Br J Haematol. 2019;185(5):987–91.
Caivano A, Laurenzana I, De Luca L, La Rocca F, Simeon V, Trino S, et al. High serum levels of extracellular vesicles expressing malignancy-related markers are released in patients with various types of hematological neoplastic disorders. Tumour Biol. 2015;36(12):9739–52.
Caivano A, La Rocca F, Laurenzana I, Trino S, De Luca L, Lamorte D, Del Vecchio L, Musto P. Extracellular Vesicles in Hematological Malignancies: From Biology to Therapy. Int J Mol Sci. 2017;18(6):1183. https://doi.org/10.3390/ijms18061183.
Gargiulo E, Paggetti J, Moussay E. Hematological Malignancy-Derived Small Extracellular Vesicles and Tumor Microenvironment: The Art of Turning Foes into Friends. Cells. 2019;8(5):511. https://doi.org/10.3390/cells8050511.
Forte D, Sollazzo D, Barone M, Allegri M, di Martella OA, Romano M, et al. Mobilized Peripheral Blood versus Cord Blood: Insight into the Distinct Role of Proinflammatory Cytokines on Survival, Clonogenic Ability, and Migration of CD34(+) Cells. Mediators Inflamm. 2018;2018:5974613.
Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50.
Takov K, Yellon DM, Davidson SM. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: yield, purity and functional potential. J Extracell Vesicles. 2019;8(1):1560809.
Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, et al. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43(W1):W460–6.
Wiklander OPB, Bostancioglu RB, Welsh JA, Zickler AM, Murke F, Corso G, et al. Systematic Methodological Evaluation of a Multiplex Bead-Based Flow Cytometry Assay for Detection of Extracellular Vesicle Surface Signatures. Front Immunol. 2018;9:1326.
McLornan DP, Harrison CN. Forging ahead or moving back: dilemmas and disappointments of novel agents for myeloproliferative neoplasms. Br J Haematol. 2020. https://doi.org/10.1111/bjh.16573.
Desterke C, Martinaud C, Ruzehaji N, Le Bousse-Kerdiles MC. Inflammation as a Keystone of Bone Marrow Stroma Alterations in Primary Myelofibrosis. Mediators Inflamm. 2015;2015:415024.
Zidi B, Vincent-Fabert C, Pouyet L, Seillier M, Vandevelde A, N'Guessan P, et al. TP53INP1 deficiency maintains murine B lymphopoiesis in aged bone marrow through redox-controlled IL-7R/STAT5 signaling. Proc Natl Acad Sci U S A. 2019;116(1):211–6.
Zhou JD, Yao DM, Li XX, Zhang TJ, Zhang W, Ma JC, et al. KRAS overexpression independent of RAS mutations confers an adverse prognosis in cytogenetically normal acute myeloid leukemia. Oncotarget. 2017;8(39):66087–97.
Braun BS, Tuveson DA, Kong N, Le DT, Kogan SC, Rozmus J, et al. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101(2):597–602.
Seiki Y, Sasaki Y, Hosokawa K, Saito C, Sugimori N, Yamazaki H, et al. Increased plasma thrombopoietin levels in patients with myelodysplastic syndrome: a reliable marker for a benign subset of bone marrow failure. Haematologica. 2013;98(6):901–7.
Barraco D, Mora B, Guglielmelli P, Rumi E, Maffioli M, Rambaldi A, et al. Gender effect on phenotype and genotype in patients with post- polycythemia vera and post-essential thrombocythemia myelofibrosis: results from the MYSEC project. Blood Cancer J. 2018;8:89 United States.
Lupia E, Bosco O, Bergerone S, Dondi AE, Goffi A, Oliaro E, et al. Thrombopoietin contributes to enhanced platelet activation in patients with unstable angina. J Am Coll Cardiol. 2006;48(11):2195–203.
Liu D, Dong Z, Wang J, Tao Y, Sun X, Yao X. The existence and function of mitochondrial component in extracellular vesicles. Mitochondrion. 2020;54:122–7.
Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14):2173–83.
Torralba D, Baixauli F, Sanchez-Madrid F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front Cell Dev Biol. 2016;4:107.
Buckman JF, Hernandez H, Kress GJ, Votyakova TV, Pal S, Reynolds IJ. MitoTracker labeling in primary neuronal and astrocytic cultures: influence of mitochondrial membrane potential and oxidants. J Neurosci Methods. 2001;104(2):165–76.
Gökerküçük EB, Tramier M, Bertolin G. Imaging Mitochondrial Functions: from Fluorescent Dyes to Genetically-Encoded Sensors. Genes (Basel). 2020;11(2):125. https://doi.org/10.3390/genes11020125.
Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, et al. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for "inflamm-aging". Eur J Immunol. 2014;44(5):1552–62.
Schwarzenbach H, Gahan PB. MicroRNA Shuttle from Cell-To-Cell by Exosomes and Its Impact in Cancer. Noncoding RNA. 2019;5(1):28. https://doi.org/10.3390/ncrna5010028.
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, et al. Exosomal microRNA signatures in multiple sclerosis reflect disease status. Sci Rep. 2017;7(1):14293.
Bianchi E, Ruberti S, Rontauroli S, Guglielmelli P, Salati S, Rossi C, Zini R, Tagliafico E, Vannucchi AM, Manfredini R. Role of miR-34a-5p in Hematopoietic Progenitor Cells Proliferation and Fate Decision: Novel Insights into the Pathogenesis of Primary Myelofibrosis. Int J Mol Sci. 2017;18(1):145. https://doi.org/10.3390/ijms18010145.
Kaehler M, Ruemenapp J, Gonnermann D, Nagel I, Bruhn O, Haenisch S, et al. MicroRNA-212/ABCG2-axis contributes to development of imatinib-resistance in leukemic cells. Oncotarget. 2017;8(54):92018–31.
Liu Y, Wei B, Zhang X, Xu D, Wang B, Yin G, et al. Identification of potential therapeutic target genes and miRNAs for primary myelofibrosis with microarray analysis. Exp Ther Med. 2017;14(4):2743–50.
Thounaojam MC, Jadeja RN, Warren M, Powell FL, Raju R, Gutsaeva D, Khurana S, Martin PM, Bartoli M. MicroRNA-34a (miR-34a) Mediates Retinal Endothelial Cell Premature Senescence through Mitochondrial Dysfunction and Loss of Antioxidant Activities. Antioxidants (Basel). 2019;8(9):328. https://doi.org/10.3390/antiox8090328.
Chen J, Wang M, Guo M, Xie Y, Cong YS. miR-127 regulates cell proliferation and senescence by targeting BCL6. PLoS One. 2013;8(11):e80266.
Zhu J, Ding H, Wang X, Lu Q. PABPC1 exerts carcinogenesis in gastric carcinoma by targeting miR-34c. Int J Clin Exp Pathol. 2015;8(4):3794–802.
Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and 'Garb-aging'. Trends Endocrinol Metab. 2017;28(3):199–212.
Xu D, Dong P, Xiong Y, Yue J, Ihira K, Konno Y, Kobayashi N, Todo Y, Watari H. MicroRNA-361: A Multifaceted Player Regulating Tumor Aggressiveness and Tumor Microenvironment Formation. Cancers (Basel). 2019;11(8):1130. https://doi.org/10.3390/cancers11081130.
Ihira K, Dong P, Xiong Y, Watari H, Konno Y, Hanley SJ, et al. EZH2 inhibition suppresses endometrial cancer progression via miR-361/Twist axis. Oncotarget. 2017;8(8):13509–20.
Acknowledgments
The authors acknowledge the supports from AIL Bologna ODV. We would like to thank Jorge A. Formaro at Beckman Coulter for his support for the CytoFLEX.
Funding
This research was partially funded by SIE-Società Italiana di Ematologia e Associazione “Amici di Beat Leukemia Dr. Alessandro Cevenini ONLUS” and by a fellowship from Associazione Italiana Ricerca sul Cancro (AIRC-Fellowship Abroad, Rif. 20930). This work has been also funded by Roberto and Cornelia Pallotti Foundation to M.C3.
Author information
Authors and Affiliations
Contributions
D.F., F.F., and L.C. contributed to the conception, research design, drafting of the manuscript, and conceptualization methodology; D.F., M.B., G.A., D.S. collected human samples; M.B., C.M., S.C. and M.C.3 performed the miRNA evaluation; S.B. and G.S. performed GEP analysis and S.B. performed WB analysis; F. F. and E. B. performed the experiment with Nanosight; M. C. D. and G. C. performed TEM analysis; D.F. and L.C. performed data curation; E.O. performed the mutational molecular analysis; G.A., N.V., F.P. and M.C.1–2 provided clinical data and enrolled patients for the study; F.P., M.C.3 and L.C. reviewed and edited the manuscript; D.F. acquired the funding; G.C., C.F., M.C.3, M.C.1–2, F.P. and L.C. provided the supervision of the study. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted after the acquisition of written informed consent from the patients and the HD. The study received the approval of the local Ethics Committee (CE N° 7/2019/Sper/AOUBo).
Consent for publication
All contributing authors agree to the publication of this article.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Characteristics of the patient population. Table S2. Differentially expressed transcripts. Table S3. List of the selected miRNAs with their relative fold-changes (FC). Figure S1. Characterization by TME and NTA of EVs. Figure S2. EV characterization by flow cytometry. Figure S3. Representative western blot and quantification graph for TOMM20.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Forte, D., Barone, M., Morsiani, C. et al. Distinct profile of CD34+ cells and plasma-derived extracellular vesicles from triple-negative patients with Myelofibrosis reveals potential markers of aggressive disease. J Exp Clin Cancer Res 40, 49 (2021). https://doi.org/10.1186/s13046-020-01776-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-020-01776-8