Abstract
Background
The incidence of spontaneous abortion (SA), which affects approximately 15–20% of pregnancies, is the most common complication of early pregnancy. Pathogenic copy number variations (CNVs) are recognized as potential genetic causes of SA. However, CNVs of variants of uncertain significance (VOUS) have been identified in products of conceptions (POCs), and their correlation with SA remains uncertain.
Results
Of 189 spontaneous abortion cases, trisomy 16 was the most common numerical chromosome abnormality, followed by monosomy X. CNVs most often occurred on chromosomes 4 and 8. Gene Ontology and signaling pathway analysis revealed significant enrichment of genes related to nervous system development, transmembrane transport, cell adhesion, and structural components of chromatin. Furthermore, genes within the VOUS CNVs were screened by integrating human placental expression profiles, PhyloP scores, and Residual Variance Intolerance Score (RVIS) percentiles to identify potential candidate genes associated with spontaneous abortion. Fourteen potential candidate genes (LZTR1, TSHZ1, AMIGO2, H1-4, H2BC4, H2AC7, H3C8, H4C3, H3C6, PHKG2, PRR14, RNF40, SRCAP, ZNF629) were identified. Variations in LZTR1, TSHZ1, and H4C3 may contribute to embryonic lethality.
Conclusions
CNV sequencing (CNV-seq) analysis is an effective technique for detecting chromosomal abnormalities in POCs and identifying potential candidate genes for SA.
Similar content being viewed by others
Background
Spontaneous abortion is one of the most common complications of pregnancy, occurring in approximately 15% of pregnancies, defined as pregnancy loss before 28 weeks of gestation without human intervention [1, 2]. The etiology of SA is complex, involving genetic factors, autoimmune diseases, endocrine disorders, thrombophilias, and environmental factors [3,4,5]. Embryo chromosomal abnormalities, including numerical and structural chromosome abnormalities, as well as pathogenic copy number variations (pCNVs), play a primary role in early SA(< 12 weeks of gestation) [6, 7]. Numerical chromosome abnormalities are the most prevalent type of chromosome abnormalities [8], with pCNVs following closely [9]. CNVs which are the increase or decrease of DNA fragments larger than 1 kb bases on a chromosome, mainly in the form of deletions and duplications at the submicroscopic level are recognized as significant genetic variations strongly associated with the risk of SA [10,11,12,13,14]. In recent years, an increasing number of studies have demonstrated the association of CNVs with various complex and common disorders [10], such as neurodevelopmental disorders, autism, cancer, and Parkinson’s disease, by altering gene function [15,16,17,18,19].
Chromosome karyotype analysis, a fundamental test for identifying chromosome abnormalities as the underlying cause of malformations or diseases, has been utilized in POC samples for years. However, it has encountered increasing limitations such as low resolution, long cell culture cycles, and difficulty in detecting pathogenic microdeletions and microduplications smaller than 5 Mb [20]. The emergence of copy number variation sequencing based on next-generation sequencing (NGS) technology has addressed the shortcomings of traditional genetic detection methods, significantly improving detection efficiency and reducing misdiagnosis rates. Although pathogenic CNVs are recognized as causes of SA, the presence of numerous CNVs of uncertain significance detected in POCs remains to be explored in clinical trials [21, 22].
This study aims to systematically investigate the frequency and distribution differences of chromosomal abnormalities in SA and to explore the role of CNVs with unknown clinical significance. CNV-seq was employed to detect POC samples, and gene functions of both pathogenic CNVs and CNVs of uncertain significance were analyzed through enrichment and signaling pathway analyses. Genes within the VOUS region were further examined alongside gene conservation scores (PhyloP), tissue-specific gene expression, RVIS scores, and percentiles. The objective is to identify candidate genes associated with embryonic development or abortion and offer meaningful molecular genetic guidance for high-risk pregnancies.
Materials and methods
Participants
A total of 189 POC samples were collected from pregnant women experiencing spontaneous abortions, who were admitted to the First People’s Hospital of Changde City between January 2020 and November 2022. Informed consent was obtained from all participants, and the study was approved by the Medical Ethics Committee of the First People’s Hospital of Changde City. POC samples inclusion criteria (1) all patients with unexplained spontaneous abortion within 28 weeks of gestation, (2) no history of smoking or alcohol consumption, and (3) no history of taking teratogenic drugs and no history of exposure to toxic substances in the first three months of pregnancy or during pregnancy. POC samples exclusion criteria (1) significant maternal cell contamination, (2) coagulation disorders, endocrine abnormalities, and immune function abnormalities prior to pregnancy, (3) anatomical and structural malformations of the reproductive tract, (4) history of infectious diseases during pregnancy.
CNV sequencing
Genomic DNA from peripheral blood cells was extracted using the DNeasy Blood & Tissue Kit (Qiagen) following the manufacturer’s instructions. The DNA sample concentration is greater than 8ng/μl (Qubit assay) and the total amount is not less than 50ng. A sequencing library was prepared using 50 ng of genomic DNA as a template. Initially, DNA was fragmented to an average size of 300 bp, followed by ligation of a 9 bp barcode sequencing adapter. Modified fragments underwent PCR amplification, and fragments were then selected and purified using bead purification to remove interference from primer dimers. Subsequently, a DNA library was constructed and the purified DNA library concentration should not be less than 25nM. CNV-seq was performed on the NextSeq CN500 platform (Berry Genomics). Sequences were mapped to the GRCh37 reference genome, which was conducted by the Burrows-Wheeler Alignment tool. Reads were processed and CNVs were evaluated by an in-house pipeline using read counts based on a smoothness model (Berry Genomics, Beijing, China) [23]. Copy number gains or losses were compared with in-house database of copy number variants (CNVs) and with public CNV databases, including Genomic Variants (http://dgv.tcag.ca/dgv/app/home), UCSC(https://genome.ucsc.edu/cgi-bin/hgGateway), NCBI(https://www.ncbi.nlm.nih.gov/), Decipher(http://decipher.sanger.ac.uk/),Online Mendelian Inheritance in.
Man (OMIM, http://www.omim.org/) and ClinGen (https://www.clinicalgenome.org/) [24, 25]. CNV segments with microdeletions or microduplications greater than 100 kb were recorded. All genomic coordinates were based on the Human GRCh37/hg19 Genome Assembly. The American College of Medical Genetics and Genomics (ACMG 2019) standard was utilized as the final criterion for evaluating the pathogenicity of CNVs. Finally, the distribution map of pCNVs and VOUS CNVs on chromosomes was generated using R version 4.02 software.
Statistical analysis
Data analysis was conducted using SPSS software (version 29.0, IBM Corp., Armonk, NY, USA). Descriptive statistical methods were employed to present the data, with measurement data expressed as mean ± SD. A significance level of P < 0.05 was considered statistically significant.
Functional enrichment analysis
Protein-coding genes within pathogenic CNVs, likely pathogenic CNVs, and VOUS regions were referenced from the DECIPHER (http://decipher.sa-nger.ac.uk/) and Clingen (http://www.ncbi.nlm.nih.gov/projects/dbvar/clingen/) databases. Gene ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were conducted using the DAVID bioinformatics database (https://david.ncifcrf.gov). The top ten results of each analysis were selected for plotting. GO analysis encompasses gene function across cellular component (CC), biological process (BP), and molecular function (MF) terms. KEGG, established in 1995 by the Kanehisa Laboratory at the Center for Bioinformatics, Kyoto University, Japan, serves as a database resource for comprehending advanced functional and biological systems, particularly those derived from large molecular datasets generated by genome sequencing and other high-throughput experimental techniques. Finally, the gene enrichment map was generated using R version 4.02 software.
Identification of candidate genes
Human placental expression profiles, PhyloP scores, and Residual Variance Intolerance Score percentiles of the genes were integrated to screen candidate genes from the VOUS region. PhyloP scores were obtained using the UCSC genome browser (https://genome.ucsc.edu/), and genes with scores ≥ 0.4 were considered conserved. Gene expression profiles in the human placenta were retrieved from the Expression Atlas (https://www.ebi.ac.uk/). RVIS scores, downloaded from the RVIS website (http://genic-intolerance.org/), were filtered to include scores ≤ 25th percentile for identifying candidate genes.
Results
Characteristics of subjects
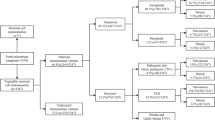
All 189 POC samples (comprising 244 experimental results) were successfully detected. Chromosomal abnormalities were identified in 121 POC samples (with 176 results from among these samples), while no abnormalities were observed in 68 POC samples, resulting in an overall abnormal detection rate of 64.02% (121/189). The average gestational duration of abortion was 10.5 ± 3.6 weeks (ranging from 4 to 26.6 weeks), with pregnant women having an average age of 30.3 ± 4.4 years (ranging from 21 to 42 years) and undergoing an average of 1.6 ± 0.8 abortions (ranging from 1 to 6 times) (see Table 1). Interestingly, the study indicated the frequency of CNV abnormalities in the early abortion group was significantly higher than that in the late abortion group(P < 0.05)(see Table 1).
Results of numerical chromosome abnormalities and CNVs
Among the 121 POC samples, a total of 176 abnormal results (72.13% of 244) were detected, comprising 59 cases (33.52% of 176) of numerical chromosome abnormalities, 73 cases (41.48% of 176) of CNVs, and 44 cases (25.00% of 176) of complex abnormalities where both numerical abnormalities and CNVs were detected (see Tables 2 and 3). Aneuploidy was the most common abnormality among numerical chromosome abnormalities, predominantly involving sex chromosomes and chromosome 16, followed by sex chromosomes in the present POC samples study. Among the CNVs observed, there were 71 duplications and 25 deletions, including 22 pathogenic CNVs (including likely pathogenic CNVs), 66 variants of uncertain significance (VOUS), and 8 likely benign variations. CNVs were detected in all chromosomes except for chromosome 21, with chromosomes X, 8, and 2 being the most frequently affected (see Fig. 1 Fig. 2). Among these cases, Xp22 microduplication (3/71) and 4q3 deletion (3/25) were found (see Supplementary Table 3).
Functional enrichment analysis of pCNVs and VOUS
We conducted gene enrichment analysis of genes from pathogenic CNVs (including likely pathogenic CNVs) and VOUS regions. The analysis revealed 4277 genes in pathogenic CNVs and 188 genes in VOUS CNVs. GO analysis indicated significant enrichment of 205 different functions (P < 0.05) among the 4277 genes from pathogenic CNVs (see Fig. 3 and Supplementary Table 1), and 29 different functions (P < 0.05) among the 188 genes from VOUS CNVs, with the most significant functions being “homophilic cell adhesion via plasma membrane adhesion molecules” (P = 1.35 × 10^-29) and “structural constituent of chromatin” (P = 3.76 × 10^-42), respectively (see Fig. 4 and Supplementary Table 2). KEGG analysis of pathogenic CNVs identified “Neuroactive ligand-receptor interaction” (P = 0.002) as the most commonly enriched signaling pathway among the 11 pathways identified (P < 0.05), while KEGG analysis of VOUS CNVs revealed “Systemic lupus erythematosus” (P = 9.42 × 10^-26) as the most enriched among the 5 pathways identified (P < 0.05).
The GO and KEGG analysis results mentioned above indicated the enrichment of several biological processes, including nervous system development, transmembrane transport, cell adhesion, and structural constituent of chromatin.
Identification of candidate genes from VOUS CNVs
We used human placental expression profiles, PhyloP scores, and RVIS percentiles of genes encompassed in detected CNVs to identify potential candidate genes. PhyloP scores reflect the evolutionary conservation of genes, with higher scores indicating greater conservation. RVIS percentiles assess the susceptibility of genes to genetic variations, with values below 25% indicating intolerance to mutations, implying a higher probability that disruption of the gene is pathogenic. After excluding cases with complex abnormalities, we further analyzed a total of 31 CNVs in 24 cases. These CNVs encompassed 188 genes. Ultimately, we identified 14 genes with PhyloP scores greater than 0.4 and RVIS percentiles below 25%, each found in four cases (LZTR1, TSHZ1, AMIGO2, H1-4, H2BC4, H2AC7, H3C8, H4C3, H3C6, PHKG2, PRR14, RNF40, SRCAP, ZNF629), with the LZTR1 gene possibly associated with SA (see Table 4).
Discussion
Chromosomal abnormalities in embryos are well recognized as being associated with the risk of spontaneous abortion. In the past, molecular diagnosis of POCs was conducted using chromosome karyotype analysis, but its lengthy experimental period and low resolution resulted in numerous misdiagnoses. With the rapid advancement of next-generation sequencing technology, CNV-seq has become widely adopted in clinical practice, significantly improving the rate of abnormality detection. According to the guidelines of the American College of Medical Genetics and Genomics (ACMG) [26], results are interpreted as pathogenic CNVs, likely pathogenic CNVs, variants of uncertain significance CNVs, likely benign, and benign CNVs. VOUS CNVs have been identified in many embryos or fetuses with developmental abnormalities or abortion. The impact of these VOUS CNVs on the normal development of embryos or fetuses remains unknown and requires further investigation through extensive clinical cases and studies. Some genes, such as THSD1, have already been associated with embryo development; mutations in these genes can lead to improper blood vessel formation, resulting in embryo death [27]. In this study, we aimed to investigate whether any of the 188 genes were related to embryo and fetus development or spontaneous abortion. We analyzed these genes and ultimately identified 14 candidate genes in 4 cases. Among the 14 genes screened, only the chromosome regions involved in LZTR1 and TSHZ1 exhibited copy number deletion, while the others showed duplications.
LZTR1 serves as a substrate adaptor for the cullin 3 (CUL3) ubiquitin ligase complex and acts as a negative regulator of the Receptor Tyrosine Kinase/Ras GTPase/MAP kinase (RTK/Ras/MAPK) signaling pathway activation [28]. Previous studies have implicated LZTR1 mutations in conditions such as glioblastoma (GBM), schwannomatosis (SWNMT), and Noonan syndrome (NS) [29,30,31]. We identified a 0.74 Mb deletion from case 1 at chromosome 22q11.2 region, which contains the LZTR1 gene located in the central region of 22q11.2 deletion syndrome (Velocardiofacial/DiGeorge syndrome) manifested as severe congenital heart disease, cleft palate and other specialized facial features, and severe immunodeficiency. Previous studies have reported two cases with similar microdeletions to the present case with clinical phenotype of developmental delay, language developmental disorders, mental retardation, and peculiar facial features [32, 33]. We hypothesize that the microdeletion of the central chromosome 22q11.2 region may lead to severe cardiovascular problems and immune deficiencies that result in embryo termination. The RTK/Ras/MAPK pathway plays a significant role in regulating cell proliferation and survival, apoptosis, differentiation, and nervous system function. Inactivation of LZTR1 leads to decreased ubiquitination, resulting in the overactivation of the RTK/Ras/MAPK signaling pathway [34]. The overactivation of Ras/MAPK pathways leads to increased cell division and proliferation, resulting in the excessive accumulation of reactive oxygen species (ROS), ultimately activating the apoptotic pathway. During embryonic cell development, metabolic reactions occur, leading to the production of aging mitochondria. When this pathway becomes dysregulated, excessive accumulation of aging mitochondria and ROS ensues, leading to cellular toxicity and enhanced oxidative stress, ultimately resulting in cell apoptosis. Numerous studies have suggested that oxidative stress plays a crucial role in early pregnancy loss [35]. ROS is closely linked to various aspects of the female reproductive process, particularly in the ovaries and embryos [36]. ROS exerts biological effects on various reproductive processes. It can be inferred that the loss of LZTR1 leads to the inability to negatively regulate the signaling pathway, resulting in excessive pathway activation and ROS accumulation. Oxidative stress disrupts placental trophoblast function [37], and also plays a role in regulating the reproductive process signaling pathway, altering the uterine immune system and leading to embryo failure [38].
The histone family comprises histones H1, H2A, H2B, H3, and H4, representing evolutionarily conserved protein families. This family is associated with developmental disorders and various neoplasms [39,40,41]. Histones play crucial roles in transcriptional regulation and DNA replication [42, 43]. Mutations in H1-4 and H4C3 are implicated in syndromes characterized by intellectual disability. H4C3 is particularly crucial in embryonic development [39], with mutations in this gene in zebrafish models resulting in severe embryonic developmental defects. TSHZ1 is linked to congenital aural atresia and anosmia; Tshz1-/- leads to neonatal lethality in mouse experiments [44]. Among other genes, AMIGO2 and PHKG2 are associated with gastric adenocarcinoma and glycogen storage disease, respectively [45, 46]. SRCAP encodes an ATPase and is linked to developmental delays and Floating-Harbor syndrome (FHS) when this gene loses function [47], FHS is a rare genetic disease typically manifesting in early childhood, characterized by short stature and facial dysmorphism. While this study suggests that PRR14, RNF40, and ZNF629 genes may be associated with embryonic development, neonatal lethality, or abortion, the sample size is not sufficient. Otherwise, we did not investigate whether these CNVs were inherited or de novo, and follow-up studies remained to be continued. Thus, more CNV-seq results of POCs are required to identify genes associated with SA. Some genes have demonstrated roles in embryonic development in animal studies; however, further basic experiments are necessary to validate their functions and mechanisms in vivo.
Conclusion
In this study, we sequenced the tissue samples from 189 cases of spontaneous abortion and integrated various gene scores to screen for genes involved in the VOUS CNVs region detected in spontaneous abortion samples. Among 188 genes analyzed, we identified 14 potential developmental genes, with most being associated with neurodevelopment and signaling pathway regulation. Our findings suggest that LZTR1, TSHZ1 and H4C3 genes are likely linked to embryonic development, offering new insights into the pathogenesis of SA.
Data availability
No datasets were generated or analysed during the current study.
References
Colley E, Hamilton S, Smith P, Morgan NV, Coomarasamy A, Allen S. Potential genetic causes of miscarriage in euploid pregnancies: a systematic review. Hum Reprod Update. 2019;25(4):452–72.
Smith LK, Dickens J, Atik RB, Bevan C, Fisher J, Hinton L. Parents’ experiences of care following the loss of a baby at the margins between miscarriage, stillbirth and neonatal death: a UK qualitative study. BJOG: Int J Obstet Gynecol. 2020;127(7):868–74.
RPL E G G o R, Bender Atik OB, Christiansen J, Elson AM, Kolte S, Lewis, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod open. 2018;2018(2):hoy004.
Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US preventive services task force recommendation statement. JAMA. 2020;323(13):1286–92.
Hamulyák EN, Scheres LJ, Marijnen MC, Goddijn M, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database of Systematic Reviews. 2020; (5).
Zhang X, Fan J, Chen Y, Wang J, Song Z, Zhao J, et al. Cytogenetic analysis of the products of conception after spontaneous abortion in the first trimester. Cytogenet Genome Res. 2021;161(3–4):120–31.
Hu P, Wang Y, Sun R, Cao L, Chen X, Liu C, et al. Copy number variations with isolated fetal ventriculomegaly. Curr Mol Med. 2017;17(2):133–9.
Zhang R, Chen X, Wang D, Chen X, Wang C, Zhang Y, et al. Prevalence of chromosomal abnormalities identified by copy number variation sequencing in high-risk pregnancies, spontaneous abortions, and suspected genetic disorders. J Int Med Res. 2019;47(3):1169–78.
Luo S, Chen X, Yan T, Ya J, Xu Z, Cai P, et al. Application of copy number variation sequencing in genetic analysis of miscarriages in early and middle pregnancy. Cytogenet Genome Res. 2021;160(11–12):634–42.
Almal SH, Padh H. Implications of gene copy-number variation in health and diseases. J Hum Genet. 2012;57(1):6–13.
Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, et al. Global variation in copy number in the human genome. Nature. 2006;444(7118):444–54.
Hastings PJ, Lupski JR, Rosenberg SM, Ira G. Mechanisms of change in gene copy number. Nat Rev Genet. 2009;10(8):551–64.
Lauer S, Gresham D. An evolving view of copy number variants. Curr Genet. 2019;65(6):1287–95.
Zarrei M, MacDonald JR, Merico D, Scherer SW. A copy number variation map of the human genome. Nat Rev Genet. 2015;16(3):172–83.
Auwerx C, Lepamets M, Sadler MC, Patxot M, Stojanov M, Baud D, et al. The individual and global impact of copy-number variants on complex human traits. Am J Hum Genet. 2022;109(4):647–68.
Hujoel ML, Sherman MA, Barton AR, Mukamel RE, Sankaran VG, Terao C, et al. Influences of rare copy-number variation on human complex traits. Cell. 2022;185(22):4233–48. e4227.
Collins RL, Glessner JT, Porcu E, Lepamets M, Brandon R, Lauricella C, et al. A cross-disorder dosage sensitivity map of the human genome. Cell. 2022;185(16):3041–55. e3025.
Auwerx C, Jõeloo M, Sadler MC, Tesio N, Ojavee S, Clark CJ, et al. Rare copy-number variants as modulators of common disease susceptibility. Genome Med. 2024;16(1):5.
Hujoel ML, Handsaker RE, Sherman MA, Kamitaki N, Barton AR, Mukamel RE et al. Protein-altering variants at copy number-variable regions influence diverse human phenotypes. Nat Genet. 2024:1–10.
Zhang J, Tang X, Hu J, He G, Wang J, Zhu Y, et al. Investigation on combined copy number variation sequencing and cytogenetic karyotyping for prenatal diagnosis. BMC Pregnancy Childbirth. 2021;21:1–14.
Wu H, Huang Q, Zhang X, Yu Z, Zhong Z. Analysis of genomic copy number variation in miscarriages during early and middle pregnancy. Front Genet. 2021;12:732419.
Chau MHK, Cao Y, Kwok YKY, Chan S, Chan YM, Wang H, et al. Characteristics and mode of inheritance of pathogenic copy number variants in prenatal diagnosis. Am J Obstet Gynecol. 2019;221(5):493. e491–493. e411.
Zhou X, Chen X, Jiang Y, Qi Q, Hao N, Liu C, et al. A rapid PCR-free next-generation sequencing method for the detection of copy number variations in prenatal samples. Life. 2021;11(2):98.
Wang L, Wang X, Zhang J, Song Z, Wang S, Gao Y, et al. Detection of chromosomal aneuploidy in human preimplantation embryos by next-generation sequencing. Biol Reprod. 2014;90(5):95.
Liang D, Peng Y, Lv W, Deng L, Zhang Y, Li H, et al. Copy number variation sequencing for comprehensive diagnosis of chromosome disease syndromes. J Mol Diagn. 2014;16(5):519–26.
Riggs ER, Andersen EF, Cherry AM, Kantarci S, Kearney H, Patel A, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Elsevier; 2020.
Shamseldin HE, Tulbah M, Kurdi W, Nemer M, Alsahan N, Mardawi EA, et al. Identification of embryonic lethal genes in humans by autozygosity mapping and exome sequencing in consanguineous families. Genome Biol. 2015;16:1–7.
Bigenzahn JW, Collu GM, Kartnig F, Pieraks M, Vladimer GI, Heinz LX, et al. LZTR1 is a regulator of RAS ubiquitination and signaling. Science. 2018;362(6419):1171–7.
Frattini V, Trifonov V, Chan JM, Castano A, Lia M, Abate F, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45(10):1141–9.
Piotrowski A, Xie J, Liu YF, Poplawski AB, Gomes AR, Madanecki P, et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat Genet. 2014;46(2):182–7.
Yamamoto GL, Aguena M, Gos M, Hung C, Pilch J, Fahiminiya S, et al. Rare variants in SOS2 and LZTR1 are associated with Noonan syndrome. J Med Genet. 2015;52(6):413–21.
Verhagen JM, Diderich KE, Oudesluijs G, Mancini GM, Eggink AJ, Verkleij-Hagoort AC, et al. Phenotypic variability of atypical 22q11. 2 deletions not including TBX1. Am J Med Genet Part A. 2012;158(10):2412–20.
Rump P, N de Leeuw AJ, van Essen CC, Verschuuren-Bemelmans HE, Veenstra‐Knol ME, Swinkels, et al. Central 22q11. 2 deletions. Am J Med Genet Part A. 2014;164(11):2707–23.
Steklov M, Pandolfi S, Baietti M, Batiuk A, Carai P, Najm P, et al. Mutations in LZTR1 drive human disease by dysregulating RAS ubiquitination. Science. 2018;362(6419):1177–82.
Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–22.
Hussain T, Murtaza G, Metwally E, Kalhoro DH, Kalhoro MS, Rahu BA, et al. The role of oxidative stress and antioxidant balance in pregnancy. Mediat Inflamm. 2021;2021:1–11.
Jauniaux E, Gulbis B, Burton GJ. Physiological implications of the materno–fetal oxygen gradient in human early pregnancy. Reprod Biomed Online. 2003;7(2):250–3.
Wu F, Tian F-J, Lin Y. Oxidative stress in placenta: health and diseases. Biomed Res Int. 2015;2015.
Tessadori F, Giltay JC, Hurst JA, Massink MP, Duran K, Vos HR, et al. Germline mutations affecting the histone H4 core cause a developmental syndrome by altering DNA damage response and cell cycle control. Nat Genet. 2017;49(11):1642–6.
Qi HH, Sarkissian M, Hu G-Q, Wang Z, Bhattacharjee A, Gordon DB, et al. Histone H4K20/H3K9 demethylase PHF8 regulates zebrafish brain and craniofacial development. Nature. 2010;466(7305):503–7.
Kallappagoudar S, Yadav RK, Lowe BR, Partridge JF. Histone H3 mutations—a special role for H3.3 in tumorigenesis? Chromosoma. 2015;124:177–89.
Albig W, Kioschis P, Poustka A, Meergans K, Doenecke D. Human histone gene organization: nonregular arrangement within a large cluster. Genomics. 1997;40(2):314–22.
Marzluff WF, Gongidi P, Woods KR, Jin J, Maltais LJ. The human and mouse replication-dependent histone genes. Genomics. 2002;80(5):487–98.
Coré N, Caubit X, Metchat A, Boned A, Djabali M, Fasano L. Tshz1 is required for axial skeleton, soft palate and middle ear development in mice. Dev Biol. 2007;308(2):407–20.
Izutsu R, Osaki M, Nemoto H, Jingu M, Sasaki R, Yoshioka Y, et al. AMIGO2 contained in cancer cell-derived extracellular vesicles enhances the adhesion of liver endothelial cells to cancer cells. Sci Rep. 2022;12(1):792.
Burwinkel B, Tanner MS, Kilimann MW. Phosphorylase kinase deficient liver glycogenosis: progression to cirrhosis in infancy associated with PHKG2 mutations (H144Y and L225R). J Med Genet. 2000;37(5):376–7.
Messina G, Prozzillo Y, Delle Monache F, Santopietro MV, Atterrato MT, Dimitri P. The ATPase SRCAP is associated with the mitotic apparatus, uncovering novel molecular aspects of floating-Harbor syndrome. BMC Biol. 2021;19:1–15.
Acknowledgements
We are grateful to all patients and their families who participated in this study.
Funding
This work was supported by the open research fund of Hunan Provincial Key Laboratory of Regional Hereditary Birth Defects Prevention and Control (HPKL2023023), the science and technology innovation Program of Changde city (2023ZD65), Scientific Research Project of Hunan Provincial Health Commission (D202301037694) and Hunan Provincial Natural Science Foundation Of China (2024JJ7014).
Author information
Authors and Affiliations
Contributions
Study design and data statistical analysis: AHL, LYZ; YZH and DP conceived the ideas and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was performed according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Changde Hospital, Xiangya School of Medicine, Central South University (The first people’s hospital of Changde city) (protocol code 2022-185-01).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, A., Zhou, L., Huang, Y. et al. Analysis of copy number variants detected by sequencing in spontaneous abortion. Mol Cytogenet 17, 13 (2024). https://doi.org/10.1186/s13039-024-00683-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13039-024-00683-3