Abstract
Background
BCR::ABL1-like acute lymphoblastic leukaemia (BCR::ABL1-like ALL) is characterized by inferior outcomes. Current efforts concentrate on the identification of molecular targets to improve the therapy results. The accessibility to next generation sequencing, a recommended diagnostic method, is limited. We present our experience in the BCR::ABL1-like ALL diagnostics, using a simplified algorithm.
Results
Out of 102 B-ALL adult patients admitted to our Department in the years 2008–2022, 71 patients with available genetic material were included. The diagnostic algorithm comprised flow cytometry, fluorescent in-situ hybridization, karyotype analysis and molecular testing with high resolution melt analysis and Sanger Sequencing. We recognized recurring cytogenetic abnormalities in 32 patients. The remaining 39 patients were screened for BCR::ABL1-like features. Among them, we identified 6 patients with BCR::ABL1-like features (15.4%). Notably, we documented CRLF2-rearranged (CRLF2-r) BCR::ABL1-like ALL occurrence in a patient with long-term remission of previously CRLF2-r negative ALL.
Conclusions
An algorithm implementing widely available techniques enables the identification of BCR::ABL1-like ALL cases in settings with limited resources.
Similar content being viewed by others
Introduction
B-cell acute lymphoblastic leukaemia (B-ALL) is a malignancy resulting from the transformation of a B-cell lineage progenitor cell [1]. The hallmark of B-ALL cases is the presence of genetic abnormalities, including chromosomal rearrangements, DNA copy number variations (CNV) and sequence mutations [2]. The 5th edition of the WHO classification divides B-ALL entity on the basis of refined diagnostic criteria and emphasis on therapeutically and/or prognostically actionable biomarkers [3]. The recently updated classification delineates a newly identified molecular subtype—B-ALL with BCR::ABL1-like features as a separate entity. It is characterized by a similar gene expression profile to the ALL with BCR::ABL1-fusion, but lacks the BCR::ABL1 fusion gene [4, 5]. It is exclusive of well-known drivers of B-ALL, including BCR::ABL1 fusion, KMT2A rearrangement, ETV6::RUNX1 and TCF3::PBX1 fusions [5]. The prevalence of the BCR::ABL1-like ALL is impacted by the age and ethnicity of distinct cohorts and the identification methodology. The incidence increases with age, with a peak in young adults population [6,7,8]. It is characterized by inferior outcomes due to a high rate of nonresponse to induction therapy, higher relapse risk, lower overall survival rates and the persistence of minimal residual disease (MRD) [9,10,11].
Diverse genetic alterations dysregulating kinase and receptor signaling are the hallmark of the BCR::ABL1-like ALL and can be divided into several classes: (1) alterations activating JAK-STAT pathway signaling (including rearrangements of cytokine receptor-like factor 2 (CRLF2) gene, Janus kinase 2 (JAK2) gene and erythropoietin receptor (EPOR) gene); (2) rearrangements of ABL-class genes (ABL1, ABL2, PDGFRα, PDGFRβ, CSF1R); (3) Ras pathway mutations (KRAS, NRAS, NF1, PTPN11) and other uncommon rearrangements [6, 7, 12].
The underlying molecular changes in the BCR::ABL1-like ALL remain of significant interest due to the possibility of incorporation of targeted therapy with tyrosine kinase inhibitors (TKI) and JAK inhibitors [13, 14]. Several ongoing clinical studies are evaluating the effectiveness of addition of targeted therapy to chemotherapy to improve the prognosis [15]. Current scientific efforts concentrate on the identification of molecular targets, and numerous algorithms have been proposed for the recognition of the BCR::ABL1-like ALL subtype, including targeted fusion testing, tiered algorithms and broad-based testing [16,17,18,19]. Nevertheless, the principal aim of the diagnostic approach is to recognize the underlying genetic feature, since they are determinative for the prognosis and targeted therapy. In smaller, real-world groups with constrained resources, the access to comprehensive sequencing strategy is limited. Hence, in those centers, the testing methods should be tailored.
Herein, we present our experience in the BCR::ABL1-like ALL diagnostics. We applied an integrated algorithm which allowed a cost-effective detection of this entity. The frequency and clinical outcome of BCR::ABL1-like ALL cases were analyzed and compared with the existing literature data, with a particular emphasis on the potential therapeutic options.
Materials and methods
Patients
The study was conducted at the Department of Hematology and Bone Marrow Transplantation of Poznan University of Medical Sciences. Adult patients diagnosed with B-cell ALL treated at our Department in the years 2008–2022 were included (n = 102). Thirty-one patients were excluded from further analysis due to the lack of cytogenetic material or essential clinical data. We performed a retrospective analysis of the clinical data, cytogenetic and molecular characteristics in patients treated in the years 2008–2020 (n = 63). Independently, a prospective analysis of cases diagnosed in the years 2020–2022 was performed (n = 8). This study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Poznań University of Medical Sciences Bioethical Committee (Resolution No. 705/20). 63 patients (88.7%) enrolled in the study were treated with B-ALL protocols according to the Polish Adult Leukemia Group (PALG) guidelines. Remaining patients were treated according to hyper-CVAD protocol.
Methods
The expression of TSLPR (predictive of the rearrangement of the CRLF2) with an anti-TSLP antibody (Invitrogen™, clone 1F11/TSLPR PE) was performed using the 10-color multiparameter flow cytometry method (FCM; BD FacsCanto II Ilyric™) using the strategy of internal negative control.
The karyotype analysis was performed using G banding (GTG). The results were described according to the International System for Human Cytogenetic Nomenclature (ISCN). FISH studies were performed on the interface nuclei using break-apart probes for TCF3::PBX1, CRFL2, JAK2, EPOR, ABL1, ABL2 (Cytocell, Cambridge, UK) and for BCR::ABL1, KMT2A, and PDGFRb (Vysis, IL, USA) and, additionally, for IGH and P2RY8 in the CRLF2 rearranged (CRLF2-r) cases (Cytocell, Cambridge, UK). At least 200 interphase nuclei were scored for each probe by two independent examiners. The cut-off threshold for the BCR::ABL1-like FISH probes of > 10% of cells was established.
The analysis of the JAK2 exon 16 sequence was conducted using DNA extracted from whole-blood leukocytes at the time of diagnosis QIAmp DNA Mini Kit (Qiagen) and high resolution melt analysis (HRMA). For the variant type identification screened by HRMA, Sanger sequencing was applied using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Thermo Fisher Scientific) and the following primers—forward: 5ʹ-TGCTCCAGTACTTGTGGACTGA-3ʹ and reverse: 5ʹ-CCACTGCCCAAGTAAAGCTTAG-3ʹ.
Diagnostic algorithm
For the identification of BCR::ABL1-like ALL cases, we implemented a stepwise algorithm integrating all the above-mentioned techniques (Fig. 1).
Firstly, the presence of the recurring cytogenetic features was evaluated. Patients with recurring cytogenetic lesions were excluded from screening for BCR::ABL1-like ALL. The expression of TSLPR was evaluated by FCM (in a prospective analysis only). Patients expressing TSLPR on leukemic blasts were enrolled for the FISH analysis with a CRLF2 break-apart probe. Patients lacking the TSLPR expression were recognized as non-CRLF2-rearranged (non-CRLF2-r) and subsequently proceeded to the analysis with remaining FISH probes (JAK2, EPOR, ABL1, ABL2, PDGFRb). In a case of retrospective analysis, the patients were primarily examined for the presence of CRLF2 rearrangement with a FISH probe. In CRLF2-r cases, the next step included an analysis with IGH and P2RY8 FISH probes to identify the fusion gene. Non-CRLF2-r cases proceeded to the analysis with the remaining FISH probes (JAK2, EPOR, ABL1, ABL2, PDGFRb).
Additionally, all patients with CRLF2-r B-ALL with available DNA were enrolled in the analysis of the JAK2 exon 16 mutational status.
Results
The median age of the patients at the time of initial diagnosis was 40 (range 18–69 years). Most of the individuals were diagnosed with the B-common phenotype (n = 50). 32 patients from the study group were recognized as B-ALL with recurring cytogenetic abnormalities. The remaining patients (n = 39) were screened for BCR::ABL1-like features. Out of the prospectively analyzed subjects (n = 8), we revealed high expression of CRLF2 in 3 cases in FCM. Second step analysis with FISH revealed CRLF2::IGH fusion in all 3 patients. In a retrospectively analyzed group, we revealed CRLF2-r in one patient and ABL-class genes rearrangements in 2 patients.
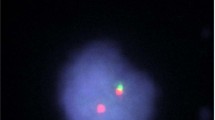
Interestingly, one of the patients was enrolled in the study due to a relapse of B-ALL within 13 years after the treatment with chemotherapy and an allogeneic hematopoietic stem cell transplantation (alloHSCT) in the first complete remission (CR1). Notably, we performed a retrospective analysis on the basis of cytogenetic material obtained at the time of the initial diagnosis, however, the rearrangement of CRLF2 was absent. The results of the cytogenetic analysis in the relapsed case is presented in Fig. 2.
The results of a diagnostic work-up of a patient with CRLF2-rearranged BCR::ABL1-like ALL which occurred during a relapse after a prolonged remission despite the absence of CRLF2 rearrangement at the initial diagnosis. Top left side: FISH analysis with CRLF2 break-apart probe (CytoCell®) on leukemic blasts at the initial diagnosis. In the normal cell, 2 fused red/green signals (2 R/G) or 2 yellow signals (2Y) are observed. Top right side: FISH analysis with CRLF2 break-apart probe (CytoCell®) on leukemic blasts at the relapse after prolonged remission (13 years). A translocation resulting in 1R, 1G, 1R/G. Bottom: Second step analysis with IGH probe (CytoCell®). In a normal cell, 2 fused red/green signals (2 R/G) or 2 yellow signals (2Y) are expected. Cells with 1R, 1G, 1R/G are indicative of IGH rearrangement. ALL, acute lymphoblastic leukemia; CRLF2, cytokine receptor-like factor 2; FISH, fluorescent in-situ hybridization
The incidence of the BCR::ABL1-like ALL among patients lacking recurrent cytogenetic features was 15.4% and in the whole study group of B-ALL patients it was 8.5%. Most of the cases were CRLF2-r (n = 4; 66.7%). Overall, we distinguished 5 subtypes of B-ALL in the study group: BCR::ABL1 positive ALL, BCR::ABL1-like ALL, ALL with KMT2A-r, TCF3::PBX1 positive ALL and other B-ALL. In Table 1 we present a brief summary of clinical characteristics of distinguished cytogenetic subtypes of B-ALL. In Table 2 we present the clinical characteristics of BCR::ABL1-like ALL patients. The incidence of distinct entities is presented in Fig. 3.
Additionally, patients with CRLF2-r were enrolled in the analysis of the JAK2 exon 16 mutational status. HRMA revealed different melting profile in one studied sample (CRLF2-r case). We confirmed the presence of the variant LRG_612:c.2049A>C(p.Arg683Ser) using Sanger sequencing in this case (Fig. 4). Overall, the incidence of point mutation in the JAK2 exon 16 within CRLF2-r cases was 25%.
The high resolution melt analysis (HRMA)—top field, and Sanger sequencing result (bottom field) in the CRLF2-r patients. HRMA revealed abnormal melting profile in one studied sample, in contrast to the normal double-stranded DNA dissociation characteristics during heating in control samples (wild type, WT). In the presented case, Sanger sequencing study revealed the presence of the variant LRG_612:c.2049A>C(p.Arg683Ser). Reference transcript ID (RefSeq): NM_004972.4:c.2049A>C, NP_004963.1:p.(Arg683Ser)
Discussion
Herein we present a strategy to identify cases with potentially targetable genomic lesions which can be applied in a limited resource setting. A similar approach integrating FISH and FCM has been implemented by Sharma and Virk [20, 21]. In the material from our Department, high expression of CRLF2 was indicative of the presence of CRLF2-r, similarly to observations from a larger cohort in the study of Virk et al. [21]. The frequency of BCR::ABL1-like cases in our material was 15.1% of B-ALL patients. The incidence of this entity in our cohort appears to be lower than in the literature data [6, 7]. It might be the result of both substantial number of cases excluded lacking adequate material and of limited techniques applied in the study. Sharma used a similar cost-effective approach in a larger cohort and revealed a slightly lower incidence of BCR::ABL1-like cases in − 11.4% of B-ALL in the screened group. Notably, this study group included adults, as well as the pediatric population, in which BCR::ABL1-like ALL is less frequently reported [20]. Among JAK-STAT pathway fusions, the rearrangements of CRLF2 account for the majority of cases [8, 9, 20]. The overexpression of CRLF2 observed in FCM, may be the result of either cryptic deletion of the pseudoautosomal region 1 of chromosomes X and Y leading to the gene fusion P2RY8::CRLF2, or the translocation resulting in the gene fusion IGH::CRLF2 [21, 22]. Approximately 50% of patients with CRLF2-r ALL harbor mutations in the JAK family genes, mainly in the JAK2 gene [6]. In our group, one patient harbored a point mutation within the exon 16 of the JAK2 gene, JAK2 c.2049A>C (p.R683S), accounting for 25% of CRLF2-r cases. Notably, the mutation occurred in the patient with a relapse after a prolonged remission post-alloHSCT. The relatively low frequency of JAK2 mutations in the study may be explained by the applied technique, which is less sensitive than the next generation sequencing implemented in numerous reports, a small study group and the fact that JAK2 mutations in the BCR::ABL1-like ALL may occur in other coding regions.
The optimal treatment strategy of the BCR::ABL1-like ALL is debatable. As far as the molecular background of this subtype is concerned, the combination of standard chemotherapy with TKI remains promising. Several preclinical studies and case studies reported safety, activity and efficacy of the JAK inhibitor, ruxolitinib, in BCR::ABL1-like ALL harboring JAK-STAT-activating aberrations and ABL-class inhibitors in cases with rearrangements of ABL-class genes [6, 10, 14, 23,24,25,26,27,28]. The studies by Steeghs et al., on the other hand, revealed that proliferation of JAK2 mutated ALL cells depended on several signaling pathways activity [29]. Hence, while JAK2-r leukemic cells were found to be susceptible to JAK inhibitors, both ruxolitinib and momelotinib, the efficacy of JAK specific therapy may be limited in JAK2 mutated cells. Similar results were observed by Schwartzman et al. [30]. Furthermore, the study of Steeghs et al. provides rationale for the hypothesis that JAK2 mutations may be secondary lesions in the leukemic process, while JAK2 rearrangements are leukemic drivers. Therefore, it is suggested to combine JAK inhibitors with Ras pathway inhibitors to avoid clonal selection [29,30,31,32]. A synergistic effect of combination of TKIs with antagonists of the BCL-2 anti-apoptotic protein, venetoclax and navitoclax, was also reported [24].
The role of alloHSCT in the first CR is also a subject of debate, since the prognostic impact of MRD negativity post-induction remains questioned [33, 34]. An analysis of Koller et al. suggests that alloHSCT may overcome the poor prognosis of CRLF2-r ALL [35]. It is postulated that patients with the presence of CRLF2-r and JAK2-r should be considered as candidates for alloHSCT [36]. On the other hand, relapses post-alloHSCT are often driven by CRLF2-r clones. These relapses occur irrespective of the MRD-negativity achievement, since CRLF2 fusions are considered early events in the leukemogenesis and CRLF2-r malignant clone may persist in a quiescence during the treatment, and eventually escape the immune system or gain a proliferative state trough acquired mutations [37,38,39]. Notably, herein we report a case of a patient with CRLF2-r which occurred during a relapse after a prolonged remission, despite the absence of CRLF2-r in at the initial diagnosis. Conversely, we could not exclude the possibility of overexpression of CRLF2 on leukemic blast at the original diagnosis, since it was not evaluated in flow cytometry at that time. Shah et al. described a similar case of an individual with a relapse of CRLF2-r ALL after a prolonged remission, however, the authors did not analyze the presence of CRLF2-r in the material from the initial diagnosis [39]. Studies by Aldoss et al. revealed that during a late relapse of ALL after alloHSCT, novel cytogenetic aberrations might occur as a manifestation of a genetic evolution of the disease or clonal selection, or even due to de novo secondary leukemogenesis related to former therapy [40].
Although our study provided valuable results regarding BCR::ABL1-like ALL diagnosis, it did have some drawbacks. The first is the small number of patients enrolled in the study and its mainly retrospective nature due to a relatively low incidence of B-ALL in the adult population. A substantial proportion of patients was eventually excluded as a result of lack of adequate cytogenetic material, which could impact the overall incidence of BCR::ABL1-like cases in the analyzed group. The second limitation is the use of standard diagnostic techniques which, however, are acceptable, if next generation techniques are unattainable [41]. Another limitation of the study is the fact that enrolled patients were treated over a long time period of time. Although most of the subjects were diagnosed and treated according to the guidelines of PALG, the therapeutic protocol evolved over the last decade, hence the patients were not uniformly treated. Finally, this is a single-center study, therefore, it presents a cohort which is not large enough to show significance. On the other hand, our results remain useful for future meta-analysis on BCR::ABL1-like ALL incidence and outcomes from real-world settings. Our study demonstrates that smaller centers can potentially provide useful information regarding BCR::ABL1-like ALL, regardless of the limited techniques employed. Our results remains also essential considering the potential advent of molecularly targeted therapy in BCR::ABL1-like patients.
Conclusions
The diagnostic strategy implementing widely available techniques enables the identification of high risk and therapeutically targetable cases of BCR::ABL1-like ALL. The presented approach may be particularly appropriable in settings with limited resources.
Availability of data and materials
Data available upon request from the authors.
References
Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. https://doi.org/10.1038/bcj.2017.53.
Iacobucci I, Mullighan CG. Genetic basis of acute lymphoblastic leukemia. J Clin Oncol. 2017;35(9):975–83. https://doi.org/10.1200/JCO.2016.70.7836.
The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms | Leukemia. Accessed 13 Nov 2022. https://www.nature.com/articles/s41375-022-01620-2
Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80. https://doi.org/10.1056/NEJMoa0808253.
Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–34. https://doi.org/10.1016/S1470-2045(08)70339-5.
Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–15. https://doi.org/10.1056/NEJMoa1403088.
Roberts KG, Gu Z, Payne-Turner D, et al. High frequency and poor outcome of Philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol. 2017;35(4):394–401. https://doi.org/10.1200/JCO.2016.69.0073.
Jain N, Roberts KG, Jabbour E, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129(5):572–81. https://doi.org/10.1182/blood-2016-07-726588.
Chiaretti S, Messina M, Della Starza I, et al. Philadelphia-like acute lymphoblastic leukemia is associated with minimal residual disease persistence and poor outcome. First report of the minimal residual disease-oriented GIMEMA LAL1913. Haematologica. 2021;106(6):1559–68. https://doi.org/10.3324/haematol.2020.247973.
Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22(2):153–66. https://doi.org/10.1016/j.ccr.2012.06.005.
Boer JM, Koenders JE, van der Holt B, et al. Expression profiling of adult acute lymphoblastic leukemia identifies a BCR-ABL1-like subgroup characterized by high non-response and relapse rates. Haematologica. 2015;100(7):e261-264. https://doi.org/10.3324/haematol.2014.117424.
Pui CH, Roberts KG, Yang JJ, Mullighan CG. Philadelphia chromosome-like acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(8):464–70. https://doi.org/10.1016/j.clml.2017.03.299.
Mullighan CG. How advanced are we in targeting novel subtypes of ALL? Best Pract Res Clin Haematol. 2019;32(4):101095. https://doi.org/10.1016/j.beha.2019.101095.
Bӧhm JW, Sia KCS, Jones C, et al. Combination efficacy of ruxolitinib with standard-of-care drugs in CRLF2-rearranged Ph-like acute lymphoblastic leukemia. Leukemia. 2021. https://doi.org/10.1038/s41375-021-01248-8.
Płotka A, Lewandowski K. BCR/ABL1-like acute lymphoblastic leukemia: from diagnostic approaches to molecularly targeted therapy. AHA. 2022;145(2):122–31. https://doi.org/10.1159/000519782.
Harvey RC, Kang H, Roberts KG, et al. Development and validation of a highly sensitive and specific gene expression classifier to prospectively screen and identify B-precursor acute lymphoblastic leukemia (ALL) patients with a philadelphia chromosome-like (“Ph-like” or “BCR-ABL1-Like”) signature for therapeutic targeting and clinical intervention. Blood. 2013;122(21):826–826. https://doi.org/10.1182/blood.V122.21.826.826.
Roberts KG, Reshmi SC, Harvey RC, et al. Genomic and outcome analyses of Ph-like ALL in NCI standard-risk patients: a report from the Children’s Oncology Group. Blood. 2018;132(8):815–24. https://doi.org/10.1182/blood-2018-04-841676.
Chiaretti S, Messina M, Grammatico S, et al. Rapid identification of BCR/ABL1-like acute lymphoblastic leukaemia patients using a predictive statistical model based on quantitative real time-polymerase chain reaction: clinical, prognostic and therapeutic implications. Br J Haematol. 2018;181(5):642–52. https://doi.org/10.1111/bjh.15251.
Reshmi SC, Harvey RC, Roberts KG, et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017;129(25):3352–61. https://doi.org/10.1182/blood-2016-12-758979.
Sharma P, Rana S, Virk H, et al. The frequency, hematological characteristics, and end-of induction residual disease in B-acute lymphoblastic leukemia with BCR-ABL1-like chimeric gene fusions in a high-risk cohort from India. Leuk Lymphoma. 2022;63(10):2474–8. https://doi.org/10.1080/10428194.2021.1929964.
Virk H, Rana S, Sharma P, et al. Hematological characteristics, cytogenetic features, and post-induction measurable residual disease in thymic stromal lymphopoietin receptor (TSLPR) overexpressed B-cell acute lymphoblastic leukemia in an Indian cohort. Ann Hematol. 2021;100(8):2031–41. https://doi.org/10.1007/s00277-021-04574-0.
Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–21. https://doi.org/10.1182/blood-2009-09-245944.
Russell LJ, Capasso M, Vater I, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114(13):2688–98. https://doi.org/10.1182/blood-2009-03-208397.
Konoplev S, Lu X, Konopleva M, et al. CRLF2-positive B-cell acute lymphoblastic leukemia in adult patients: a single-institution experience. Am J Clin Pathol. 2017;147(4):357–63. https://doi.org/10.1093/ajcp/aqx005.
Jain N, Jabbour EJ, McKay PZ, et al. Ruxolitinib or dasatinib in combination with chemotherapy for patients with relapsed/refractory Philadelphia (Ph)-like acute lymphoblastic leukemia: a phase I–II trial. Blood. 2017;130(Supplement 1):1322–1322. https://doi.org/10.1182/blood.V130.Suppl_1.1322.1322.
Roberts KG, Yang YL, Payne-Turner D, et al. Oncogenic role and therapeutic targeting of ABL-class and JAK-STAT activating kinase alterations in Ph-like ALL. Blood Adv. 2017;1(20):1657–71. https://doi.org/10.1182/bloodadvances.2017011296.
Ding YY, Stern JW, Jubelirer TF, et al. Clinical efficacy of ruxolitinib and chemotherapy in a child with Philadelphia chromosome-like acute lymphoblastic leukemia with GOLGA5-JAK2 fusion and induction failure. Haematologica. 2018;103(9):e427–31. https://doi.org/10.3324/haematol.2018.192088.
Mayfield JR, Czuchlewski DR, Gale JM, et al. Integration of ruxolitinib into dose-intensified therapy targeted against a novel JAK2 F694L mutation in B-precursor acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017. https://doi.org/10.1002/pbc.26328.
Bayram N, Yaman Y, Özdilli K, et al. Clinical efficacy of ruxolitinib monotherapy and haploidentical hematopoeitic stem cell transplantation in a child with Philadelphia chromosome-like relapsed/refractory acute lymphoblastic leukemia. Pediatr Transplant. 2021;25(4):e14024. https://doi.org/10.1111/petr.14024.
Duployez N, Grzych G, Ducourneau B, et al. NUP214-ABL1 fusion defines a rare subtype of B-cell precursor acute lymphoblastic leukemia that could benefit from tyrosine kinase inhibitors. Haematologica. 2016;101(4):e133-134. https://doi.org/10.3324/haematol.2015.136499.
Steeghs EMP, Jerchel IS, de Goffau-Nobel W, et al. JAK2 aberrations in childhood B-cell precursor acute lymphoblastic leukemia. Oncotarget. 2017;8(52):89923–38. https://doi.org/10.18632/oncotarget.21027.
Schwartzman O, Savino AM, Gombert M, et al. Suppressors and activators of JAK-STAT signaling at diagnosis and relapse of acute lymphoblastic leukemia in down syndrome. Proc Natl Acad Sci USA. 2017. https://doi.org/10.1073/pnas.1702489114.
Vesely C, Frech C, Eckert C, et al. Genomic and transcriptional landscape of P2RY8-CRLF2-positive childhood acute lymphoblastic leukemia. Leukemia. 2017;31(7):1491–501. https://doi.org/10.1038/leu.2016.365.
Winter PS, Sarosiek KA, Lin KH, et al. RAS signaling promotes resistance to JAK inhibitors by suppressing BAD-mediated apoptosis. Sci Signal. 2014;7(357):ra122. https://doi.org/10.1126/scisignal.2005301.
Aldoss I, Kamal MO, Forman SJ, Pullarkat V. Adults with Philadelphia chromosome-like acute lymphoblastic leukemia: considerations for allogeneic hematopoietic cell transplantation in first complete remission. Biol Blood Marrow Transplant. 2019;25(2):e41–5. https://doi.org/10.1016/j.bbmt.2018.09.041.
Aldoss I, Advani AS. Have any strategies in Ph-like ALL been shown to be effective? Best Pract Res Clin Haematol. 2021;34(1):101242. https://doi.org/10.1016/j.beha.2021.101242.
Koller P, Saliba RM, Ledesma C, et al. Outcomes in patients with CRLF2 overexpressed acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2021;56(7):1746–9. https://doi.org/10.1038/s41409-021-01262-5.
El Fakih R, Savani B, Mohty M, Aljurf M. Hematopoietic cell transplant consideration for Philadelphia chromosome-like acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant. 2020;26(1):e16–20. https://doi.org/10.1016/j.bbmt.2019.08.010.
Tracy SI, Cao Q, Bachan B, et al. Ph-like gene alterations and complex chromosomal abnormalities are frequent in patients with acute lymphoblastic leukemia experiencing relapse after allogeneic hematopoietic cell transplantation. Eur J Haematol. 2022. https://doi.org/10.1111/ejh.13814.
Potter N, Jones L, Blair H, et al. Single-cell analysis identifies CRLF2 rearrangements as both early and late events in down syndrome and non-down syndrome acute lymphoblastic leukaemia. Leukemia. 2019;33(4):893–904. https://doi.org/10.1038/s41375-018-0297-4.
Shah G, Mikhail FM, Carroll AJ, Kutny M, Papadantonakis N. Relapse after prolonged remission in Philadelphia-like acute lymphoblastic leukemia. Case Rep Hematol. 2019;2019:3536517. https://doi.org/10.1155/2019/3536517.
Acknowledgements
The authors would like to thank Katarzyna Lewandowska for editing and proofreading the final version of the manuscript.
Funding
The study was funded by Poznań University of Medicals Sciences statutory funds No. 2705 and by Poznań University of Medical Sciences Doctoral School Large Research Grant No. SDUM-GB13/03/21.
Author information
Authors and Affiliations
Contributions
Conception and design: AP and KL. Methodology AP, KL, AP–Ch, ZK; Acquisition of the clinical data: AP, KL, AP–Ch; Investigation, AP, KL, ZK, AP–Ch, MK, BR, JK-P, AM, KG. Analysis and interpretation of the clinical and laboratory data, AP, KL, ZK, MK, AP–Ch, BR, KG MJ-S, LG; Project administration, AP, KL; Formal analysis, AP, KL; Writing—original draft, AP. Writing-review and editing; AP, KL, LG, MJ-S, AP–Ch. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. The genetic material was utilized subject to patients’ written informed consent. The study was approved by the Poznań University of Medical Sciences Bioethical Committee (Resolution No. 705/20).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Płotka, A., Przybyłowicz-Chalecka, A., Korolczuk, M. et al. BCR::ABL1-like acute lymphoblastic leukaemia: a single institution experience on identification of potentially therapeutic targetable cases. Mol Cytogenet 16, 14 (2023). https://doi.org/10.1186/s13039-023-00645-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13039-023-00645-1