Abstract
Background
Cognitive impairment presents in both adolescent-onset(ado-OP) and adult-onset psychosis(adu-OP). Age and neurodevelopmental factors likely contribute to cognitive differences. This study aimed to characterize cognitive functions in ado-OP compared to adu-OP in a clinical population with drug-naive first-episode psychosis(FEP).
Methods
A total of 788 drug-naive patients with FEP and 774 sex- and age-matched healthy controls(HCs) were included. Participants were divided into four groups by whether they were under or over 21 years of age: adolescent-onset FEP(ado-FEP, n = 380), adult-onset FEP(adu-FEP, n = 408), adolescent HC(ado-HC, n = 334), and adult HC(adu-HC, n = 440). Comprehensive cognitive assessments were performed using the MATRICS Cognitive Consensus Battery(MCCB), covers six cognitive domains: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning, and problem-solving. Data analyses were conducted using correlation analyses and binary logistic regression.
Results
The patterns of cognitive domain differences between ado-FEP and adu-FEP were found to be similar to those between ado-HC and adu-HC, whereas cognitive impairments appeared to be more pronounced in patients with adu-OP than ado-OP. The mazes subtest had the maximum effect size(ES) in the FEP(ES = 0.37) and HC(ES = 0.30) groups when comparing the adolescent and adult groups. Cognitive subtests were mostly significantly correlated with negative symptoms, especially for adolescents with FEP, in which all the subtests were significantly correlated with negative symptoms in the ado-FEP group. Better performance in the domains of spatial cognition and problem-solving abilities was more likely in the ado-FEP group than in the adu-FEP group.
Conclusions
These findings suggest cognitive differences between adolescents and adults but similar patterns of affected domains in HCs and patients with FEP. Therefore, the development of targeted cognitive interventions tailored to the specific needs of different age groups appears warranted.
Similar content being viewed by others
Introduction
Adolescent-onset psychosis (ado-OP) [1] is characterized by the manifestation of psychotic symptoms during adolescence. When psychosis emerges during adolescence, it presents unique challenges and considerations specific to the adolescent population. Ado-OP may exhibit a range of symptoms similar to those seen in adult-onset psychosis (adu-OP), including hallucinations, delusions, disorganized speech, and negative symptoms, such as social withdrawal and decreased motivation. However, the presence of these symptoms during a critical period of cognitive, emotional, and social development can have a profound impact on overall functioning and psychosocial adjustment [2, 3].
Studying ado-OP presents several challenges. Adolescence is a period of significant neurodevelopmental changes, making it difficult to disentangle the effects of psychosis from typical developmental processes [4]. Additionally, adolescents may have varying levels of cognitive and emotional maturity, which can complicate the assessment of symptoms and cognitive deficits [5]. Moreover, more negative symptoms [6] and prodromal symptoms [7], as well as different premorbid characteristics [8], have been observed in early-onset psychosis compared to adult-onset psychosis. Furthermore, ethical considerations arise when working with this vulnerable population, particularly in relation to consent and the potential impact of research participation on their mental health. Despite these challenges, research in this area is crucial for understanding the unique aspects of ado-OP and developing age-appropriate interventions.
Ado-OP is characterized by a more severe course of illness and poorer long-term outcomes than adu-OP [3, 9]. Cognitive impairment is a common feature and can manifest before the onset of psychotic symptoms [10, 11]. Research suggests that these cognitive deficits are present early in the course of illness [12, 13], especially during adolescence and childhood [14]. De la Serna et al. [15] found that patients with an earlier age of psychosis onset showed greater impairment in global cognition, executive functioning, and sustained attention. White et al. [16] reported that first-episode adolescent schizophrenia patients performed worse than adult patients on working memory, language, and motor function tasks, suggesting the onset of schizophrenia during adolescence may lead to a cessation in the development of specific cognitive domains. The causes of cognitive impairment in ado-OP are multifaceted and include neurodevelopmental abnormalities [17, 18], genetic factors [19], and altered brain connectivity [20, 21]. In addition, environmental factors [22] such as early life stress, substance abuse, and poor social support, can further impact cognitive functioning in individuals with ado-OP.
Understanding specific cognitive functioning in ado-OP is important for early detection, accurate diagnosis, and appropriate intervention strategies [23]. Identifying cognitive deficits early in the course of ado-OP can help clinicians develop targeted treatment plans that address these impairments and potentially improve long-term outcomes. For example, targeted cognitive remediation [24] programs and comprehensive treatment approaches that address cognitive deficits may help mitigate the impact of these impairments and improve the overall functional outcomes of individuals with ado-OP. Moreover, understanding the cognitive profiles of ado-OP can aid in distinguishing ado-OP from other psychiatric disorders, leading to more accurate diagnoses and personalized interventions.
Although cognitive deficits have been widely reported in patients with ado-OP, few studies have directly compared adolescent and adult patients with FEP [25] with well-matched controls. Previous studies had small sample sizes [2, 14], confounding effects of concomitant antipsychotic use [3], and participants with substance abuse [9, 26]. In this study, we aimed to perform a comprehensive analysis of cognitive functions in patients with first-episode psychosis (FEP) that onset in adolescence or adulthood. By evaluating a larger sample size, including both clinical and healthy controls (HCs) and carefully controlling for potential confounding factors, our study sought to provide a more robust understanding of the specific cognitive characteristics associated with ado-OP.
Methods
Subjects
Participants in the current study were recruited from ten psychiatric tertiary hospitals in China based on the National Key R&D Program of the Ministry of Science and Technology of China (2016YFC1306800) conducted between 2016 and 2021. This project aimed to explore behavioral and biological markers for the stage identification of psychosis and to develop treatments for early intervention. A total of 788 consecutive patients with FEP (males: 399; females: 389) in these hospitals and 774 well-matched HCs from local communities were enrolled. Participants must be under 35 years of age. Participants were included in the study with an established diagnosis of FEP, as identified by a certified psychiatrist in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). The diagnoses considered as psychotic disorders in this study included schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, and psychotic disorder not otherwise specified (NOS). To be eligible for inclusion, patients had to be within their first 2 years of experiencing psychotic symptoms, as determined by their first presentation to a clinical setting, and were required to have not received any prescribed antipsychotic medication prior to the study. The presence of frank psychotic symptoms, such as hallucinations, delusions, and disorganized thinking, was essential for inclusion in the study. However, FEP participants must be in a relatively stable condition, as judged by a clinician, with no significant risk of agitation or impulsivity, enabling them to complete clinical assessments and cognitive testing. They did not have any history of substance abuse or dependence according to the specific exclusion criteria.
The HC group was recruited from the communities surrounding the 10 centers participating in the study. Each center was responsible for recruiting sex-, age-, and education-matched participants from their respective cities. The recruitment process was carefully designed to ensure that the HCs were representative of the general population in terms of demographic factors, thereby allowing for meaningful comparisons with the FEP group. The inclusion criteria for the HC group were otherwise identical to those of the FEP group, with the primary exception being the absence of a psychotic disorder diagnosis. Exclusion Criteria for HC Group: (1) A history of any psychiatric disorder; (2) Current or previous use of psychiatric medications; (3) A history of substance abuse or dependence; (4) A first-degree relative with a history of psychosis; (5) Sensory impairments (e.g., visual or auditory) that could interfere with the completion of cognitive assessments; (6) Any physical health condition that could prevent the participant from completing the cognitive tests.
The project was led by the Shanghai Mental Health Center (SMHC), and all procedures involving human subjects/patients were approved by the Research Ethics Committee of SMHC (IRB2016-009). The relevant research ethics committees at different sites approved these studies. All participants provided written informed consent during recruitment. All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and the 1975 Declaration of Helsinki, as revised in 2008.
Symptomatic assessments
The clinical assessment was completed on the same day as enrollment. Face-to-face interviews were conducted using the Positive and Negative Syndrome Scale (PANSS) [27]. The PANSS consists of 30 items divided into three subscales: positive, negative, and general psychopathology. Each item is rated on a 7-point Likert scale (1 = absent to 7 = extreme). Structured clinical interviews were conducted with 23 senior psychiatrists who had completed the training required for this type of investigation. The inter-rater reliability for the DSM-IV-TR FEP diagnosis and PANSS ranged from 0.76 to 0.92 among the trained interviewers.
Cognitive assessments
Cognitive testing was conducted when the patients’ clinical symptoms were relatively stable, with no significant risk of agitation or impulsivity. This ensured that the patients were in a suitable state to participate in the assessments effectively. The Chinese version of the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB) [28,29,30] was used for the cognitive assessments. The Chinese version of the MCCB included the following eight subtests: (1) Part A of the Trail-Making Test (Trail-Making A), (2) Symbol Coding of the Brief Assessment of Cognition in Schizophrenia (BACS symbol coding), (3) Category Fluency Test (category fluency), (4) Continuous Performance Test–Identical Pairs (CPT-IP), (5) Spatial Span of the Wechsler Memory Scale-III (WMS-3 spatial span), (6) Revised Hopkins Verbal Learning Test (HVLT-R), (7) Revised Brief Visuospatial Memory Test (BVMT-R), and (8) Neuropsychological Assessment Battery: Mazes (NAB mazes). Test-retest reliability in a previous Chinese psychosis sample ranged from 0.73 to 0.94 [30]. The MCCB covers six cognitive domains: speed of processing (Trail-Making A, BACS symbol coding, and category fluency), attention/vigilance (CPT-IP), working memory (WMS-3 spatial span), verbal learning (HVLT-R), visual learning (BVMT-R), reasoning, and problem-solving (NAB mazes).
The cognitive assessments were performed by trained researchers at each center. At the start of the project, all cognitive assessors underwent standardized training on the MCCB. The training was conducted by recognized experts in MCCB testing in China. The training included practice with no fewer than 10 test cases. Only those assessors who successfully passed an on-site evaluation by the cognitive assessment trainer were certified to conduct the assessments.
Data analysis
SPSS for Windows (version 20.0; IBM, Armonk, NY, USA) and the R statistical software package (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria) were both utilized for the data analysis. Participants were divided into four groups based on adolescent and adult groups according to their age: 21 years or younger and older than 21 years [31], including adolescent-onset FEP (ado-FEP), adult-onset FEP (adu-FEP), adolescent HC (ado-HC), and adult HC (adu-HC). To determine the differences in performance on neurocognitive subtests in the MCCB, we calculated z-scores for the ado- and adu-FEP groups based on the means and standard deviations (SD) of the ado- and adu-HC participants and compared them using an independent t-test analysis of variance. The effect size (ES) was evaluated as η2 = 0.01 (small), η2 = 0.06 (medium), and η2 = 0.14 (large). Spearman’s correlation analysis was conducted to explore the association between the severity of clinical symptoms and cognitive functions. Statistical comparisons between the ado- and adu-FEP groups were conducted using the package cocor [32] in the R programming language (http://comparingcronbachalphas.org). Binary logistic regression was used to determine adjusted associations of cognitive performance between the ado-FEP, adu-FEP, ado-HC, and adu-HC groups. A backward selection procedure was used to find the most parsimonious model, and the Hosmer–Lemeshaw goodness of fit test was used to determine the model fitness. The odds ratios (OR) and 95% confidence intervals (CIs) for covariates were reported.
Results
The demographic and clinical characteristics of the 788 FEP and 774 HC participants are shown in Table 1. The mean age was not significantly different between the FEP (22.7 ± 6.3) and HC (22.4 ± 4.9) groups. The age range for the entire sample, including both FEP and HC participants, was 10 to 35 years (subgroup age ranges: ado-FEP, 11 to 21 years; adu-FEP, 22 to 35 years; ado-HC, 10 to 21 years; adu-HC, 22 to 35 years). The ado-FEP group had a higher proportion of males and a lower educational level than the adu-FEP group. Additionally, the adu-FEP group had higher positive symptom scores compared to the ado-FEP group.
Comparative analyses
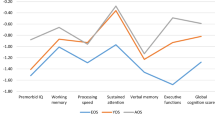
In the HC groups, the mean scores of the NAB mazes (t = 4.060, p < 0.001) and WMS-3 spatial span (t = 3.608, p < 0.001) were significantly higher, and those of CPT-IP (t=-4.683, p < 0.001) and category fluency (t=-2.879, p = 0.004) were significantly lower in the adolescent groups than in the adult groups (Fig. 1), (Table 2). In the patients with FEP, the mean scores of the NAB mazes (t = 5.223, p < 0.001), BVMT-R (t = 3.107, p = 0.002), and BACS symbol coding (t = 3.062, p = 0.002) were significantly higher in the adolescent group than in the adult group.
Neuropsychological comparisons between first-episode psychosis (FEP) and healthy controls (HC), stratified by adolescent and adult groups. z-scores for the ado- and adu-FEP groups based on the means and standard deviations (SD) of ado- and adu-HC participants. BACS, Brief Assessment of Cognition in Schizophrenia symbol coding; BVMT-R, Brief Visuospatial Memory Test–Revised; CPT-IP, Continuous Performance Test–Identical Pairs; HVLT-R, Hopkins Verbal Learning Test–Revised; NAB, Neuropsychological Assessment Battery mazes; WMS-3, Wechsler Memory Scale–Third Edition spatial span
ES analyses
In comparing the adolescent and adult groups, the NAB mazes test had the maximum ES in the FEP and HC groups (Fig. 2). Specifically, adolescent participants performed better in the NAB mazes test but worse in the CPT-IP and BACS category fluency tests than adult participants. In comparing the HC and FEP groups, BACS category fluency and CPT-IP—the top two tests—had the maximum ES in the adolescent and adult groups.
Effect sizes (Cohen d) for cognitive comparisons among healthy controls (HC) groups included ado-HC and adu-HC, and first-episode psychosis (FEP) groups included ado-FEP and adu-FEP groups Note: BACS, Brief Assessment of Cognition in Schizophrenia symbol coding; BVMT-R, Brief Visuospatial Memory Test–Revised; CPT-IP, Continuous Performance Test–Identical Pairs; HVLT-R, Hopkins Verbal Learning Test–Revised; NAB, Neuropsychological Assessment Battery mazes; WMS-3, Wechsler Memory Scale–Third Edition spatial span
Correlation analyses
Except for the NAB mazes test, all MCCB subtests were insignificantly correlated with positive symptoms, and the only significant correlation with general symptoms was found in the category fluency in the overall FEP group (Table 3). Meanwhile, the cognitive subtests were mostly significantly correlated with negative symptoms, particularly in adolescents with FEP. All MCCB subtests were significantly correlated with negative symptoms in the ado-FEP group. In the adu-FEP group, the Trail-Making A, BACS symbol coding, NAB maze, category fluency, and CPT-IP subtests were significantly correlated with negative symptoms. The HVLT-R subtest score showed more significant correlations with negative and general symptoms in the ado-FEP group than in the adu-FEP group.
Logistic regression analyses
According to the binary logistic regression analysis, poorer performance in the category fluency and CPT-IP tests and better performance in the BACS symbol coding, NAB mazes, and BVMT-R were more likely in patients with adolescent-onset than adult-onset FEP. A similar pattern was found in the comparison of the adolescent and adult HC groups (Table 4). When stratified by adolescents and adults, BACS symbol coding and CPT-IP were the top two significant discriminators between the FEP and HC groups.
Disscussion
This study is based on large-scale controlled research and has identified some valuable findings. First, the patterns of cognitive domain differences between adolescent and adult-onset psychosis were found to be similar to the patterns of cognitive domain differences between adolescent and adult HCs. Second, upon visual inspection, it appears that cognitive impairments are more pronounced in adult-onset patients than in adolescent-onset patients. Third, there is a close relationship between negative symptoms and cognitive functioning. Lastly, adolescents tended to outperform adults in specific cognitive tests, such as the NAB maze and BVMT-R tests in both the FEP and HC groups.
The similarities in cognitive function differences between adolescents and adults in both psychosis and HC groups may be attributed to neurodevelopmental factors, which play a significant role in cognitive differences [33, 34], During adolescence and early adulthood, the brain undergoes substantial structural and functional changes, including synaptic pruning [35], myelination [36], and refinement of neural circuits [34]. These developmental processes are closely linked to cognitive functions, such as attention, memory, executive functions, and social cognition. In FEP patients, disruptions in these neurodevelopmental processes may lead to similar patterns of cognitive impairment in both adolescents and adults [37, 38].
Contrary to previous findings suggesting greater cognitive impairments in ado-OP patients [14, 39], this study found more pronounced impairments in adu-OP patients. This could be due to the greater neuroplasticity of the adolescent brain, which may offer some resilience against the cognitive impairments associated with psychosis [40]. Additionally, psychosocial factors unique to adolescence, such as strong social support systems and active educational engagement [41], may contribute to better cognitive functioning and offset some of the impairments associated with psychosis.
The NAB maze and BVMT-R tests, which assess spatial and problem-solving abilities, showed better performance in adolescents than adults. This finding aligns with research by Nitzburg et al. [42], which showed that visual memory domains and problem-solving abilities were better in adolescents (17–20 years) than in adults (20–23 years). Adolescents’ higher levels of neuroplasticity and learning capacity may lead to better performance on these tests [43], as they can adapt more quickly and use more flexible strategies. In addition, adolescents may have had more recent exposure to maze-like tasks or video games [44], which could enhance their familiarity with spatial navigation tasks.
Although this study included a large sample of clinical and control participants and minimized the influence of medications, several limitations should be acknowledged. The use of a cross-sectional design limited our ability to establish causal relationships or determine the trajectory of cognitive impairment over time. Longitudinal studies should provide more insight into the developmental patterns of cognitive deficits in different age groups. The intelligence quotient (IQ) is an important factor to consider when examining cognitive differences [45, 46]. The absence of IQ testing in this study prevented us from accounting for individual variations in baseline cognitive abilities, which could have impacted the interpretation of the results. The duration of untreated psychosis [47] or prodromal symptoms [48, 49] is a crucial variable in understanding the impact of early intervention on cognitive outcomes. The absence of these data limits our understanding of its potential influence on cognitive impairment. To address these limitations and further enhance our understanding of cognitive impairments in patients with adu-OP and adu-OP, longitudinal studies [50] that follow individuals from adolescence into adulthood should be conducted. Such studies would allow for a more comprehensive evaluation of cognitive trajectories and the identification of potential critical periods for intervention.
Conclusion
This study demonstrates that cognitive differences between patients with ado-OP and adu-OP and HCs exhibit similar patterns across cognitive domains. In addition, our results suggest that cognitive impairments may be more pronounced in patients with adu-OP. Future research should focus on developing targeted cognitive interventions tailored to the specific needs of different age groups. Longitudinal studies are needed to better understand the trajectory of cognitive impairment and its impact on functional outcomes over time.
Data availability
Data generated during this study are not publicly available because participants did not agree for their data to be shared publicly. Individual deidentified, anonymized data are available from the authors upon reasonable request.
Abbreviations
- Ado-OP:
-
Adolescent-onset
- Ado-HC:
-
Adolescent healthy control
- Ado-FEP:
-
Adolescent-onset first-episode psychosis
- Adu-OP:
-
Adult-onset psychosis
- Adu-HC:
-
Adult healthy control
- Adu-FEP:
-
Adult-onset first-episode psychosis
- BACS :
-
Brief assessment ofcCognition in schizophrenia
- BVMT-R:
-
Revised brief visuospatial memory test
- CIs:
-
Confidence intervals
- CPT-IP:
-
Continuous performance test–identical pairs
- DSM-IV-TR:
-
Diagnostic and statistical manual of mental disorders, fourth edition, text revision
- ES:
-
Effect size
- FEP:
-
First-episode psychosis
- HCs:
-
Healthy controls
- HVLT-R:
-
Revised Hopkins verbal learning test
- MCCB:
-
Measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery
- NAB:
-
Neuropsychological assessment battery
- OR:
-
Odds ratios
- PANSS:
-
Positive and negative syndrome scale
- SMHC:
-
Shanghai Mental Health Center
- Trail-Making A :
-
Part A of the trail-making test
- WMS-3:
-
Wechsler memory scale-III
References
Boeing L, Murray V, Pelosi A, McCabe R, Blackwood D, Wrate R. Adolescent-onset psychosis: prevalence, needs and service provision. Br J Psychiatry. 2007;190:18–26.
Lay B, Blanz B, Hartmann M, Schmidt MH. The psychosocial outcome of adolescent-onset schizophrenia: a 12-year followup. Schizophr Bull. 2000;26(4):801–16.
Hui CL, Li AW, Leung CM, Chang WC, Chan SK, Lee EH, Chen EY. Comparing illness presentation, treatment and functioning between patients with adolescent- and adult-onset psychosis. Psychiatry Res. 2014;220(3):797–802.
Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 2011;41(1):1–6.
Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–57.
Ballageer T, Malla A, Manchanda R, Takhar J, Haricharan R. Is adolescent-onset first-episode psychosis different from adult onset? J Am Acad Child Adolesc Psychiatry. 2005;44(8):782–9.
Baeza I, de la Serna E, Mezquida G, Cuesta MJ, Vieta E, Amoretti S, Lobo A, Gonzalez-Pinto A, Diaz-Caneja CM, Corripio I, et al. Prodromal symptoms and the duration of untreated psychosis in first episode of psychosis patients: what differences are there between early vs. adult onset and between schizophrenia vs. bipolar disorder? Eur Child Adolesc Psychiatry. 2024;33(3):799–810.
Baeza I, de la Serna E, Amoretti S, Cuesta MJ, Diaz-Caneja CM, Mezquida G, Lobo A, Gonzalez-Pinto A, Corripio I, Vieta E et al. Premorbid Characteristics as predictors of early onset Versus Adult Onset in patients with a first episode of psychosis. J Clin Psychiatry 2021, 82(6):21m13907.
Veru F, Jordan G, Joober R, Malla A, Iyer S. Adolescent vs. adult onset of a first episode psychosis: impact on remission of positive and negative symptoms. Schizophr Res. 2016;174(1–3):183–8.
Zhang T, Cui H, Wei Y, Tang X, Xu L, Hu Y, Tang Y, Chen T, Li C, Wang J. Neurocognitive assessments are more important among adolescents than adults for Predicting psychosis in clinical high risk. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7(1):56–65.
Cui H, Giuliano AJ, Zhang T, Xu L, Wei Y, Tang Y, Qian Z, Stone LM, Li H, Whitfield-Gabrieli S, et al. Cognitive dysfunction in a psychotropic medication-naive, clinical high-risk sample from the ShangHai-At-Risk-for-psychosis (SHARP) study: associations with clinical outcomes. Schizophr Res. 2020;226:138–46.
Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, et al. Association of Neurocognition with Transition to psychosis: baseline functioning in the second phase of the North American Prodrome Longitudinal Study. JAMA Psychiatry. 2016;73(12):1239–48.
Mensi MM, Orlandi M, Casini E, Catalan A, de Pablo GS, Fusar-Poli P, Borgatti R. Neurocognition and functioning in adolescents at clinical high risk for psychosis. Child Adolesc Psychiatry Ment Health. 2023;17(1):22.
Grover S, Sahoo S, Nehra R. A comparative study of childhood/adolescent and adult onset schizophrenia: does the neurocognitive and psychosocial outcome differ? Asian J Psychiatr. 2019;43:160–9.
De la Serna E, Puig O, Mezquida G, Moreno-Izco L, Merchan-Naranjo J, Amoretti S, Ruiz P, Gonzalez-Pinto A, Molina-Garcia M, Corripio I, et al. Relationship between cognition and age at onset of first-episode psychosis: comparative study between adolescents, young adults, and adults. Eur Child Adolesc Psychiatry. 2023;32(4):639–49.
White T, Ho BC, Ward J, O’Leary D, Andreasen NC. Neuropsychological performance in first-episode adolescents with schizophrenia: a comparison with first-episode adults and adolescent control subjects. Biol Psychiatry. 2006;60(5):463–71.
Arango C, Fraguas D, Parellada M. Differential neurodevelopmental trajectories in patients with early-onset bipolar and schizophrenia disorders. Schizophr Bull. 2014;40(Suppl 2):S138–146.
Lee H, Dvorak D, Kao HY, Duffy AM, Scharfman HE, Fenton AA. Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron. 2012;75(4):714–24.
Kuo SS, Musket CW, Rupert PE, Almasy L, Gur RC, Prasad KM, Roalf DR, Gur RE, Nimgaonkar VL, Pogue-Geile MF. Age-dependent patterns of schizophrenia genetic risk affect cognition. Schizophr Res. 2022;246:39–48.
James A, Joyce E, Lunn D, Hough M, Kenny L, Ghataorhe P, Fernandes HM, Matthews PM, Zarei M. Abnormal frontostriatal connectivity in adolescent-onset schizophrenia and its relationship to cognitive functioning. Eur Psychiatry. 2016;35:32–8.
Zhao J, Zhang Y, Liu F, Chen J, Zhao J, Guo W. Abnormal global-brain functional connectivity and its relationship with cognitive deficits in drug-naive first-episode adolescent-onset schizophrenia. Brain Imaging Behav. 2022;16(3):1303–13.
Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S7–13.
Puig O, Penades R, Baeza I, De la Serna E, Sanchez-Gistau V, Bernardo M, Castro-Fornieles J. Cognitive remediation therapy in adolescents with early-onset schizophrenia: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2014;53(8):859–68.
Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res. 2007;94(1–3):221–30.
Fagerlund B, Pagsberg AK, Hemmingsen RP. Cognitive deficits and levels of IQ in adolescent onset schizophrenia and other psychotic disorders. Schizophr Res. 2006;85(1–3):30–9.
Hanna RC, Shalvoy A, Cullum CM, Ivleva EI, Keshavan M, Pearlson G, Hill SK, Sweeney JA, Tamminga CA, Ghose S. Cognitive function in individuals with psychosis: moderation by adolescent Cannabis Use. Schizophr Bull. 2016;42(6):1496–503.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–76.
Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RS, Mesholam-Gately RI, Seidman LJ, Lee C, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1–3):124–31.
Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214–20.
Shi C, He Y, Cheung EF, Yu X, Chan RC. An ecologically valid performance-based social functioning assessment battery for schizophrenia. Psychiatry Res. 2013;210(3):787–93.
Hardin AP, Hackell JM, Committe on practice, Ambulatory medicine. Age Limit of Pediatrics. Pediatr. 2017;140(3):e20172151.
Diedenhofen B, Musch J. Cocor: a comprehensive solution for the statistical comparison of correlations. PLoS ONE. 2015;10(3):e0121945.
Goldberg X, Fatjo-Vilas M, Penades R, Miret S, Munoz MJ, Vossen H, Fananas L. Neurodevelopmental liability to schizophrenia: the complex mediating role of age at onset and premorbid adjustment. Schizophr Res. 2011;133(1–3):143–9.
Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37(3):514–23.
Germann M, Brederoo SG, Sommer IEC. Abnormal synaptic pruning during adolescence underlying the development of psychotic disorders. Curr Opin Psychiatry. 2021;34(3):222–7.
Wei W, Zhang Y, Li Y, Meng Y, Li M, Wang Q, Deng W, Ma X, Palaniyappan L, Zhang N, et al. Depth-dependent abnormal cortical myelination in first-episode treatment-naive schizophrenia. Hum Brain Mapp. 2020;41(10):2782–93.
Guo P, Hu S, Jiang X, Zheng H, Mo D, Cao X, Zhu J, Zhong H. Associations of Neurocognition and Social Cognition with Brain structure and function in early-onset Schizophrenia. Front Psychiatry. 2022;13:798105.
Chen J, Wei Y, Xue K, Han S, Wang C, Wen B, Cheng J. The interaction between first-episode drug-naive schizophrenia and age based on gray matter volume and its molecular analysis: a multimodal magnetic resonance imaging study. Psychopharmacology. 2023;240(4):813–26.
Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Cognitive deficits distinguish patients with adolescent- and adult-onset schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10(2):107–12.
Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of Brain Development. Trends Cogn Sci. 2015;19(10):558–66.
Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207.
Nitzburg GC, Derosse P, Burdick KE, Peters BD, Gopin CB, Malhotra AK. MATRICS cognitive consensus battery (MCCB) performance in children, adolescents, and young adults. Schizophr Res. 2014;152(1):223–8.
Contreras MJ, Escrig R, Prieto G, Elosua MR. Spatial visualization ability improves with and without studying Technical drawing. Cogn Process. 2018;19(3):387–97.
Lopes AS, Silva KS, Barbosa Filho VC, Bezerra J, de Oliveira ES, Nahas MV. Trends in screen time on week and weekend days in a representative sample of Southern Brazil students. J Public Health (Oxf). 2014;36(4):608–14.
Stone WS, Mesholam-Gately RI, Giuliano AJ, Woodberry KA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, et al. Healthy adolescent performance on the MATRICS Consensus Cognitive Battery (MCCB): developmental data from two samples of volunteers. Schizophr Res. 2016;172(1–3):106–13.
Mohn C, Sundet K, Rund BR. The relationship between IQ and performance on the MATRICS consensus cognitive battery. Schizophr Res Cogn. 2014;1(2):96–100.
Ito S, Nemoto T, Tsujino N, Ohmuro N, Matsumoto K, Matsuoka H, Tanaka K, Nishiyama S, Suzuki M, Kinoshita H, et al. Differential impacts of duration of untreated psychosis (DUP) on cognitive function in first-episode schizophrenia according to mode of onset. Eur Psychiatry. 2015;30(8):995–1001.
Zhang T, Xu L, Tang Y, Cui H, Wei Y, Wang J, Tang X, Li C, Wang J. Duration of untreated prodromal symptoms in a Chinese sample at a high risk for psychosis: demographic, clinical, and outcome. Psychol Med. 2018;48(8):1274–81.
Zhang T, Xu L, Tang Y, Cui H, Tang X, Wei Y, Wang Y, Hu Q, Qian Z, Liu X, et al. Relationship between duration of untreated prodromal symptoms and symptomatic and functional recovery. Eur Arch Psychiatry Clin Neurosci. 2019;269(8):871–7.
Ropcke B, Eggers C. Early-onset schizophrenia: a 15-year follow-up. Eur Child Adolesc Psychiatry. 2005;14(6):341–50.
Acknowledgements
None.
Funding
This study was supported by National Key R&D Program of the Ministry of Science and Technology of China (2023YFC2506800), National Natural Science Foundation of China (82171544, 82371505, 82151314, 82101623).
Author information
Authors and Affiliations
Contributions
THZ and JJW. conceptualized the study, wrote the first draft of manuscript and conducted the statistical analyses. HRC, YYT and XCT helped in the design of the study and edited the manuscript. LHX, ZXW, and YYW, interviewed participants and collected and organized the primary data. CBL, TC, YGH, and HCL, managed the literature searches, statistical analyses and edited the manuscript THZ and JJW designed the study and provided supervision in the implementation of the study. All authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This observational study was conducted following the principles of good clinical practice, the Declaration of Helsinki, and current ethical standards. Data are collected within the framework of guideline-based routine care. The study was approved by the research ethics committee of Shanghai Mental Health Center (IRB approval number: IRB2016-009). Consent from patients and parents/legal guardians (depending on the patients’ age) was obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, T., Wei, Y., Tang, X. et al. Cognitive impairment in adolescent and adult-onset psychosis: a comparative study. Child Adolesc Psychiatry Ment Health 18, 122 (2024). https://doi.org/10.1186/s13034-024-00815-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13034-024-00815-y