Abstract
Background
Human papillomavirus (HPV) genotypes differ by geographic location. With the advent of HPV vaccination and HPV-based cervical screening tests in Ethiopia, a nationwide dataset on the genotype distribution of HPV among women has paramount importance in the fight against cervical cancer. However, there is limited data in this regard in the northwest part of the country. Therefore, this study aimed to identify the genotype distribution of high-risk HPVs among women presenting with cervical abnormalities.
Methods
A health facility-based cross-sectional study was conducted at Felege Hiwot Comprehensive Specialized Hospital (FHCSH), Bahir Dar–Ethiopia. Women aged ≥ 30 years who visited the hospital gynecology unit from 01 March 2019 to 30 October 2021 were included. Following general and pelvic examinations, a senior gynecologist collected cervical punch biopsies for histopathological examinations and cervical swabs for HR-HPV detection using the Abbott Alinity m system (Abbott Molecular, Des Plaines, IL, USA). Extended genotyping was carried out with the INNO-LiPA HPV Genotyping Extra II assay (INNO-LiPA; Fujirebio Europe, Ghent, Belgium) as per the manufacturer protocols at the Institute of Virology, Leipzig University Hospital, Germany.
Results
We included 355 women with a mean age of 46.4 ± 11.4 years. The majority of the participants, 277 (79.4%) were sexually active before the age of 18 years and 180 (51.6%) had multiple sexual partners. Forty-eight (13.5%) of the participants were HIV positive. The proportion of HR-HPV was 53.0% (n = 188; 95%CI: 47.8–58.1%). From these samples, 13 different HR-HPV types with a total of 258 sequences were identified. The detection of HR-HPV increased significantly with an increase in the age of the participants. The predominant identified HR-HPV was HPV16, 50.4% followed by HPV31 (9.7%), HPV33 (8.5%), HPV39, and HPV68 each (5.8%) and HPV18 (4.7%). Of the total HR-HPV-positive women, 23.9% (45/188) were infected with multiple HR-HPV types. All HPV16, HPV18, HPV35, and HPV45 genotypes (as a single or in coinfections) were found to be associated with either high-grade lesions or cervical cancer.
Conclusions
HR-HPV infection was reportedly higher among women in the present study area. Based on our findings, we strongly recommend the nonavalent HPV vaccine for immunization and any HPV-based screening method to take into consideration the predominant genotypes circulating in the country. The role of multiple HPV infections in high-grade cervical lesions entails further study in Ethiopia.
Similar content being viewed by others
Background
Cervical cancer is one of the most frequent malignancies worldwide. Almost nine out of ten deaths occur in developing countries, where there is inefficient vaccination and cervical screening [1]. Cervical cancer is one of the emerging public health challenges in Ethiopia. The incidence and prevalence are increasing [2]. According to the international agency for research on cancer assessments, the estimated number of new cervical cancer cases at 7500 in 2020 could intensify to 15,300 in 2040. Similarly, the mortality from the disease could increase from 5340 in 2020 to 11,000 in 2040 yearly in Ethiopia [3].
Epidemiological, clinical, and molecular-based studies confirmed that persistent infection with oncogenic types or high-risk (HR) human papillomaviruses (HPVs) is the main cause of cervical cancer [4, 5]. HR-HPVs are also linked with a large number of other types of cancers (anus, vulva, vagina, and penis) and a growing number of head and neck tumors [6,7,8,9,10]. So far, more than 200 different HPVs are characterized and completely sequenced. Of all types, about 40 are sexually transmitted [8, 11,12,13,14]. HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 grouped under HR-HPV because of their strong association in carcinogenesis. Infection with HPV type 16 or HPV18 is associated with a higher risk of disease progression compared to other HR-HPVs causing more than 50% of all cervical cancer burdens globally [15,16,17,18]. According to a report from a recent systematic review, HPV16 followed by HPV52, HPV35, HPV18, and HPV56 were the most common types identified from cervical samples in Ethiopia. The combined prevalence of HPV 16/18 was 53.7% [19].
Cervical cancer is preventable. Early detection of precancerous lesions and vaccinating schoolchildren with optimal coverage could save lives. HPV-based screening is rarely available in Ethiopia. Vaccination was introduced in 2018 using Gardasil-4® recombinant vaccine that targets HPV 6, 11, 16, and HPV18 for 14-year-old girls and according to the 2020 estimation, the first and last dose coverage was 76% and 95%, respectively [1, 20]. Despite its proven effectiveness and safety, the currently available vaccine in Ethiopia presents some limitations in terms of incorporating the most important oncogenic HPV variants circulating in the country.
HPV types differ by geographical location and the host’s background [21]. The burden and heterogeneity in HPV distribution between and among countries and even among specific regions within a country are significantly different [22], which directly influence the HPV-based screening and vaccination approaches. With the advent of HPV HPV-based screening tests and vaccination in Ethiopia, it is crucial to map the distribution of HPV genotypes in areas where there is no previous report. Even though there are few studies conducted in Ethiopia on HPV genotyping [23,24,25,26,27,28,29,30,31], to the best of our knowledge, there are only a couple of health facility-based reports in the northern part of the country [24, 31].
Therefore, this study was conducted to identify the HR-HPV genotype circulating in northwest Ethiopia among women presenting with various cervical lesions. The finding will be an important input to help guide in the revision of the current national cervical screening and vaccination program as national data and context is important in planning full-pledged cervical cancer prevention services.
Methods and materials
Study setting
A hospital-based cross-sectional study was conducted at Felege Hiwot Comprehensive Specialized Hospital (FHCSH) between 01 March 2019 and 30 Oct 2021. The hospital is located in Bahir Dar city, northwest Ethiopia, which is the capital of Amhara National Regional State, positioned about 565 km away from Addis Ababa. The FHCSH, with more than 500 beds, is a tertiary health care facility that provides several types of specialized referral services for more than ten million people of northwest Ethiopia.
Study population
Cervical specimens were collected from 355 women presented with suggestive signs and symptoms of abnormal cervical lesions including abnormal vaginal discharge, vaginal bleeding, and women complaining of painful sexual intercourse [32, 33]. Participants with the following characteristics were considered to take part in the study: Age ≥ 30, had sexual history, were not pregnant, have an intact uterus and cervix, and not on menses. On the other hand, women who were seriously ill and those who were under treatment for invasive cervical cancer were excluded.
Data and sample collection
An interviewer-administered structured questionnaire was used to collect data on the participants’ demographic and gynecologic history. The tool was prepared following previous similar works [6, 34,35,36,37,38,39]. Trained nurses who were working in the hospital gynecology department collected the questionnaire-based data. Each participant underwent a general and pelvic examination in a compassionate and respectful process after obtaining an informed consent. Then, from the grossly visible lesion, a licensed gynecologist collected a punch biopsy for histopathological examinations and cervical swabs with a single-use broom-type brush (Digene HC2 DNA collection device: Qiagen, Hilden, Germany) following the manufacturer’s instruction for HPV DNA testing. Specimen Transport Medium (STM) that contains 0.05% sodium azide as a preservative accompanied the sample collection brush. Cervical swabs were labeled with a unique code and the patient card number and were stored at − 80 °C at Bahir Dar University, College of Medicine and Health Science Research Laboratory. Later, the frozen specimens were transported on dry ice packs to the Institute of Virology, Leipzig University Hospital, Germany for molecular analysis.
HPV DNA detection and genotyping
The molecular detection and characterization of HPVs from cervical lesions were based on the principle that the viral DNA is present in the epithelial layers of the affected tissue and can be detected easily with PCR-based technologies [40]. HR-HPV detection and characterization were made using Alinity m HR HPV AMP Kit on Alinity m System (software version: 1.6.3) (Abbott Molecular, Des Plaines, IL, USA). The Alinity m System offers fully automated continuous random access to different assays. It is a fully integrated and automated molecular analyzer introduced in 2019 [41]. Alinity m HR-HPV assay fulfills the international consensus guideline criteria for primary cervical cancer screening [42]. The time to the first result is less than two hours [43]. Using real-time PCR and ReadiFlex® technology, the Alinity m HR HPV assay is a qualitative in vitro test targeting the conserved L1 region for the detection of DNA from 14 HR-PV genotypes; HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 from clinical specimen [42]. HR-HPVs were detected with genotype-specific probes in five distinct channels: HPV16, HPV18, HPV45, and other high-risk genotypes group A (HPV31, 33, 52, and 58), and other high-risk genotypes group B (HPV35, 39, 51, 56, 59, 66, and 68) at clinically relevant infection levels [41]. To ensure its quality performance, the Alinity m system in addition to testing external positive and negative controls, detects the endogenous human beta-globin sequence as a Cellular Control signal to evaluate cell adequacy, sample extraction, and amplification efficiency [43].
The steps of the Alinity m HR HPV assay consist of sample preparation, real-time PCR assembly, amplification/detection, and result calculation and reporting. All steps of the assay are performed automatically by the Alinity m system. During sample preparation, 400 µL of cervical swabs were pretreated and lysed with chaotropic reagents, allowing HPD DNA to be captured on magnetic microparticles. The bound purified DNA was then washed and eluted. A lyophilized amplification master mix consisting of DNA polymerase, primers, probes, and dNTPs rehydrated using the eluate and activation reagent. The resultant PCR mixture was then transferred to a reaction vessel, which was subsequently capped and transferred to the amplification and detection unit. Results are reported automatically [44, 45].
Samples (n = 49) that tested positive for HR-HPV A and HR-HPV B with the Abbott Alinity m test were further processed using the INNO-LiPA HPV Genotyping Extra II assay (INNO-LiPA; Fujirebio Europe, Ghent, Belgium) for identification of the specific genotypes following the manufacturer instructions and as described previously [46, 47].
With regard to the histological examination, after collection, cervical biopsies were placed in screw-capped and labeled bottles that contained 20 ml of 10% formol-saline fixative solution and transported to the hospital pathology laboratory for downstream processing by the senior pathologist as described previously [48]. Lastly, the microscopic examination of slides was reported as normal histology, cervicitis, Cervical intraepithelial neoplasia (CIN)1, CIN2, CIN3, cancer, and other miscellaneous findings.
Data analysis
Generated data were entered and analyzed using SPSS V25. Descriptive statistics were used to describe the demographic and clinical characteristics of the study participants. The type distribution of the identified HR-HPVs with a 95% confidence interval (CI) was calculated. The results are presented as simple counts, percentages, and mean with standard deviations.
Results
Demographic and other characteristics of the study participants
In this study, 355 women were included. At enrolment, the study participants were in the age range of 30–80 years with a mean age of 46.4 ± 11.4 years. The majority of the participants were married 272 (76.6%), housewives 314 (88.5%), were from rural settings 232 (65.9%), and did not have formal education 272 (76.6%) (Table 1).
The majority of the participants 277 (79.4%) were sexually active before the age of 18 years. The mean age at first sexual intercourse was 15.7 ± 2.6. Furthermore, 48 (13.5%), 106 (30.0%), and 180 (51.6%) of the participants were HIV positive, had a history of vaginal discharge, and multiple sexual partners, respectively.
More than half of the participants 211 (59.4) were unaware of cervical cancer. The participants’ cervical screening practice and screening in the last five years were 97 (27.3%) and 88 (24.8%), respectively. Besides, their knowledge about the disease was very limited and only 11 (8.9%) of them know that the disease is caused by a sexually transmitted pathogen (Table 1).
Type of the identified HR-HPVs
The prevalence of high-risk HPV (HR-HPV) was 53.0% (188/355; 95% CI: 47.8–58.1%). From the processed samples, 13 different HR-HPVs with a total of 258 sequences were identified. The detection of HR-HPV increased significantly with an increase in the age of participants. Similarly, HR-HPV detection was different based on the educational status and screening history of the study participants (p-value < 0.05) (Table 2).
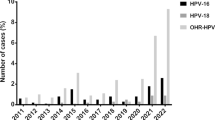
The predominant identified HR-HPV was HPV16 that accounted for 50.4% (130/258: 95% CI: 29.4–39.2%) followed by HPV31 9.7% (25/258: 95% CI: 6.7–13.9%), HPV33 8.5% (22/258: 95% CI: 5.7–12.6%), HPV39 and HPV68 each 5.8% (15/258: 95% CI: 3.6–9.4%) and HPV18 4.7% (12/258: 95% CI: 2.7–8.0%). The least detected genotype was HPV58 (0.4%). The combined prevalence of HPV 16 and HPV18 was 55.1% (142/258: 95% CI: 48.9–61.0%) (Fig. 1).
HR-HPV co-infections
Among HR-HPV positive samples (n = 188), mono-infection was documented in 143 (76.1%) whereas 27 (14.4%) and 18 (9.6%) samples were positive for two and multiple (≥ 3) HR-HPV types, respectively. We noted up to five different HR-HPVs infecting a single patient. The most common HR-HPV coinfection was HPV31 with HPV33 followed by HPV39 with HPV59 and HPV68.
The distribution of HR-HPV in cervical lesions of different histopathological grades
Concerning the types of HR-HPVs involved in different histopathological grades, all HPV16, HPV18, HPV35, and HPV45 genotypes (as a single or in coinfections) were found to be associated with either high-grade lesions (CIN2+) or cervical cancer. Likewise, 79 (80.6%), 7 (87.5%), 3 (75%), and 5 (100%) of HPV16, HPV18, HPV35, and HPV45 mono infections were identified in histopathologically confirmed cancer cases, respectively. Similarly, 11 (78.6%) HPV31&33 coinfections were recovered from cancer cases.
Specifically, the detection of rate of HPV16 was increased with an increase in the degree of cervical lesions in which the proportion was 12.5% (n = 5), 16.7% (n = 2), 41.7% (n = 5), and 85.3% (n = 58) in Cervical Intraepithelial Neoplasia (CIN) 1, CIN2, CIN3, and cancer cases, respectively. In 9.7% (13/133) of cervical cancer cases, HR-HPVs were not detected (Table 3).
Discussion
A nationwide dataset on the prevalence and genotype distribution of HPV among Ethiopian women is crucial as it affects the current vaccination and HPV-based screening. This, however, is not adequately available despite the high burden of cervical cancer-related morbidity and mortality in the country. Vaccination and screening-led elimination of cervical cancer are highly dependent on optimizing HPV data of a particular country. Such information is decisive to appraise the lasting impact of HPV vaccines and HPV-based screening, and informing policymakers on the best alternative options for cervical and other HPV-associated cancer prevention and control activities [49].
In the present study, the prevalence of HPV among women who presented with cervical abnormalities was 53.0% (188/355). In this regard, previously conducted similar studies in Ethiopia revealed wide-ranging findings. The proportion of HR-HPV among women who visited the gynecology clinics and who were recruited from the general population in different parts of Ethiopia previously ranged from 16 to 100% [23, 26, 27, 30, 31, 50]. A study by Gebremeskel et al. [31] that specifically included women from the northern part of the country, reported the proportion of HR-HPVs among the identified HPVs to be 55.5%, which is almost the same as our finding. However, according to our recent systematic review, the proportion of HR-HPV among women with different kinds of cervical abnormalities was 77.5% in Ethiopia [19]. The HR-HPV prevalence reported in other African countries was also diverse; in Togo 53.3% [51], and Zimbabwe 96% [52]. Variation in the proportion of HR-HPV across different studies might be because of the difference in the methods of HPV detection, study population, degree of cervical lesions, sociodemographic and other related factors [23, 26, 27, 30, 31, 50]
In our study, the HR-HPV proportion was higher among women aged above fifty years and HIV-positive women compared to their counterparts (p-value < 0.005) (Table 2). This implies that these groups of women should get priority for public health interventions such as, screening by the use of HPV-based and other similar methods and a positive HPV result at this age might implies persistence for a number of reasons [53].
In the present study, the leading identified HR-HPV was HPV16 (50.4%). In other similar reported series in Ethiopia, other parts of Africa, and globally at large, HPV16 was the single most common genotype identified in cervical samples. The second, third, fourth, and the rest most commonly reported genotypes were usually different. Specifically, HR-HPV types from cervical samples in Ethiopia were reported to be diverse [23, 26, 27, 30, 31, 50]. This implies that HPV genotype distribution is diverse at different places and periods even within the same population [54]. Similarly, the distribution of HR-HPVs among women in other African countries was reported to heterogeneous [25, 49, 52, 55, 56]. For instance, HPV16 is not the first-ranked genotype from cervical samples in Kenya [53], Togo [51, 56], and Nigeria [22]. According to studies, not only HPV16 but also HPV18 is not that important in some African countries [51, 56]. However, systematic reviews in Africa, Asia, Latin America, and North America consistently reported that HPV16 is the most frequently identified genotype among women with various degrees of cervical lesions [57,58,59,60]. The genotype distribution of other HPVs is heavily dependent on the type of cervical lesions. Most often, HPV18, HPV31, 33, 52 and 56 are found as confecting genotypes [61].
Globally, HPV18 is the most frequently detected HR-HPV next to HPV16 from advanced cervical lesions including cancer [18]. However, it was rare in our study and we reported a similar result in a recently published systematic review [19]. The reason behind the low involvement of HPV18 in cancer and high-grade cervical lesions in Ethiopia requires further studies. Generally, the difference in the proportion and type of HR-HPVs among different studies in Ethiopia and other parts of the world might vary partly due to study-specific characteristics, heterogeneity in geographical location [62], age, life style and socio-economic status of the study participants and differences in study population, and most importantly methods used for HPV detection [49, 53].
The understanding that persistent infection with certain HR-HPVs is mainly a cause of cervical cancer has resulted in the development of new prospects for cervical screening and vaccination globally. HPV-based testing for cervical screening is becoming a cost-effective approach in most countries around the world [63]. In 2020, the World Health Organization (WHO) launched a global strategy to accelerate the elimination of cervical cancer as a public health problem. According to this strategy, priority is given to vaccinating schoolgirls, screening, and treatment of precancerous lesions [64]. This milestone approach is primarily dependent on the availability of nationwide HPV genotype data for countries, like Ethiopia. Our finding together with other previous HPV genotype reports could be used to partly evaluate the impact of vaccination and in the future the impact of the screening program in Ethiopia [62].
The current vaccine cocktail used in the country since 2018 for schoolgirls is Gardasil-4® that targets HPV6, 11, 16, and 18. The vaccine does not target other HR-HPVs circulating in the country. In the present study, for example, the combined prevalence of HPV16 and HPV18 was 55.1%, which implies that a significant proportion of girls might not be protected against other types even though they are vaccinated. So far, HPV vaccination is not part of the national immunization program in Ethiopia. One reason may be the cost of the vaccine. A recent study revealed that compared to the Gardasil-4™, a nonavalent Gardasil®9 that targets close to 90% of all HR-HPVs is a profitable option for Ethiopia [65].
A large proportion of HPV infections are sustained by multiple genotypes [66]. Of the total HR-HPV-positive women, 23.9% (45/188) were infected with two and more (up to five) multiple HR-HPVs in the present study. A study in China similarly reported 29.8% HR-HPV multiple infections from women with abnormal cervical cytology [67]. Multiple HPV infections are usually common at a younger age [66]. However, the role of such multiple infections in cervical carcinogenesis has not been well explained in Ethiopia. Wentzensen et al. reported up to 14 HPV types from a single cervical specimen although they did not observe type interactions among multiple HPVs [68]. In the present study, we noted multiple HR-HPV infections without the development of high-grade lesions. For instance, infections with HPV31, 52, 66, and infections with HPV31, 52, 39, 59, and 68 were not found to be associated with any form of high-grade cervical lesions. The role of multiple HR-HPV infections in the development of an advanced form of cervical lesions including the potential efficacy of HPV vaccines on such infections warrants further research in Ethiopia [30].
There are contradicting reports about the role of multiple HR-HPV infections in cervical carcinogenesis. Adcock and his colleague reported that HPV genotype and viral load, but not a multiplicity of HPV infections are important predictors of high-grade lesions including cervical cancer [69]. In contrast, a recently published study (2021) by Kim et al. showed that multiple HPV infections were found significantly associated with high-grade cervical lesions compared to infections with single HPV types. Furthermore, according to Kim et al. report, patients with multiple HPV infections exhibited a persistent and longer duration of the infection compared to patients with a single HPV infection [70].
Concerning the identified HR-HPVs and histopathologic grades, all HPV16, HPV18, HPV35, and HPV45 were associated with either high-grade lesions (CIN2+) or cervical cancer in the present study. Likewise, 79 (80.6%), 7 (87.5%), 3 (75%), and 5 (100%) of HPV16, HPV18, HPV35, and HPV45 were respectively noted in histopathologically confirmed cervical cancers. Specifically, the detection of HPV16 was increasing with the degree of cervical lesions in which the proportion was 5 (12.5%), 2 (16.7%), 5 (41.7%), and 58 (85.3%) in Cervical intraepithelial neoplasia (CIN)1, CIN2, CIN3, and cancer, respectively. A statistically significant association between HPV16 and the progression of cervical lesions was reported by a previous study [71]. Similarly, in our study 11 (78.6%) HPV31&33 coinfections were recovered from cancer cases. A study showed that next to HPV16, the hierarchy of HPV types based on their carcinogenic potential was reported to be HPV33 followed by HPV31 [69]. Song et al. in China also discovered that the prevalence of HPV16 and HPV33 increased significantly with the degree of cervical lesions [67].
Finally, according to the latest WHO report (2022), a large proportion of cervical cancer cases (> 95%) are due to infection with HPV [18]. In our study, 9.7% (13/133) of cervical cancer cases were without HR-HPVs. A study in Belgium showed that up to 15% of cervical cancers were reported to be without HPV infection [72]. Additional study is required in this regard in Ethiopia.
Conclusions
This study provided a health facility-based estimate of HR-HPV infection of the uterine cervix and the common genotypes associated with the infection in northwest Ethiopia. More than half of the study participants tested positive for HR-HPV mainly infected with HPV16, HPV31 and HPV33. About 24% of cervical samples were found to be infected with two and more (up to five) multiple HR-HPV types. The HR-HPV proportion was higher among women aged above 50 years and HIV-positive once. Therefore, all forms of cervical cancer prevention strategies including multivalent HPV vaccination and screening should be expanded in the study area. Community-based similar studies with better HPV detection methods should be considered for a better appreciation of the HPVs circulating in northwest Ethiopia.
Availability of data and materials
The original data source could be shared upon the request of the principal investigator.
References
Bruni LAG, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. In: Summary report 22 October 2021; 2021.
WHO. Global strategy to accelerate the elimination of cervical cancer as a public health problem 2020. https://apps.who.int/iris/bitstream/handle/10665/336583/9789240014107-eng.pdf
Cancer WIAfRo. Cancer tomorrow: estimated number of cervical cancer incidence and mortality in Ethiopia 2020. https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=23&single_unit=500&populations=231&group_populations=1&multiple_populations=1&sexes=0&types=1&age_start=0
Santos-Lopez G, Marquez-Dominguez L, Reyes-Leyva J, Vallejo-Ruiz V. General aspects of structure, classification and replication of human papillomavirus. Rev Med Inst Mex Seguro Soc. 2015;53(2):S166–71.
WHO. Human papillomavirus (HPV) and cervical cancer 2016. http://www.who.int/mediacentre/factsheets/fs380/en/
Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 2017. 2017 http://www.hpvcentre.net/statistics/reports/XWX.pdf
Chow LT, Broker TR, Steinberg BM. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010;118(6–7):422–49.
Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;20(30):083.
Wallace NA, Galloway DA. Novel functions of the human papillomavirus E6 oncoproteins. Annu Rev Virol. 2015;2(1):403–23.
Centre IIICoHaCHI. Human Papillomavirus and Related Diseases Report 2017. http://www.hpvcentre.net/statistics/reports/XWX.pdf
Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–60.
Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ. HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Futur Virol. 2011;6(1):45–57.
Kajitani N, Satsuka A, Kawate A, Sakai H. Productive lifecycle of human papillomaviruses that depends upon squamous epithelial differentiation. Front Microbiol. 2012;3:152.
Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98(10):1505–11.
WHO. Human papil-lomavirus and related cancers in world. Summary Report 2010. 2010.
FMoH. Federal Ministry of Health: guideline for cervical cancer prevention and control in Ethiopia. 2013.
WHO. Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland 2013.
WHO. Cervical Cancer 2022. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
Derbie A, Mekonnen D, Nibret E, Maier M, Woldeamanuel Y, Abebe T. Human papillomavirus genotype distribution in Ethiopia: an updated systematic review. Virol J. 2022;19(1):13.
Health EMo. Expanded Program on Immunization (EPI) 2022. https://www.moh.gov.et/site/Expanded_Program_on_Immunization_%28EPI%29
Fitzpatrick MB, Hahn Z, Mandishora RSD, Dao J, Weber J, Huang C, et al. Whole-genome analysis of cervical human papillomavirus type 35 from rural Zimbabwean women. Sci Rep. 2020;10(1):7001.
Emeribe AU, Abdullahi IN, Etukudo MH, Isong IK, Emeribe AO, Nwofe JO, et al. The pattern of human papillomavirus infection and genotypes among Nigerian women from 1999 to 2019: a systematic review. Ann Med. 2021;53(1):944–59.
Leyh-Bannurah S-R, Prugger C, de Koning MNC, Goette H, Lellé RJ. Cervical human papillomavirus prevalence and genotype distribution among hybrid capture 2 positive women 15 to 64 years of age in the Gurage zone, rural Ethiopia. Infect Agents Cancer. 2014;9:33.
Fanta B. The distribution of Human Papilloma Virus infection in women with cervical histological abnormalities from an area with high incidence of cervical cancer. Ethiop Med J. 2005;43(3):151–8.
Abate E, Aseffa A, El-Tayeb M, El-Hassan I, Yamuah L, Mihret W, et al. Genotyping of human papillomavirus in paraffin embedded cervical tissue samples from women in ethiopia and the Sudan. J Med Virol. 2013;85(2):282–7.
Bekele A, Baay M, Mekonnen Z, Suleman S, Chatterjee S. Human papillomavirus type distribution among women with cervical pathology: a study over 4 years at Jimma Hospital, southwest Ethiopia. Trop Med Int Health. 2010;15(8):890–3.
Mihret W, Yusuf L, Abebe M, Yamuah LK, Bekele L, Abate E, et al. A pilot study on detection and genotyping of humanpapilloma virus isolated from clinically diagnosed Ethiopian women having cervical intraepithelial neoplasia. Ethiop Med J. 2014;1:49–52.
Eshetu K. Prevalence and genotype distribution of high risk human papilloma virus and cervical cytology abnormalities at selected obstetrics and gynecology clinics, in Addis Ababa. Ethiopia: Addis Ababa University; 2015.
Ruland R, Prugger C, Schiffer R, Regidor M, Lellé R. Prevalence of human papilloma virus infection in women in rural Ethiopia. Eur J Epidemiol. 2006;21(9):727–9.
Teka B, Gizaw M, Ruddies F, Addissie A, Chanyalew Z, Skof AS, et al. Population-based human papillomavirus infection and genotype distribution among women in rural areas of South Central Ethiopia. Int J Cancer. 2021;148(3):723–30.
Gebremeskel TA. Molecular Epidemiology of HPV in Northern Ethiopia. 2018.
Cancer.Net. Cervical Cancer: Symptoms and Signs 2017. https://www.cancer.net/cancer-types/cervical-cancer/symptoms-and-signs
Health FDRoEMo. Guideline for cervical cancer prevention and control in Ethiopia 2015. https://www.iccp-portal.org/system/files/plans/Guideline%20Eth%20Final.pdf
Tifaoui N, Maudelonde T, Combecal J, Vallo R, Doutre S, Didelot MN, et al. High-risk HPV detection and associated cervical lesions in a population of French menopausal women. J Clin Virol. 2018;108:12–8.
Wang Z, Wang J, Fan J, Zhao W, Yang X, Wu L, et al. Risk factors for cervical intraepithelial neoplasia and cervical cancer in Chinese women: large study in Jiexiu, Shanxi Province. China J Cancer. 2017;8(6):924–32.
Barchitta M, Maugeri A, Quattrocchi A, Agrifoglio O, Scalisi A, Agodi A. The association of dietary patterns with high-risk human papillomavirus infection and cervical cancer: a cross-sectional study in Italy. Nutrients. 2018;10(4):469.
Luhn P, Walker J, Schiffman M, Zuna RE, Dunn ST, Gold MA, et al. The role of co-factors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol. 2013;128(2):265–70.
WHO. Integrated Africa Cancer Factsheet Focusing on Cervical Cancer 2014. http://www.who.int/pmnch/media/events/2014/africa_cancer_factsheet.pdf
Niccolai LM, Meek JI, Brackney M, Hadler JL, Sosa LE, Weinberger DM. Declines in human papillomavirus (HPV)-associated high-grade cervical lesions after introduction of HPV vaccines in Connecticut, United States, 2008–2015. Clin Infect Dis. 2017;65(6):884–9.
Kambouris M, Chini V, Daskalaki A. HPV Detection and genotyping using the Luminex xMAP Technology 2010. http://www.irma-international.org/viewtitle/40440/
Dhillon SK, Oštrbenk Valenčak A, Xu L, Poljak M, Arbyn M. Clinical and analytical evaluation of the Alinity m HR HPV assay within the VALGENT-3 framework. J Clin Microbiol. 2021;59(6):e00286-e321.
Oštrbenk Valenčak A, Šterbenc A, Seme K, Poljak M. Alinity m HR HPV assay fulfills criteria for human papillomavirus test requirements in cervical cancer screening settings. J Clin Microbiol. 2019;58(1):e01120-19.
Abbott. ALINITY m HR HPV ASSAY 2022. https://www.molecular.abbott/int/en/products/infectious-disease/alinity-m-hr-hpv-assay
Jang D, Ratnam S, Smieja M, Speicher DJ, Arias M, Clavio A, et al. Comparison of alinity m HPV and cobas HPV assays on cervical specimens in diverse storage media. Tumour Virus Res. 2021;12:200224.
Abott. HR HPV AMP kit 2020. https://www.steinberg-partner.de/files/dr-steinberg/dokumente/downloads/PI_HR%20HPV%20AMP%20Kit.pdf
INNO-LiPA N.VI HPV Genotyping Extra 2011. https://search.cosmobio.co.jp/cosmo_search_p/search_gate2/docs/IGT_/8106381064.20130815.pdf
Tewari P, Banka P, Kernan N, Reynolds S, White C, Pilkington L, et al. Prevalence and concordance of oral HPV infections with cervical HPV infections in women referred to colposcopy with abnormal cytology. J Oral Pathol Med. 2021;50(7):692–9.
Ameya G, Yerakly F. Characteristics of cervical disease among symptomatic women with histopathological sample at Hawassa University referral hospital, Southern Ethiopia. BMC Womens Health. 2017;17(1):91.
Ardhaoui M, Ennaifer E, Letaief H, Salsabil R, Lassili T, Chahed K, et al. Prevalence, genotype distribution and risk factors for cervical human papillomavirus infection in the Grand Tunis Region, Tunisia. PLoS ONE. 2016;11(6):e0157432.
Wolday D, Derese M, Gebressellassie S, Tsegaye B, Ergete W, Gebrehiwot Y, et al. HPV genotype distribution among women with normal and abnormal cervical cytology presenting in a tertiary gynecology referral Clinic in Ethiopia. Infect Agent Cancer. 2018;13(28):018–0201.
Kuassi-Kpede AP, Dolou E, Zohoncon TM, Traore IMA, Katawa G, Ouedraogo RA, et al. Molecular characterization of high-risk human papillomavirus (HR-HPV) in women in Lomé, Togo. BMC Infect Dis. 2021;21(1):278.
Kuguyo O, Dube Mandishora RS, Thomford NE, Makunike-Mutasa R, Nhachi CFB, Matimba A, et al. High-risk HPV genotypes in Zimbabwean women with cervical cancer: comparative analyses between HIV-negative and HIV-positive women. PLoS ONE. 2021;16(9):e0257324.
Sweet K, Bosire C, Sanusi B, Sherrod CJ, Kwatampora J, Waweru W, et al. Prevalence, incidence, and distribution of human papillomavirus types in female sex workers in Kenya. Int J STD AIDS. 2020;31(2):109–18.
Jeudin P, Abebe T, Butler R, Hooi D, Watt A, Capo-Chichi CD, et al. Human papilloma virus distribution across the African diaspora. JCO Glob Oncol. 2021;7:1206–8.
Tagne Simo R, Djoko Nono AG, Fogang Dongmo HP, Seke Etet PF, Fonyuy BK, Kamdje AHN, et al. Prevalence of precancerous cervical lesions and high-risk human papillomavirus types in Yaounde. Cameroon J Infect Dev Ctries. 2021;15(9):1339–45.
Dolou E, Kuassi-Kpede A, Zohoncon TM, Traore IM, Katawa G, Ouedraogo AR, et al. Molecular characterization of high-risk human papillomavirus genotypes in women with or without cervical lesions at VIA/VILI in Kara. Togo African Health Sci. 2021;21(4):1715–21.
Ogembo RK, Gona PN, Seymour AJ, Park HS, Bain PA, Maranda L, et al. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. PLoS One. 2015;10(4):e122488.
Peng RR, Li HM, Chang H, Li JH, Wang AL, Chen XS. Prevalence and genotype distribution of cervical human papillomavirus infection among female sex workers in Asia: a systematic literature review and meta-analysis. Sex Health. 2012;9(2):113–9.
Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1157–64.
Ciapponi A, Bardach A, Glujovsky D, Gibbons L, Picconi MA. Type-specific HPV prevalence in cervical cancer and high-grade lesions in Latin America and the Caribbean: systematic review and meta-analysis. PLoS ONE. 2011;6(10):4.
Conesa-Zamora P, Ortiz-Reina S, Moya-Biosca J, Doménech-Peris A, Orantes-Casado FJ, Pérez-Guillermo M, et al. Genotype distribution of human papillomavirus (HPV) and co-infections in cervical cytologic specimens from two outpatient gynecological clinics in a region of southeast Spain. BMC Infect Dis. 2009;9(1):124.
González-Yebra B, Mojica-Larrea M, Alonso R, González AL, Romero-Morelos P, Taniguchi-Ponciano K, et al. HPV infection profile in cervical lesions. Gac Med Mex. 2022;158(4):222–8.
Sehnal B, Sláma J. What next in cervical cancer screening? Ceska Gynekol. 2020;85(4):236–43.
World Health O. Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Health Organization; 2020. p. 2020.
Wondimu A, Postma MJ, van Hulst M. Cost-effectiveness analysis of quadrivalent and nonavalent human papillomavirus vaccines in Ethiopia. Vaccine. 2022.
Bello BD, Spinillo A, Alberizzi P, Cesari S, Gardella B, D’Ambrosio G, et al. Cervical infections by multiple human papillomavirus (HPV) genotypes: prevalence and impact on the risk of precancerous epithelial lesions. J Med Virol. 2009;81(4):703–12.
Song L, Lyu Y, Ding L, Li X, Gao W, Wang M, et al. Prevalence and genotype distribution of high-risk human papillomavirus infection in women with abnormal cervical cytology: a population-based study in Shanxi Province. China Cancer Manag Res. 2020;12:12583–91.
Wentzensen N, Schiffman M, Dunn T, Zuna RE, Gold MA, Allen RA, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125(9):2151–8.
Adcock R, Cuzick J, Hunt WC, McDonald RM, Wheeler CM. Role of HPV Genotype, Multiple Infections, and Viral Load on the Risk of High-Grade Cervical Neoplasia. Cancer Epidemiol Biomark Prevent. 2019;28(11):1816–24.
Kim M, Park NJ-Y, Jeong JY, Park JY. Multiple human papilloma virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses. 2021;13(7):1342.
Joharinia N, Farhadi A, Hosseini SY, Safaei A, Sarvari J. Association of HPV16 and 18 genomic copies with histological grades of cervical lesions. Virusdisease. 2019;30(3):387–93.
Tjalma W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis Obgyn. 2018;10(2):107–13.
Acknowledgements
Authors would like to thank the study participants and the data collectors.
Funding
This research was partially supported by Addis Ababa University and the Institute of Biotechnology, Bahir Dar University, and received funding from the Centre for Innovative Drug Development & Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University.
Author information
Authors and Affiliations
Contributions
AD, TA, and YW designed the study protocol. AD and MM carried out the molecular laboratory analysis. EM participated in cervical sample collection. Likewise, BA involved in the histopathological examination of cervical biopsies. AD performed the data analysis and drafted the manuscript. EM, EN, MM, LU, YW, and TA critically reviewed the manuscript. All authors read and approved the final version of the manuscript for submission to a journal.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the institutional review board (IRB) of the College of Health Sciences, Addis Ababa University (Protocol number: 087/19/DMIP) and by the Ethiopian National Research Ethics Review Committee at the Ministry of Education (Ref number: 7/2-149/m259/35). Written informed consent was ensured from all study participants to take part in the study voluntarily after they get informed about the objective and purpose of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Derbie, A., Maier, M., Amare, B. et al. High-risk human papillomavirus genotype distribution among women with gynecology complaints in northwest Ethiopia. Infect Agents Cancer 18, 4 (2023). https://doi.org/10.1186/s13027-023-00481-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-023-00481-3