Abstract
Background
Very little is known about the characteristics of echocardiographic abnormalities and joint hypermobility in Chinese patients with osteogenesis imperfecta (OI). The aim of our study was to investigate the characteristics, prevalence and correlation of echocardiographic abnormalities and joint hypermobility in Chinese patients with OI.
Methods
A cross-sectional comparative study was conducted in pediatric and adult OI patients who were matched in age and sex with healthy controls. Transthoracic echocardiography was performed in all patients and controls, and parameters were indexed for body surface area (BSA). The Beighton score was used to evaluate the degree of joint hypermobility.
Results
A total of 48 patients with OI (25 juveniles and 23 adults) and 129 age- and sex-matched healthy controls (79 juveniles and 50 adults) were studied. Four genes (COL1A1, COL1A2, IFITM5, and WNT1) and 39 different mutation loci were identified in our study. Mild valvular regurgitation was the most common cardiac abnormality: mild mitral and tricuspid regurgitation was found in 12% and 36% of pediatric OI patients, respectively; among 23 OI adults, 13% and 17% of patients had mild mitral and tricuspid regurgitation, respectively, and 4% had mild aortic regurgitation. In multiple regression analysis, OI was the key predictor of left atrium diameter (LAD) (β=-3.670, P < 0.001) and fractional shortening (FS) (β = 3.005, P = 0.037) in juveniles, whereas for adults, OI was a significant predictor of LAD (β=-3.621, P < 0.001) and left ventricular mass (LVM) (β = 58.928, P < 0.001). The percentages of generalized joint hypermobility in OI juveniles and adults were 56% and 20%, respectively. Additionally, only in the OI juvenile group did the results of the Mann‒Whitney U test show that the degree of joint hypermobility was significantly different between the echocardiographic normal and abnormal groups (P = 0.004).
Conclusions
Mild valvular regurgitation was the most common cardiac abnormality in both OI juveniles and adults. Compared with OI adults, OI juveniles had more prevalent and wider joint hypermobility. Echocardiographic abnormalities may imply that the impairment of type I collagen is more serious in OI. Baseline echocardiography should be performed in OI patients as early as possible.
Similar content being viewed by others
Introduction
Osteogenesis imperfecta (OI) is a clinically and genetically heterogeneous group of hereditary bone disorders [1]. In OI, approximately 85-90% of autosomal dominant disorders are caused by mutations in the COL1A1 and COL1A2 genes, which encode the α1(I) and α2(I) chains of type I collagen [2]. Since the first identification of the gene resulting in recessive OI in 2006, over 20 different genes involved in the biosynthesis of type I collagen to cause OI have been reported [3]; therefore, currently, OI is widely accepted as a collagen-related disease [2]. According to data from Europe and the United States, the incidence of OI is approximately 0.3–0.7 per 10,000 births, and the number varies in different regions [4].
The severity of OI ranges from individuals with a few fractures to some with severe skeletal deformities and perinatal lethality [5]. Type I collagen is the major protein component of the extracellular matrix in bone, skin, tendon, heart, and so on; therefore, extra-skeletal manifestations such as blue sclera, dentinogenesis imperfecta, hearing impairment, ligamentous laxity, and cardiovascular abnormalities are also common in patients [4, 6]. Most of the research on OI undertaken to date has focused on the characteristics of bone mineral density (BMD) and fracture rates, as well as related therapies; however, these indicators fail to adequately reflect the patient’s health status and quality of life [7]. A study of the mortality of patients with OI in Denmark has shown that diseases of the circulatory system were the most common cause of death (2.9%) [8]. Type I collagen covers approximately 74% of the collagen content of the mitral valve [9]. The aorta and most arteries contain abundant type III and type I collagen [10]. Preliminary experimental evidence based on OI mouse models has suggested that mutations in COL1A1 or COL1A2 will increase the risk of cardiovascular diseases. Previous clinical studies [11,12,13,14] have also demonstrated that patients with OI have an increased risk of heart disease compared to healthy controls, and valvular diseases and widened aortic diameter were the most frequently reported disorders. However, very little is known about the clinical characteristics of the cardiovascular system in OI, and limited attention has been given to cardiovascular screening of patients with OI in China.
Joint hypermobility is the range of movement exceeding the normal level considering age, sex, and ethnic background [15]. Ligamentous laxity is the primary cause of hypermobility. The Beighton score, an easy-to-use scoring system to measure joint hypermobility, is currently the most widely used method for assessing the presence of generalized joint hypermobility [16]. The varying degrees of joint hypermobility in different types of EDS, OI and Marfan syndrome form a heterogeneous group of hereditary connective tissue disorders [17]. Joint hypermobility is a common characteristic of patients with OI [18], which may increase the risk of falls and fractures. EDS is characterized by joint hypermobility, soft and hyperextensible skin, and abnormal scar formation after injury [19]. Recently, patients with overlapping phenotypes of OI and EDS have been found to harbor heterozygous pathogenic variants in COL1A1 or COL1A2 [20, 21], and Morlino et al. proposed the term “COL1-related overlap disorder (C1ROD)” to consider a wide spectrum of bridging phenotypes between OI and EDS [22]. The variants found in C1ROD were mainly located in the N-terminal helical region, whereas some variants were also reported to be located outside the region [23].
Very little is known about the characteristics of structural cardiovascular abnormalities and joint hypermobility in Chinese patients with OI. The primary objective of this cross-sectional study is to investigate the characteristics and prevalence of echocardiographic abnormalities and joint hypermobility in Chinese patients with OI. We also analyzed the association between these two phenotypes in our cohort.
Materials and methods
Subjects
This was a single-center cross-sectional study of patients with OI consulted at the Department of Osteoporosis and Bone Disease of Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine from June 2021 to October 2022. Patients who met all these criteria were included: (1) confirmed with OI-related gene variants, (2) underwent clinical evaluation, (3) underwent echocardiography, and (4) without hypertension or other chronic cardiovascular diseases. Individuals matched for age and sex with no history of cardiovascular disease, hypertension, or diabetes were voluntarily recruited for echocardiography as normal controls. The Ethics Committee of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine approved the study. Written informed consent was obtained from patients or legal guardians of juveniles younger than 18.
Clinical evaluation
Clinical information, including age, sex, height, weight, fracture history, non-skeletal features (such as blue sclera, dentinogenesis imperfecta, and hearing loss), typical cardiovascular symptoms (such as chest pain, dyspnea, palpitations, and syncope), and medical history were collected. Body surface area (BSA) was calculated using the Mosteller formula [24]. Blood pressure was measured with an OMRON U16 (OMRON HEALTHCARE Co. Ltd.) electronic sphygmomanometer. Hypertension was defined when systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg was measured or when the participants were administered antihypertensive medication. In addition, X-ray radiography of the upper and lower extremities and thoracolumbar vertebrae were examined. Patients were typed 1 to 5 according to the Nosology of Genetic Skeletal Disorders: 2023 Revision [25].
Echocardiographic examination
Transthoracic echocardiography was performed with Philips EPIQ 7 C. The echocardiography protocol was performed by two experienced cardiologists at the same time, and the echocardiograms were interpreted by experienced cardiologists. Atrial, ventricular, valvular, and aortic measurements were performed according to the guidelines of the American Society of Echocardiography [26]. Two-dimensional measurements, M-mode measurements, and Doppler ultrasonography were used to measure aorta diameter (AD), left atrium diameter (LAD), interventricular septum thickness (IVST), left ventricular posterior wall thickness (LVPWT), left ventricular end-diastolic diameter (LVEDd), left ventricular end-systolic diameter (LVESd), left ventricular ejection fraction (LVEF), fractional shortening (FS), and valvular regurgitation. Left ventricular mass (LVM) was calculated according to the American Society of Echocardiography’s (ASE)-recommended formula [26]. Echocardiographic parameters can be influenced by BSA, so the measurements were divided by BSA according to the recommendations of the ASE [26]. All detectable regurgitations were recorded and divided into mild, moderate, or severe [27]. Based on the echocardiographic reports, patients were divided into two subgroups: (1) normal and (2) abnormal (including but not limited to valvular regurgitation, atrioventricular enlargement, ventricular septal thickening, and ascending aortic dilatation).
Joint Hypermobility Assessment
The presence or absence of joint hypermobility and severity were assessed by the smae experienced physician to minimize variability. The Beighton scoring system was used to evaluate the joint hypermobility severity [16]. A score of four or higher was considered a positive result for generalized joint hypermobility. According to the physical examination and Beighton score, we divided the results of the joint hypermobility assessment into three subgroups: (1) normal (absence of joint hypermobility); (2) peripheral joint hypermobility (mainly hands); and (3) generalized joint hypermobility (Beighton score ≥ 4) [28].
Detection of gene mutations
Genomic DNA was extracted from the peripheral blood of all patients by standard techniques using a DNA extraction kit (Lifefeng Biotech, Shanghai). Exons and exon‒intron boundaries of COL1A1, COL1A2, IFITM5, and WNT1 genes were amplified by polymerase chain reaction (PCR) (GenBank accession NO. NC_000017.11, NO. NC_000007.14, NO.NC_000011.10, and NO.NC_000012.12). Primers were designed by Web-based Primer 3 software (https://bioinfo.ut.ee/primer3-0.4.0/). Direct sequencing was performed using BigDye Terminator Cycle Sequencing Ready Reaction Kit, v. 3.1 (Applied Biosystems, California, USA). The products were analyzed with an ABI 3730 sequencer (Foster, CA, United States). SNPs were identified using Polyphred (https://droog.gs.washington.edu/polyphred/). The results absent from the LOVD Database (https://www.lovd.nl/) were regarded as novel variants.
Statistical analysis
Continuous variables were expressed as the means ± SDs. Total counts and percentages were reported for categorical variables. The chi-square test was used to assess the differences in proportions between OI and controls. Group differences in continuous variables between OI patients and controls were completed by independent-sample t tests. Simple and multiple linear regression models were used to investigate the impacts of possible variables on echocardiographic parameters. The Mann‒Whitney U test was applied to analyze the correlation between cardiovascular abnormalities and joint hypermobility. Two-tailed P values less than 0.05 were considered statistically significant. SPSS 26.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis.
Results
Baseline characteristics and genotypes of the subjects
In all, 48 patients with OI (25 juveniles and 23 adults) and 129 age- and sex-matched healthy controls (79 juveniles and 50 adults) were studied. Twenty-one patients with OI were reported in our previous study [29]. Baseline characteristics are shown in Table 1. For juveniles, the mean age of OI patients was 11.0 ± 4.4 years when receiving echocardiographic examinations, and 18 (72%) patients were boys; the control group had 42 boys and 37 girls, with a mean age of 12.6 ± 3.5 years. For adults, the mean ages of OI patients and healthy controls were 39.54 ± 12.18 years and 41.00 ± 10.75 years, respectively; there were 6 (26%) and 25 (50%) males in the OI and control groups, respectively. The two OI groups had significantly lower BSA than the control individuals, 1.3 ± 0.5 VS 1.5 ± 0.4 m2 in juveniles (P = 0.013), and 1.5 ± 0.2 VS 1.7 ± 0.2 m2 in adults (P < 0.001), respectively. There was no difference in SBP and DBP between the OI groups and controls. Among the 48 patients with OI, only two adult patients experienced cardiac disease-related symptoms or treatments: one patient underwent aortic valve prosthetic replacement when she was 25 years old because of a congenital aortic valve defect, and the other had episodic palpitation.
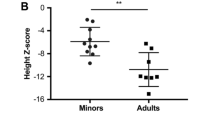
Skeletal and extra-skeletal manifestations of patients with OI are displayed in Table 1. Two (8%) juveniles and 3 (13%) adults with OI had scoliosis. Both lumbar spine and femoral neck BMD values were low in juvenile and adult patients with OI: the lumbar spine BMD Z score was − 1.80 ± 1.87 in juveniles and − 2.20 ± 1.07 in adults, and the femoral neck BMD Z score was − 3.00 ± 1.48 in juveniles and − 1.34 ± 0.96 in adults. Blue sclera was a very common extra-skeletal manifestation in the two OI groups, and hearing loss was much more frequent in adults than in juveniles with OI. According to the classification, there were 15 juvenile patients with OI type 1, 1 with type 3, 8 with type 4, and 1 with type 5; in adults, type 1 included 20 patients, type 4 contained 2, and type 5 involved 1.
A total of four genes (COL1A1, COL1A2, IFITM5, and WNT1) and 39 different mutation loci were identified in our study. Thirty patients carried COL1A1 gene mutations, including 11 nonsense mutations, 9 missense mutations, 8 frameshift mutations, one splice mutation, and one gross deletion (chr17:48262862–48,268,851, approximately 5.99 kb). Sixteen patients harbored COL1A2 gene mutations, including 14 missense mutations and 2 splice mutations. A pathogenic variant in IFITM5 was detected in two patients, and one boy carried WNT1 compound heterozygous variants. Three variants (c.1865dupC and a gross deletion in COL1A1, c.1649G > C in COL1A2) absent in the LOVD Database (https://www.lovd.nl/) were novel variants.
Echocardiographic measurements of the subjects
Mild mitral regurgitation was found in 12% and mild tricuspid regurgitation in 36% of patients in the OI juvenile group. Among 23 OI adults, 13% and 17% of patients had mild mitral and tricuspid regurgitation, respectively, 4% had mild aortic regurgitation, 4% had left atrium enlargement and increased pulmonary artery pressure, respectively, and only one (4%) had experienced aortic valve replacement. Detailed information on the patients mentioned above is displayed in Supplementary Table 1.
Echocardiographic parameters in OI patients and controls and their comparisons are shown in Table 2. There was no difference between the OI and control groups regarding echocardiographic parameters, except for AD and LVM, which were much larger in control adults than in OI adults. However, when corrected for BSA, AD, LAD, IVST, LVEDd, LVESd, and FS became significantly higher in the OI juveniles than in the control juveniles. AD, LAD, IVST, LVPWT, LVEDd, LVESd, LVEF, and FS became significantly larger in the OI adults than in the control adults when corrected for BSA.
Bold P values indicate significance.
Simple regression analysis of echocardiographic parameters in juveniles showed that age, sex, BSA, SBP, and DBP were significant predictors of AD, LAD, IVST, LVPWT, LVEDd, LVESd, and LVM (except for IVST and LVPWT); OI, however, was the only significant predictor of LVPWT (Table 3A). In adults, sex and BSA were the significant predictors of AD, LAD, IVST, LVEDd, LVESd, and LVM (except sex for LAD and IVST) displayed by simple regression analysis. Age was the key predictor of AD, LAD, LVEF, and FS, whereas OI was an important predictor of AD and LVM (Table 3B).
Multiple regression analysis of echocardiographic parameters in juveniles showed that age and BSA were significant predictors of AD, IVST, and LVPWT among juveniles; sex and BSA were key predictors of LVEDd and LVESd; and BSA and OI were important predictors of LAD and FS (Table 4A). For adults, age was an important predictor of AD, LAD, FS, and LVM, whereas sex was the only significant predictor of LAD; BSA was a significant predictor of AD, LAD, LVPWT, LVEDd, LVESd, and LVM, and OI was a significant predictor of LAD and LVM (Table 4B).
Joint hypermobility in OI and its correlation with cardiovascular abnormalities
Joint hypermobility was assessed in 18 of 25 juveniles with OI and two (11%) juveniles did not fulfill the criteria for a diagnosis of joint hypermobility, 6 (33%) had peripheral joint hypermobility, and 10 (56%) had generalized joint hypermobility. Among 23 OI adults, 20 underwent the joint hypermobility assessment: 8 (40%) adults did not have joint hypermobility, 8 (40%) had peripheral joint hypermobility, and 4 (20%) had generalized joint hypermobility.
In the OI juvenile group, the results of the Mann‒Whitney U test showed that the degree of joint hypermobility was significantly different between the echocardiographic normal and abnormal groups (P = 0.004) (Table 5A). Joint hypermobility had a wider impact on the echocardiographic abnormal group with OI. However, this was not found in the OI adult group (P = 0.153) (Table 5B).
Discussion
In the present study, it should be noted that two groups of juveniles or adults were carefully matched by investigators so that they were comparable in age, sex, race, and blood pressure. As BSA has turned out to be an important factor in cardiac structural size, the ASE recommended correcting parameters by dividing by BSA [26]. By enrolling 25 juveniles and 23 adults with OI, we investigated the characteristics of cardiovascular abnormalities and joint hypermobility in Chinese patients with OI and analyzed the correlation between these two phenotypes in our cohort. Our results mainly suggest that mild valve regurgitation was the most common cardiovascular abnormality in both juveniles and adults with OI. Compared with healthy controls, some echocardiographic parameters, such as AD, LAD and IVST, were much larger and LVEF and LVM were significantly smaller in patients with OI only if corrected for BSA. In addition, joint hypermobility was more common in juveniles than in adults, and we also found that joint hypermobility had a wider impact on the echocardiographic abnormal group in juveniles with OI.
The cardiovascular connective tissues of the heart valves, aortic wall and heart chambers have a high concentration of type I collagen [9, 30]. Cardiac disorders are often associated with an accumulation, depletion, or restructuring of the collagen matrix [30]. Type I collagen is a heterotrimer composed of two α1 and one α2 chain that are assembled into a triple helix [4]. Most of the patients with OI result from structural or quantitative defects in the COL1A1 or COL1A2 genes that encode the α1 and α2 chains of collagen type I [2]. Preliminary experimental evidence based on OI mouse models has suggested that mutations in COL1A1 or COL1A2 will increase the risk of cardiovascular diseases. Weis and his colleagues revealed that [31] Col1a2 mutation in the oim model produces significant alternations in the structural and mechanical properties of the ventricular myocardium, and a mild increase in LV wall thickness may represent compensation for reduced collagen. In addition, Pfeiffer et al. [32] researched the role of α2(I) collagen in aortic integrity in the oim model and suggested that the reduced thoracic aortic integrity was associated with the absence of the α2(I) collagen chains and a reduced collagen content. Based on the OI murine model Aga2, Thiele et al. [33] found that the downregulation of Col1a1 transcripts caused a loss of extracellular matrix integrity and cardiopulmonary dysfunction.
Three previous BSA-matched studies [13, 34, 35] suggested that echocardiographic parameters were much larger (such as AD, LAD, IVST, LVEDd, and LVESd) or smaller (including LVEF and LVM) in OI than in controls. Another BSA-unmatched study [36] also revealed the same results, specifically when some parameters were indexed for BSA. The research mentioned above showed that LVEF or LVEF/BSA was much smaller in patients with OI, which means that cardiac function was impaired in OI. In our study, however, the corrected LVEF was larger in OI adults than in controls. A study in 58 children with OI found no cardiac abnormalities in type I patients, but cardiac alternations detected in type III [37]. Hence, we speculated the divergence of our study was attributed to the majority of our patients were clinical type 1, the insufficient sample size of our OI cohort, and the variations in the genetic backgrounds of different ethnic groups. Parameters such as AD, LAD, and IVST were significantly larger in patients with OI, implying compensation for reduced collagen, as verified by Weis [31]. Valvular regurgitation may occur when leaflets are thin, compliant, and slightly deformed [38]. Aortic root dilation and mitral regurgitation are the most commonly reported echocardiographic abnormalities in OI [39]. Radunovic et al. [36] studied the echocardiography of 99 adults with OI and found that 57.5% of patients had mild mitral regurgitation, 7.1% had moderate mitral regurgitation, 10.1% had mild aortic valve regurgitation, and 10.1% had moderate aortic valve regurgitation. In our study, mild mitral and tricuspid regurgitation were the most common abnormalities, especially the tricuspid regurgitation, whereas mild aortic valve regurgitation was only shown in OI adults. Two previous studies [14, 40] revealed that the mean arterial pressure (MAP) was much higher in OI groups than in controls. Radunovic et al. [36] also reported that approximately one-third of adult patients with OI had hypertension. Because of the lack of large-scale studies, however, there is still little understanding of hypertension prevalence in adults with OI. In our study, we excluded patients with hypertension as it may interfere with the assessment of echocardiography results. A 36-year-old female was the only patient in our study who had received cardiac surgery because of a congenital aortic valve defect when she was 25 years old. There was a lack of cardiac-related symptoms in her previous 25 years, and she even gave birth to a baby safely when she was 20 years old.
By multiple regression analysis in our study, age, sex and BSA were the main significant predictors of echocardiographic parameters, whereas OI was an important predictor only for LAD and FS in juveniles and for LAD and LVM in adults. Radunovic et al. [36] showed that SBP was also a significant predictor of some echocardiographic parameters in adults in multiple regression analysis. However, in our study, SBP and DBP showed significant impacts only in simple regression analysis. Zhao et al. [34] also researched the correlation between cardiovascular alterations and genotypes in OI through multiple regression analysis, and they showed that COL1A1 mutation and defects in type I collagen were key predictors of cardiovascular abnormalities. They speculated that the difference of echocardiographic abnormalities might be correlated to the different changes in the structure and metabolism of type I collagen induced by various pathogenic gene mutations. In our study, most patients with echocardiographic abnormalities carried triple-helical structure change of type I collagen. However, we still need larger samples to test the effect of different genotypes on echocardiographic parameters.
Joint hypermobility is a shared feature of a group of genetic disorders affecting connective tissue matrix proteins, classically including EDS, Marfan syndrome, and OI [16]. Jansson et al. [41] revealed that females tend to be laxer than males and that the degrees of laxity decrease with age. In the European pediatric population, ligamentous laxity is considered to be present in 5–35% of children [42]. Arponen et al. [18] reported that joint hypermobility was found in 70% of pediatric OI patients, which was consistent with the research of Brizola et al. [43], who revealed joint hypermobility in 58–100% of pediatric OI patients. In our study, generalized joint hypermobility was 56% and 20% in OI juveniles and adults, respectively, which was similar to previous findings. Two patients in our study met the diagnostic criteria of C1ROD [22], who had blue sclera, generalized joint hypermobility, significantly soft and hyperextensible skin, atrophic scars and fracture history. The pathogenic variants were COL1A1 (p. Arg1026*) and COL1A2 (p. Cys1195Arg), respectively. In our study, we also investigated the correlation between cardiovascular abnormalities and the degree of joint hypermobility. Our results showed that joint hypermobility has a wider impact when echocardiography is abnormal in pediatric OI patients, which may suggest that type I collagen impairment is more severe when OI patients have abnormal cardiac manifestations, at the same time resulting in generalized ligament laxity. Radunovic et al. [36] showed that the same LV changes seem to be more pronounced in the OI type III group. A study of 58 OI children found no cardiac abnormalities in type I OI patients; however, cardiac alternations were detected in type III OI children [37]. In our study, among 9 juvenile patients with OI who had cardiovascular abnormalities, 6/9 patients were clinical type 3–4 (moderate to severe), which suggests that they had relatively more severe phenotypes. The results of these studies can strengthen our hypothesis. This finding also suggests that when doctors notice that a patient with OI who has prominent joint hypermobility, they should be alert for cardiovascular abnormalities. Larger samples and further research are needed to investigate the correlation.
Although preliminary studies and our research have demonstrated that most echocardiographic abnormalities are mild, asymptomatic and without the need for medical intervention, we still recommend that OI patients receive baseline echocardiography as early as possible. Like the female we mentioned above in our study who had a congenital aortic valve defect but not identified until her 25 years, even though it was lucky for her to be pregnant without accidents, as a doctor, we mustn’t gamble with patients’ luck. Joint hypermobility is a common extra-skeletal manifestation of OI, especially for juveniles, which may increase the risk of injury and fractures. On the other hand, there are some positive factors of hypermobility for artists and athletes that should be mentioned; for instance, dancers, violinists, pianists and gymnasts with lax finger and/or wrist joints can suffer less pain than their less flexible peers [44]. The female with the IFITM5 mutation in our study said that she showed an advantage in playing the violin compared with her classmates when she was a young girl.
The samples in our study contained not only juveniles but also adults, which can add more real-world data to the relevant research. More importantly, we investigated the correlation between echocardiographic abnormalities and joint hypermobility in patients with OI for the first time. However, there are still some limitations to our study that must be considered. First, although we have studied the characteristics of echocardiography and joint hypermobility both in pediatric and adult OI patients, our study sample is not large enough to investigate the correlation between OI genotypes and echocardiographic parameters. Second, longitudinal data on echocardiography and the degree of joint hypermobility are scarce in our study, which prevents us from making concrete recommendations for cardiovascular surveillance. Third, due to the objective constraints of our study, we were unable to compare the degree of joint hypermobility between OI patients and controls.
Conclusion
Our study mainly showed that mild valve regurgitation was the most common cardiovascular abnormality in both OI juveniles and OI adults. The differences in corrected echocardiographic parameters between OI and controls suggested that cardiac structure was damaged in OI. Joint hypermobility was more prevalent in juveniles than in adults. When echocardiography was abnormal, joint hypermobility had a wider impact on pediatric OI patients, which means that echocardiographic abnormalities may imply that the impairment of type I collage is more severe in OI. Although medical and surgical interventions were rarely needed in our study, we still recommend performing baseline echocardiographic assessments as early as possible among OI patients, and patients with cardiovascular abnormalities should be followed up intensively.
Data availability
All data generated or analyzed during this study are included in the article, and further inquiries can be directed to the corresponding author.
Abbreviations
- OI:
-
osteogenesis imperfecta
- BMD:
-
bone mineral density
- EDS:
-
Ehlers‒Danlos syndrome
- C1ROD:
-
COL1-related overlap disorder
- SBP:
-
systolic blood pressure
- DBP:
-
diastolic blood pressure
- AD:
-
aortic diameter
- LAD:
-
left atrium diameter
- IVST:
-
interventricular septum thickness
- LVPWT:
-
left ventricular posterior wall thickness
- LVEDd:
-
left ventricular end-diastolic diameter
- LVESd:
-
left ventricular end-systolic diameter
- LVEF:
-
left ventricular ejection fraction
- FS:
-
fractional shortening
- LVM:
-
Left ventricular mass
References
Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28(3):209–21.
Forlino A, Marini JC, Osteogenesis imperfecta. Lancet. 2016;387(10028):1657–71. https://doi.org/10.1016/S0140-6736(15)00728-X.
Zhytnik L, Simm K, Salumets A, Peters M, Märtson A, Maasalu K. Reproductive options for families at risk of Osteogenesis Imperfecta: a review. Orphanet J Rare Dis. 2020;15(1):128. https://doi.org/10.1186/s13023-020-01404-w.
Marini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, Paepe AD, et al. Osteogenesis Imperfecta. Nat Rev Dis Primers. 2017;3:17052. https://doi.org/10.1038/nrdp.2017.52.
Kruger KM, Caudill A, Rodriguez Celin M, Nagamani SCS, Shapiro JR, Steiner RD, et al. Mobility in osteogenesis imperfecta: a multicenter north American study. Genetics in Medicine. Official J Am Coll Med Genet. 2019;21(10):2311–8. https://doi.org/10.1038/s41436-019-0491-4.
Folkestad L, Hald JD, Gram J, Langdahl BL, Hermann AP, Diederichsen AC, et al. Cardiovascular disease in patients with osteogenesis imperfecta - a nationwide, register-based cohort study. Int J Cardiol. 2016;225:250–7. https://doi.org/10.1016/j.ijcard.2016.09.107.
Murali CN, Cuthbertson D, Slater B, Nguyen D, Turner A, Harris G, et al. Pediatric Outcomes Data Collection Instrument is a useful patient-reported outcome measure for physical function in children with Osteogenesis Imperfecta. Genetics. Medicine: Official J Am Coll Med Genet. 2020;22(3):581–9. https://doi.org/10.1038/s41436-019-0688-6.
Folkestad L, Hald JD, Canudas-Romo V, Gram J, Hermann AP, Langdahl B, et al. Mortality and causes of death in patients with Osteogenesis Imperfecta: a Register-based Nationwide Cohort Study. J Bone Min Res. 2016;31(12):2159–66. https://doi.org/10.1002/jbmr.2895.
Cole WG, Chan D, Hickey AJ, Wilcken DE. Collagen composition of normal and myxomatous human mitral heart valves. Biochem J. 1984;219(2):451–60.
Vouyouka AG, Pfeiffer BJ, Liem TK, Taylor TA, Mudaliar J, Phillips CL. The role of type I collagen in aortic wall strength with a homotrimeric. J Vasc Surg. 2001;33(6):1263–70.
Folkestad L. Mortality and morbidity in patients with osteogenesis imperfecta in Denmark. Dan Med J. 2018;65(4).
Ashournia H, Johansen FT, Folkestad L, Diederichsen ACP, Brixen K. Heart disease in patients with osteogenesis imperfecta - A systematic review. Int J Cardiol. 2015;196:149–57. https://doi.org/10.1016/j.ijcard.2015.06.001.
Rush ET, Li L, Goodwin JL, Kreikemeier RM, Craft M, Danford DA, et al. Echocardiographic phenotype in osteogenesis imperfecta varies with disease severity. Heart. 2017;103(6):443–8. https://doi.org/10.1136/heartjnl-2016-310099.
Radunovic Z, Steine K. Prevalence of Cardiovascular Disease and Cardiac symptoms: left and right ventricular function in adults with Osteogenesis Imperfecta. Can J Cardiol. 2015;31(11):1386–92. https://doi.org/10.1016/j.cjca.2015.04.016.
Grahame R. Joint hypermobility and genetic collagen disorders: are they related? Arch Dis Child. 1999;80(2):188–91.
Hakim A, Grahame R. Joint hypermobility. Best Pract Res Clin Rheumatol. 2003;17(6).
Grahame R. Heritable disorders of connective tissue. Baillieres Best Pract Res Clin Rheumatol. 2000;14(2):345–61.
Arponen H, Mäkitie O, Waltimo-Sirén J. Association between joint hypermobility, scoliosis, and cranial base anomalies in paediatric Osteogenesis Imperfecta patients: a retrospective cross-sectional study. BMC Musculoskelet Disord. 2014;15:428. https://doi.org/10.1186/1471-2474-15-428.
Malfait F, Castori M, Francomano CA, Giunta C, Kosho T, Byers PH. The Ehlers-Danlos syndromes. Nat Rev Dis Primers. 2020;6(1):64. https://doi.org/10.1038/s41572-020-0194-9.
Malfait F, Symoens S, Goemans N, Gyftodimou Y, Holmberg E, López-González V, et al. Helical mutations in type I collagen that affect the processing of the amino-propeptide result in an Osteogenesis Imperfecta/Ehlers-Danlos syndrome overlap syndrome. Orphanet J Rare Dis. 2013;8:78. https://doi.org/10.1186/1750-1172-8-78.
Cabral WA, Makareeva E, Colige A, Letocha AD, Ty JM, Yeowell HN, et al. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J Biol Chem. 2005;280(19):19259–69.
Morlino S, Micale L, Ritelli M, Rohrbach M, Zoppi N, Vandersteen A, et al. COL1-related overlap disorder: a novel connective tissue disorder incorporating the osteogenesis imperfecta/Ehlers-Danlos syndrome overlap. Clin Genet. 2020;97(3):396–406. https://doi.org/10.1111/cge.13683.
Takeda R, Yamaguchi T, Hayashi S, Sano S, Kawame H, Kanki S, et al. Clinical and molecular features of patients with COL1-related disorders: implications for the wider spectrum and the risk of vascular complications. Am J Med Genet A. 2022;188(9):2560–75. https://doi.org/10.1002/ajmg.a.62887.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098.
Unger S, Ferreira CR, Mortier GR, Ali H, Bertola DR, Calder A, et al. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A. 2023;191(5):1164–209. https://doi.org/10.1002/ajmg.a.63132.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63.
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23(5). https://doi.org/10.1016/j.echo.2010.03.019.
Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. 2017;175(1):148–57. https://doi.org/10.1002/ajmg.c.31539.
Xi L, Zhang H, Zhang Z-L. Clinical and genetic analysis in 185 Chinese probands of osteogenesis imperfecta. J Bone Min Metab. 2021;39(3):416–22. https://doi.org/10.1007/s00774-020-01163-5.
Ju H, Dixon IM. Extracellular matrix and cardiovascular diseases. Can J Cardiol. 1996;12(12):1259–67.
Weis SM, Emery JL, Becker KD, McBride DJ, Omens JH, McCulloch AD. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim). Circ Res. 2000;87(8):663–9.
Pfeiffer BJ, Franklin CL, Hsieh F-h, Bank RA, Phillips CL. Alpha 2(I) collagen deficient oim mice have altered biomechanical integrity, collagen content, and collagen crosslinking of their thoracic aorta. Matrix Biol. 2005;24(7):451–8.
Thiele F, Cohrs CM, Flor A, Lisse TS, Przemeck GKH, Horsch M, et al. Cardiopulmonary dysfunction in the Osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms. Hum Mol Genet. 2012;21(16):3535–45. https://doi.org/10.1093/hmg/dds183.
Zhao D, Liu Y, Liu J, Hu J, Zhang Q, Wang O, et al. Cardiovascular abnormalities and its correlation with genotypes of children with osteogenesis imperfecta. Front Endocrinol (Lausanne). 2022;13:1004946. https://doi.org/10.3389/fendo.2022.1004946.
Pinheiro BS, Barrios PM, Souza LT, Félix TM. Echocardiographic study in children with osteogenesis imperfecta. Cardiol Young. 2020;30(10):1490–5. https://doi.org/10.1017/S1047951120002474.
Radunovic Z, Wekre LL, Diep LM, Steine K. Cardiovascular abnormalities in adults with osteogenesis imperfecta. Am Heart J. 2011;161(3):523–9. https://doi.org/10.1016/j.ahj.2010.11.006.
Vetter U, Maierhofer B, Müller M, Lang D, Teller WM, Brenner R, et al. Osteogenesis Imperfecta in childhood: cardiac and renal manifestations. Eur J Pediatr. 1989;149(3):184–7.
White NJ, Winearls CG, Smith R. Cardiovascular abnormalities in osteogenesis imperfecta. Am Heart J. 1983;106(6):1416–20.
Lamanna A, Fayers T, Clarke S, Parsonage W. Valvular and aortic diseases in osteogenesis imperfecta. Heart Lung Circ. 2013;22(10):801–10. https://doi.org/10.1016/j.hlc.2013.05.640.
Radunovic Z, Wekre LL, Steine K. Right ventricular and pulmonary arterial dimensions in adults with osteogenesis imperfecta. Am J Cardiol. 2012;109(12):1807–13. https://doi.org/10.1016/j.amjcard.2012.01.402.
Jansson A, Saartok T, Werner S, Renström P. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202–6.
Leone V, Tornese G, Zerial M, Locatelli C, Ciambra R, Bensa M, et al. Joint hypermobility and its relationship to musculoskeletal pain in schoolchildren: a cross-sectional study. Arch Dis Child. 2009;94(8):627–32. https://doi.org/10.1136/adc.2008.150839.
Brizola E, Staub ALP, Félix TM. Muscle strength, joint range of motion, and gait in children and adolescents with osteogenesis imperfecta. Pediatr Phys Ther. 2014;26(2):245–52. https://doi.org/10.1097/PEP.0000000000000042.
Larsson LG, Baum J, Mudholkar GS, Kollia GD. Benefits and disadvantages of joint hypermobility among musicians. N Engl J Med. 1993;329(15):1079–82.
Acknowledgements
We thank the patients for their participation in this study and Shanghai Genesky Biotech Co., 102 Ltd. (Shanghai, China) for technical assistance.
Funding
This study was supported by a grant from the National Key Research and Development Program of China (grant no. 2018YFA0800801) and the National Natural Science Foundation of China (grant no. 81870618).
Author information
Authors and Affiliations
Contributions
HZ and ZZ designed the research and revised the manuscript. YM and YJ contributed equally to this study. YM summarized and analyzed the clinical data and drafted the manuscript. YJ helped collect and analyze the data. LS checked the statistical analysis results. ZM offered endocrinology counseling. ZZ and HZ contributed to funding acquisition.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all adult participants and the children’s parents. All methods were performed in accordance with the Declaration of Helsinki.
Consent for publication
Written informed consent for publication was obtained from all adult participants and the Children’s parents.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mei, Y., Jiang, Y., Shen, L. et al. Echocardiographic abnormalities and joint hypermobility in Chinese patients with Osteogenesis imperfecta. Orphanet J Rare Dis 19, 116 (2024). https://doi.org/10.1186/s13023-024-03089-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-024-03089-x